Key Points
Salvage autologous transplant vs lenalidomide/dexamethasone offers no significant survival benefit in RRMM.
Time to progression after a frontline autologous transplant does not predict benefit from salvage autologous transplant.
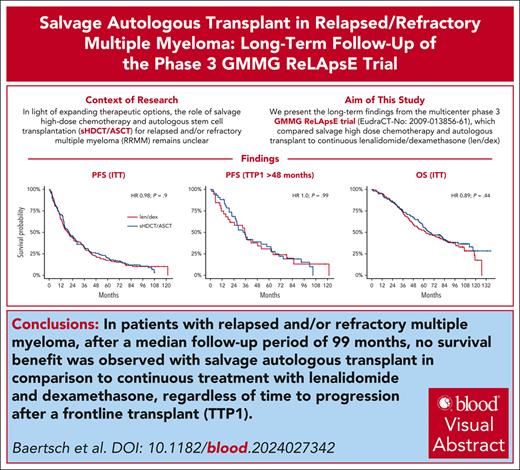
Visual Abstract
The multicenter, phase 3 German-Speaking Myeloma Multicenter Group (GMMG) ReLApsE trial randomized patients with relapsed and/or refractory multiple myeloma (RRMM) equally to lenalidomide/dexamethasone (LEN/DEX; 25 mg days 1-21, DEX 40 mg weekly, in 4-week cycles) reinduction, salvage high-dose chemotherapy (sHDCT; melphalan 200 mg/m2), autologous stem cell transplantation (ASCT), and LEN maintenance (10 mg/d; transplant arm, n = 139) vs continuous LEN/DEX (control arm, n = 138). Ninety-four percent of patients had received frontline HDCT/ASCT. We report an updated analysis of survival end points with a median follow-up of 99 months. Median progression-free survival (PFS) was 20.5 and 19.3 months in the transplant and control arm, respectively (hazard ratio [HR], 0.98; P = .9). Median overall survival (OS) was 67.1 and 62.7 months, respectively, (HR 0.89; P = .44). Landmark analyses from sHDCT and the contemporaneous LEN/DEX cycle 5 were performed because of 29% dropout of patients before sHDCT/ASCT in the transplant arm but did not reveal significant differences in PFS/OS. Time to progression after frontline HDCT/ASCT was a prognostic factor but did not predict benefit from sHDCT/ASCT. The GMMG ReLApsE trial does not support use of sHDCT/ASCT in RRMM after frontline HDCT/ASCT. This trial was registered at www.clinicaltrialsregister.eu as #EudraCT2009-013856-61.
Introduction
The role of salvage high-dose chemotherapy (sHDCT)/autologous stem cell transplantation (ASCT) for relapsed and/or refractory multiple myeloma (RRMM) in the era of novel agent treatment is poorly defined. In clinical practice, treatment options for RRMM are selected based on patient- and disease-related factors as well as prior treatment and regulatory frameworks.1,2 Major guidelines (National Comprehensive Cancer Network, European Hematology Association/European Society for Medical Oncology, American Society for Transplantation and Cellular Therapy, European Society for Blood and Marrow Transplantation, and International Myeloma Working Group) recommend considering sHDCT/ASCT for patients after frontline HDCT/ASCT if time to progression after frontline HDCT/ASCT (TTP1) was at least 18 months, or 36 months in case of maintenance treatment.1-3 Only 2 prospective, phase 3, randomized controlled trials (RCTs) on sHDCT/ASCT vs nontransplant regimens for RRMM have been published. The National Cancer Research Institute Myeloma X Relapse RCT4 demonstrated a significant progression-free survival (PFS) and overall survival (OS) benefit of sHDCT/ASCT compared with a now outdated comparator of cyclophosphamide (400 mg/m2 weekly) for 12 weeks. In the primary analysis of the German-Speaking Myeloma Multicenter Group (GMMG) phase 3 ReLApsE RCT,5 we reported the absence of a PFS and OS benefit of sHDCT/ASCT after lenalidomide/dexamethasone (LEN/DEX) reinduction and followed by LEN maintenance compared with continuous LEN/DEX. However, 29% of patients in the transplant arm dropped out of the trial before sHDCT/ASCT and landmark analyses from sHDCT suggested improved survival in patients who actually received sHDCT/ASCT, but OS data were immature. Here, we report the long-term follow-up analysis of survival end points and key post hoc subgroup analyses.
