TO THE EDITOR:
In a recent issue of Blood Advances, Yoshioka et al1 performed a model-based meta-analysis (MBMA) to evaluate the optimal dose of rivaroxaban, apixaban, and edoxaban in patients with atrial fibrillation (AF). To develop the model, they used reported population pharmacokinetic (PK) and pharmacodynamic models to estimate time profiles of changes in the prothrombin time (PT) in 5 randomized controlled trials (RCTs) that evaluated the efficacy and safety of oral factor Xa (FXa) inhibitors in AF patients. After assay normalization, the authors calculated the model-based relationships between the simulated PT ratio profiles and the rates of ischemic stroke or systemic embolism and major bleeding, and used this information to determine the dose of each oral FXa inhibitor that would achieve minimal simulated mortality.
The central assumption of this analysis is that normalized prolongation of the PT is an acceptable surrogate for efficacy and safety of the various oral FXa inhibitors. We not only disagree with this assumption, but we also believe that it was not adequately explored. Given these shortcomings, we question the validity of the conclusions derived from the work.
Fundamental to the model of Yoshioka et al is the assumption that the oral FXa inhibitors have consistent effects on the PT. There is abundant evidence that this is not the case.2-4 For example, the PT is more sensitive to rivaroxaban than to apixaban. In fact, the PT is nearly normal in patients given the 2.5-mg twice-per-day dose of apixaban, which is the approved dose for some patients with nonvalvular AF.5 Although rivaroxaban prolongs the PT, the effect is small, even with doses of 20 mg once per day.6 Thus, the PT cannot be considered to be an oral FXa inhibitor–independent surrogate for efficacy and safety. For this reason, the PT is not recommended for monitoring the anticoagulant effect of oral FXa inhibitors.7,8
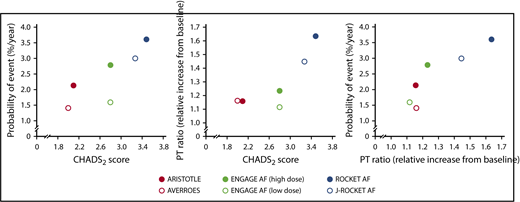
As noted by Yoshioka et al, the control treatment rates for efficacy and safety are likely to vary because of cross-trial differences in the clinical characteristics of the AF patients enrolled in the RCTs. Factors such as CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischemic attack [doubled]) score, prior stroke, age, renal function, history of diabetes, and geographical region influence the efficacy and safety of anticoagulants in AF patients.9,10 The authors explored covariate effects to explain between-trial variability, but they could not identify a statistically significant covariate. This is not surprising, because their model is based on mean values rather than on individual patient data and is therefore built on only 6 data points, resulting in low statistical power. There are 1.8- and 4.6-fold differences in mean CHADS2 score and the proportion of patients with prior stroke, respectively, across the populations studied in the 5 RCTs (Table 1). Figure 1 illustrates the hidden role of the CHADS2 score in the MBMA performed by Yoshioka et al. The left-hand graph shows the expected causal relationship between CHADS2 score (as a measure of disease severity) and the probability of major bleeding events. The middle graph reveals a noncausal association between CHADS2 score and the PT ratio observed across the studies that evaluated oral FXa inhibitors with different effects on the PT. As a logical consequence, there is also a noncausal apparent correlation between PT ratio and the probability of major bleeds, as shown in the right-hand graph of Figure 1, which was erroneously used as a basis for the exposure-response relationship described by Yoshioka et al.1 The study outcomes are influenced by patient characteristics, such as CHADS2 scores, which varied across the studies, and it is likely that the simulated PT simply confounds this relationship.
