Issue Archive
Table of Contents
BLOOD COMMENTARIES
BLOOD SPOTLIGHT
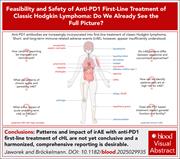
Feasibility and safety of anti-PD1 first-line treatment of classic Hodgkin lymphoma: do we see the full picture?
Clinical Trials & Observations
Immune checkpoint inhibition using antibodies blocking the action of programmed cell death protein 1 (PD1) is now standard therapy for patients with relapsed or refractory Hodgkin lymphoma (HL), and its use in combination regimens in first-line therapy increases efficacy. However, PD1 inhibitor use also brings automimmune toxicities, which need to be weighed against incremental efficacy. In this Blood Spotlight, Jaworek and Bröckelmann summarize what is known about these immune-related toxicities, emphasizing those which may persist, become chronic, or occur late. They contextualize how these toxicities need to be reported in trials and considered in weighing whether their benefits exceed their risks in first-line therapy for patients with HL who are highly curable with cytotoxic chemotherapy alone.
CLINICAL TRIALS AND OBSERVATIONS
Acalabrutinib treatment for older (aged ≥80 years) and/or frail patients with CLL: primary end point analysis of the CLL-Frail trial
Clinical Trials & Observations
Many patients with chronic lymphocytic leukemia (CLL) are older (>80 years old) and/or frail, yet they are often not included in clinical trials. Simon and colleagues report a dedicated trial for such patients, investigating the Bruton tyrosine kinase inhibitor acalabrutinib as initial therapy. They report that while two-thirds experienced severe adverse events, efficacy was high, and more than half of patients recorded an improvement in their self-perceived frailty. This trial sets the benchmark for ongoing development of safer, more effective therapy for CLL in this population.
CD371-targeted CAR T cells secreting interleukin-18 exhibit robust expansion and clear refractory acute myeloid leukemia
Clinical Trials & Observations
The efficacy of chimeric antigen receptor (CAR) T-cell therapy for acute myeloid leukemia (AML) has been limited by several factors, including the lack of a universal target. Geyer et al report design of CAR T cells armed with interleukin-18 (IL-18) secretion that target CD371, a transmembrane glycoprotein with high expression on AML and leukemia-initiating cells. In a pilot study, 3 of the first 5 patients achieved remission, with single-cell analyses indicating that response is associated with expansion of CD8+ effector memory CAR T cells and with activation of endogenous natural killer cells, consistent with the hypothesis that IL-18 will increase both innate immunity and promote CAR T-cell cytotoxicity.
GENE THERAPY
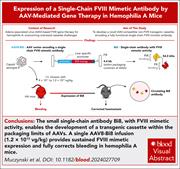
Alternative AAV gene therapy for hemophilia A using expression of Bi8, a novel single-chain FVIII-mimetic antibody
Could there be a better gene than the factor VIII (FVIII) gene to transfer for curative treatment of hemophilia A? Muczynski et al demonstrate stable correction of the bleeding diathesis in hemophilia A mice after gene therapy with an adeno-associated virus (AAV) vector encoding a novel single-chain FVIII-mimetic antibody, Bi8, expressed from hepatocytes. Similar to emicizumab, Bi8 binds FIX and FX simultaneously, enhancing FIXa-mediated FX activation. This novel approach, if translatable in clinical trials, may help overcome some of the limitations of currently approved hemophilia A gene therapies, including those in patients with FVIII-neutralizing antibodies.
HEMATOPOIESIS AND STEM CELLS
A genome-wide screen identifies Runx2 as a novel regulator of hematopoietic stem cell expansion and T-cell commitment
In vitro expansion of hematopoietic stem cells (HSCs) remains a significant goal for cellular therapy. Using a genome-wide CRISPR knockout screen, Meaker and colleagues identify that the transcription factor Runx2 is a key negative regulator of HSC expansion and T-cell lineage commitment ex vivo and in vivo. These data add to our understanding of intrinsic regulation of HSC proliferation and fate determination while also suggesting strategies to apply this to augment various cellular therapies.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
LYMPHOID NEOPLASIA
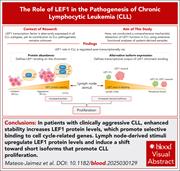
Increased LEF1 protein levels and isoform switching drive cell proliferation in chronic lymphocytic leukemia
MYELOID NEOPLASIA
Genome-wide analysis defines genetic determinants of MPN subtypes and identifies a sex-specific association at CDH22/CD40
Brief Report
Most myeloproliferative neoplasms (MPNs) arise because of 1 of 3 distinct somatic mutations (JAK2, MPL, or CALR) affecting JAK-STAT signaling, yet their phenotypes are quite diverse and not readily explained by the specific driver mutation. Increasing evidence highlights germ line predisposition as a key modifier of MPN phenotypes. Tapper et al used genome-wide association analyses to identify germ line genetic determinants of MPN phenotypes and leverage this to refine genetic risk prediction.
THROMBOSIS AND HEMOSTASIS
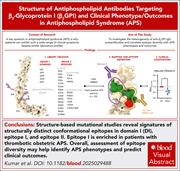
Domain and residue mapping of autoantibodies to β2GPI reveals differences among antiphospholipid syndrome phenotypes
Antiphospholipid syndrome (APS) is an autoimmune disorder defined by the occurrence of thrombosis or pregnancy-associated complications in patients who are persistently positive for antiphospholipid antibodies, many of which bind the plasma protein β2-glycoprotein I (β2GPI). By analyzing antibodies from 52 patients with APS and performing structure-based mutational studies, Kumar et al showed that these autoantibodies recognize 2 distinct epitopes in the first domain of the protein. Their work challenges the prevailing view of a single pathogenic “hot spot,” provides mechanistic insight into how these antibodies engage their target, and begins to allow association of different APS clinical phenotypes with epitope specificity.
LETTER TO BLOOD
BLOOD WORK
ERRATA
-
Cover Image
Cover Image
![issue cover]()
Bone marrow micrograph from a patient with acute myeloid leukemia (AML) at 15 days post-CD371/SAVVY/IL-18 chimeric antigen receptor T-cell therapy demonstrating markedly hypocellular marrow (<5%) and no morphologic evidence of AML (hematoxylin and eosin stain; magnification ×40). See the article by Geyer et al on page 3163.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals







Acalabrutinib to assail CLL in the frail
Clinical Trials & Observations