Study design
Trial design
A summarized trial protocol has been published.6 In brief, across 16 trial sites in Germany, 282 patients with RRMM aged ≤75 years with 1 to 3 prior lines of treatment were randomized equally to LEN/DEX (LEN 25 mg days 1-21, DEX 40 mg weekly, 4-week cycles) reinduction, sHDCT (melphalan 200 mg/m2), ASCT (≥2 × 106 CD34+ cells per kg), and LEN maintenance (10 mg daily; transplant arm) vs continuous LEN/DEX (control arm), each until progressive disease (PD) or intolerable toxicity. Major exclusion criteria were LEN-refractory disease, prior sHDCT/ASCT, and TTP1 after frontline HDCT/ASCT of <12 months.
Documented approval from the ethics committees/institutional review boards of the Medical Faculty of Heidelberg University (main institutional review board) and all participating study sites had been obtained before study start.
End points
An updated analysis of protocol-specified long-term follow-up data regarding the primary end point (PFS) and secondary end points (OS and posttrial treatment including further sHDCT/ASCT in the control arm), as well as key post hoc subgroup analyses are reported. Statistical methodology is detailed in the supplemental Methods, available on the Blood website.
Results and discussion
Between 2 December 2010 and 18 March 2016, 282 patients were randomized (intention-to-treat population, n = 277; supplemental Figure 1). Baseline patient characteristics are listed in Table 1. Nearly all patients were enrolled after 1 prior line of treatment (260/277 [94%]) and had received frontline HDCT/ASCT (259/277 [94%]), including frontline tandem HDCT/ASCT in 105 of 277 (38%) patients. Overall, 11% (30/277) of patients had previous LEN exposure.
Patient and disease characteristics (intention-to-treat population)
| . | Transplant arm (n = 139) . | Control arm (n = 138) . |
|---|---|---|
| Age, y | 61.3 (29.9-74.7) | 62.2 (41.9-74.5) |
| Interval diagnosis to randomization, y | 3.9 (0.2-19.3) | 4.1 (0.7-16.4) |
| Sex | ||
| Female | 60 (43%) | 54 (39%) |
| Male | 79 (57%) | 84 (61%) |
| WHO PS | ||
| 0 | 96 (69%) | 105 (76%) |
| 1 | 43 (31%) | 32 (23%) |
| 2 | 0 | 1 (1%) |
| ISS stage∗ | ||
| I | 82/131 (63%) | 77/129 (60%) |
| II | 32/131 (24%) | 40/129 (31%) |
| III | 17/131 (13%) | 12/129 (9%) |
| Heavy chain isotype | ||
| IgG | 79 (57%) | 71 (52%) |
| IgA | 33 (24%) | 33 (24%) |
| IgD | 1 (1%) | 0 |
| Light chain myeloma | 26 (19%) | 33 (24%) |
| Light chain isotype | ||
| κ | 87 (63%) | 102 (75%) |
| λ | 52 (37%) | 35 (26%) |
| Cytogenetic aberrations∗ | ||
| t(4;14) | 19/94 (20%) | 10/99 (10%) |
| t(11;14) | 17/88 (19%) | 20/96 (21%) |
| t(14;16) | 2/90 (2%) | 0/97 |
| del13q14 | 59/97 (61%) | 45/104 (43%) |
| del17p13 | 14/98 (14%) | 15/107 (14%) |
| Gain1q (>3 copies) | 11/97 (11%) | 12/105 (11%) |
| High risk† | 39/91 (43%) | 31/98 (32%) |
| Standard risk | 52/91 (57%) | 67/98 (68%) |
| LDH | ||
| Normal | 115 (83%) | 114 (83%) |
| Elevated | 24 (17%) | 24 (17%) |
| eGFR | ||
| ≥60 mL/min per 1.73 m2 | 108 (82%) | 106 (79%) |
| <60 mL/min per 1.73 m2 | 23 (18%) | 28 (21%) |