Mean CHADS2 score, proportion of patients with prior stroke, and dosing regimen in the trials analyzed by Yoshioka et al
| Trial . | Oral FXa inhibitors . | Regimen . | Mean CHADS2 score . | Proportion of patients with prior stroke, % . |
|---|---|---|---|---|
| J-ROCKET AF22 | Rivaroxaban | 15 mg once per d | 3.3 | 64 |
| ROCKET AF24 | Rivaroxaban | 20 mg once per d | 3.5 | 55 |
| ENGAGE20 | Edoxaban | 60 mg once per d | 2.8 | 28 |
| ARISTOTLE21 | Apixaban | 5 mg twice per d | 2.1 | 19 |
| AVERROES19 | Apixaban | 5 mg twice per d | 2.0 | 14 |
| Trial . | Oral FXa inhibitors . | Regimen . | Mean CHADS2 score . | Proportion of patients with prior stroke, % . |
|---|---|---|---|---|
| J-ROCKET AF22 | Rivaroxaban | 15 mg once per d | 3.3 | 64 |
| ROCKET AF24 | Rivaroxaban | 20 mg once per d | 3.5 | 55 |
| ENGAGE20 | Edoxaban | 60 mg once per d | 2.8 | 28 |
| ARISTOTLE21 | Apixaban | 5 mg twice per d | 2.1 | 19 |
| AVERROES19 | Apixaban | 5 mg twice per d | 2.0 | 14 |
Associations between model-estimated PT ratio, mean CHADS2score, and risk of major bleeding events. Population means of the time average of the PT ratio were digitized from Figure 2B and mean CHADS2 scores and probability of event were taken from Table 1 in Yoshioka et al.1
Associations between model-estimated PT ratio, mean CHADS2score, and risk of major bleeding events. Population means of the time average of the PT ratio were digitized from Figure 2B and mean CHADS2 scores and probability of event were taken from Table 1 in Yoshioka et al.1
Despite the fact that the oral FXa inhibitors have different effects on the PT, Yoshioka and colleagues simulated the PT profiles of apixaban and edoxaban on the basis of data with rivaroxaban. The model that was used11 is not suited for extrapolation to the rivaroxaban doses used in the ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) study or to Japanese patients in the J-ROCKET AF study because the model did not account for the previously demonstrated dose effect on rivaroxaban bioavailability or for differences in rivaroxaban PK profiles among patients of different ethnicities.12-16 Furthermore, the correlations between PK, PT, efficacy and safety outcomes, and mortality were combined in a stepwise manner, but the propagation of errors and uncertainties by doing so was not properly considered. Finally, Table 3 in the article by Yoshioka et al is problematic because the identified optimal doses of rivaroxaban lead to higher mortality rates than the currently licensed dose regimen.
In conclusion, we question the validity of the findings reported by Yoshioka et al. The central assumption that oral FXa inhibitors have similar effects on the PT is incorrect. In addition, other methodologic issues disqualify PT as a suitable surrogate for clinical outcomes. In the phase 3 trials that evaluate rivaroxaban for treating venous thromboembolism, there was no evidence of an association between PT values and clinical outcomes.8 RCTs provide the best evidence for medical decision-making.17,18 The phase 3 RCTs that compare the direct oral anticoagulants (DOACs) with vitamin K antagonists for stroke prevention in AF and for treatment of venous thromboembolism included more than 100 000 patients.19-24 In these trials, rivaroxaban, apixaban, edoxaban, and dabigatran were administered in fixed doses without routine coagulation monitoring. The positive results of these trials prompted approval of specific dose regimens for each of the DOACs for these indications. Therefore, contrary to the conclusion of Yoshioka et al, the therapeutic dose of rivaroxaban for AF should be based on the results of phase 3 trials and not on flawed surrogates such as the PT.
Acknowledgments:
This work was supported by Bayer AG, Berlin, Germany. Writing and editorial support was provided by Emma Bolton, Gemma Rogers, and Hilary Spencer of Oxford PharmaGenesis Ltd, Oxford, United Kingdom, with funding from Bayer AG.
Contribution: S.W., L.Z., H.M., H.-U.S., T.T., M.K., G.P., J.I.W., and R.B. analyzed the model, the data, and the concept of the work of Yoshioka et al and contributed to writing the reply; and S.D.B. contributed to the background and clinical interpretation of the work of Yoshioka et al and the scientific material it is based on, the evaluation performed by our team, and to coauthoring the reply.
Conflict-of-interest disclosure: S.W., R.B., H.M., and H.-U.S. are employees of Bayer AG. R.B., H.M., and H.-U.S. own stock in Bayer AG. L.Z. and G.P. are employees of Janssen Research & Development LLC and own stock in Johnson & Johnson. J.I.W. is a consultant for and has received honoraria from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Ionis, Janssen, Merck, Novartis, Pfizer, and Portola. S.D.B. is an employee of Bayer US LLC. T.T. and M.K. are employees of Bayer Yakuhin Ltd.
Correspondence: Stefan Willmann, Clinical Pharmacometrics, Bayer AG, Aprather Weg 18, Building 431, 42096 Wuppertal, Germany; e-mail: stefan.willmann@bayer.com.