| Prior lines of therapy | ||
| 1 | 131 (94%) | 129 (94%) |
| 2 | 5 (4%) | 8 (6%) |
| 3 | 3 (2%) | 1 (1%) |
| Frontline HDCT/ASCT | 129 (93%) | 130 (94%) |
| Single | 83 (64%) | 71 (55%) |
| Tandem | 46 (36%) | 59 (45%) |
| TTP1, mo‡ | 34.3 (12.3-136.8) | 31.3 (12.1-131) |
| 12-24 | 44 (34%) | 47 (36%) |
| 24-48 | 42 (33%) | 51 (39%) |
| >48 | 43 (33%) | 32 (25%) |
| Prior therapies | ||
| Bortezomib | 107 (77%) | 106 (77%) |
| Thalidomide | 31 (22%) | 25 (18%) |
| Lenalidomide | 12 (9%) | 18 (13%) |
| Interferon | 9 (6%) | 9 (7%) |
| Chemotherapy only | 14 (10%) | 10 (7%) |
| Prior maintenance therapies | ||
| Any | 46 (33%) | 50 (36%) |
| Thalidomide | 25 (18%) | 19 (14%) |
| Interferon | 9 (6%) | 9 (7%) |
| Lenalidomide | 6 (4%) | 10 (7%) |
| Bortezomib | 5 (4%) | 12 (9%) |
| Ixazomib | 4 (3%) | 2 (1%) |
| Dexamethasone | 1 (1%) | 2 (1%) |
| . | Transplant arm (n = 139) . | Control arm (n = 138) . |
|---|---|---|
| Age, y | 61.3 (29.9-74.7) | 62.2 (41.9-74.5) |
| Interval diagnosis to randomization, y | 3.9 (0.2-19.3) | 4.1 (0.7-16.4) |
| Sex | ||
| Female | 60 (43%) | 54 (39%) |
| Male | 79 (57%) | 84 (61%) |
| WHO PS | ||
| 0 | 96 (69%) | 105 (76%) |
| 1 | 43 (31%) | 32 (23%) |
| 2 | 0 | 1 (1%) |
| ISS stage∗ | ||
| I | 82/131 (63%) | 77/129 (60%) |
| II | 32/131 (24%) | 40/129 (31%) |
| III | 17/131 (13%) | 12/129 (9%) |
| Heavy chain isotype | ||
| IgG | 79 (57%) | 71 (52%) |
| IgA | 33 (24%) | 33 (24%) |
| IgD | 1 (1%) | 0 |
| Light chain myeloma | 26 (19%) | 33 (24%) |
| Light chain isotype | ||
| κ | 87 (63%) | 102 (75%) |
| λ | 52 (37%) | 35 (26%) |
| Cytogenetic aberrations∗ | ||
| t(4;14) | 19/94 (20%) | 10/99 (10%) |
| t(11;14) | 17/88 (19%) | 20/96 (21%) |
| t(14;16) | 2/90 (2%) | 0/97 |
| del13q14 | 59/97 (61%) | 45/104 (43%) |
| del17p13 | 14/98 (14%) | 15/107 (14%) |
| Gain1q (>3 copies) | 11/97 (11%) | 12/105 (11%) |
| High risk† | 39/91 (43%) | 31/98 (32%) |
| Standard risk | 52/91 (57%) | 67/98 (68%) |
| LDH | ||
| Normal | 115 (83%) | 114 (83%) |
| Elevated | 24 (17%) | 24 (17%) |
| eGFR | ||
| ≥60 mL/min per 1.73 m2 | 108 (82%) | 106 (79%) |
| <60 mL/min per 1.73 m2 | 23 (18%) | 28 (21%) |
| Prior lines of therapy | ||
| 1 | 131 (94%) | 129 (94%) |
| 2 | 5 (4%) | 8 (6%) |
| 3 | 3 (2%) | 1 (1%) |
| Frontline HDCT/ASCT | 129 (93%) | 130 (94%) |
| Single | 83 (64%) | 71 (55%) |
| Tandem | 46 (36%) | 59 (45%) |
| TTP1, mo‡ | 34.3 (12.3-136.8) | 31.3 (12.1-131) |
| 12-24 | 44 (34%) | 47 (36%) |
| 24-48 | 42 (33%) | 51 (39%) |
| >48 | 43 (33%) | 32 (25%) |
| Prior therapies | ||
| Bortezomib | 107 (77%) | 106 (77%) |
| Thalidomide | 31 (22%) | 25 (18%) |
| Lenalidomide | 12 (9%) | 18 (13%) |
| Interferon | 9 (6%) | 9 (7%) |
| Chemotherapy only | 14 (10%) | 10 (7%) |
| Prior maintenance therapies | ||
| Any | 46 (33%) | 50 (36%) |
| Thalidomide | 25 (18%) | 19 (14%) |
| Interferon | 9 (6%) | 9 (7%) |
| Lenalidomide | 6 (4%) | 10 (7%) |
| Bortezomib | 5 (4%) | 12 (9%) |
| Ixazomib | 4 (3%) | 2 (1%) |
| Dexamethasone | 1 (1%) | 2 (1%) |
Data are presented as n (%), median (range), or n/N (%).
eGFR, estimated glomerular filtration rate; IgG/A/D, immunoglobulin G/A/D/; ISS, International Staging System; LDH, lactate dehydrogenase; WHO PS, World Health Organization performance status.
Data are not available for all randomized patients.
t(4;14), t(14;16), deletion 17p13, and gain 1q21 (>3 copies).
TTP1 in the n = 259 patients with frontline HDCT/ASCT.
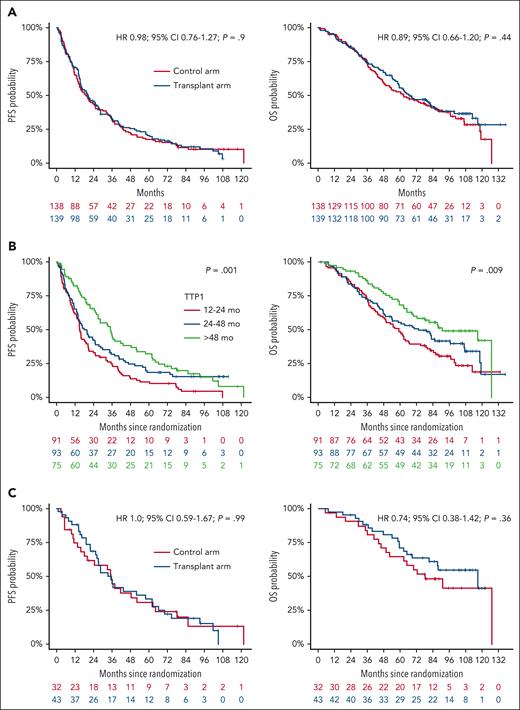
After a median follow-up of 99 months (95% confidence interval [CI], 94-105), 86% of patients (238/277) had experienced a PFS event and 63% (174/277) had died. The transplant arm was not associated with a survival benefit in the intention-to-treat population (Figure 1A). Median PFS was 20.5 (95% CI, 15.9-27.2) months in the transplant arm and 19.3 (95% CI, 14.9-25.4) months in the control arm (hazard ration [HR], 0.98; 95% CI, 0.76-1.27; P = .9); median OS was 67.1 (95% CI, 59.2-85.4) months and 62.7 (95% CI, 49.6-86.0) months, respectively (HR, 0.89; 95% CI, 0.66-1.20; P = .44). The absence of a PFS and OS benefit in the transplant arm was consistent across key disease subgroups according to frontline HDCT/ASCT status (yes/no and single/tandem), age, International Staging System/revised International Staging System, cytogenetic risk status, baseline lactate dehydrogenase, baseline renal function, prior lines of treatment, and maintenance treatment after frontline HDCT/ASCT (supplemental Figure 2). TTP1 was associated with improved survival in the overall trial population (Figure 1B). However, there was no benefit from the transplant arm in any of the subgroups according to TTP1 (Figure 1C; supplemental Figure 3).
PFS and OS from randomization. Kaplan-Meier curves of PFS and OS in the intention-to-treat population by trial arm (A) and by TTP1 (B), as well as in the subgroup with TTP1 of >48 months by trial arm (C).
PFS and OS from randomization. Kaplan-Meier curves of PFS and OS in the intention-to-treat population by trial arm (A) and by TTP1 (B), as well as in the subgroup with TTP1 of >48 months by trial arm (C).
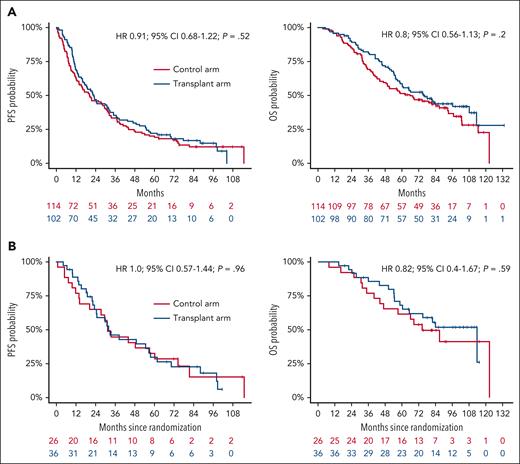
Of 139 patients (29%) in the transplant arm, 41 left the trial before sHDCT/ASCT. Post hoc landmark analyses from sHDCT and the contemporaneous LEN/DEX cycle 5 were performed to assess the treatment effect in patients that reached the sHDCT/ASCT stage. Main reasons for dropouts before sHDCT/ASCT in the transplant arm were PD (12% [17/139]), adverse events (6% [8/139]), and withdrawal of consent (5% [7/139]). Univariable landmark analyses (Figure 2A) did not reveal significant differences in PFS (23.0 [95% CI, 17.2-32.2] vs 20.3 [95% CI, 14.1-30.1] months; HR, 0.91; 95% CI, 0.68-1.22; P = .52) nor OS (76.3 [95% CI, 58.8 to not reached] vs 66.0 [95% CI, 49.7-92.9] months; HR, 0.8; 95% CI, 0.56-1.13; P = .2); neither did multivariable analyses (supplemental Table 1). The absence of a survival benefit of the transplant arm from the landmark was consistent across key disease subgroups (supplemental Figure 4) including those according to TTP1 (Figure 2B; supplemental Figure 5).
PFS and OS from the landmark at HDCT/ASCT and LEN/DEX cycle 5. PFS and OS from the landmark by trial arm as Kaplan-Meier curves in all patients that reached the landmark (A) and in the subgroup of patients with TTP1 of >48 months (B).
PFS and OS from the landmark at HDCT/ASCT and LEN/DEX cycle 5. PFS and OS from the landmark by trial arm as Kaplan-Meier curves in all patients that reached the landmark (A) and in the subgroup of patients with TTP1 of >48 months (B).
During subsequent lines of treatment after trial (supplemental Table 2), patients from the control arm more frequently underwent sHDCT/ASCT as an immediate next line of treatment (34/138 [25%] vs 12/139 [9%] patients) as well as overall (48/138 [35%] vs 19/139 [14%]). Sensitivity analyses estimating crossover adjusted OS times to account for the increased frequency of sHDCT/ASCT after PD in the control arm were consistent with the unadjusted analysis (supplemental Figure 6).
Second primary malignancies (supplemental Table 4) occurred in 24 of 135 patients [18%] and 20 of 145 patients [14%] treated according to the transplant and control arm, respectively (P = .41).
The GMMG ReLApsE trial remains, to our knowledge, the only RCT comparing sHDCT/ASCT with continuous novel agent treatment. With extended follow-up we observe a lower effect of the transplant arm across survival analyses than the initial publication5 (supplemental Table 3). This reduction in effect size is most pronounced in landmark analyses for OS, likely because of smallest sample size and least mature data at the time of the initial publication.5 Consequently, no significant PFS or OS difference between trial arms is observed in the overall trial population, and the previously reported, significant survival benefit in patients who actually received sHDCT/ASCT is not confirmed with extended follow-up.
Moreover, based on our data, TTP1, which is frequently used to stratify for sHDCT/ASCT in clinical practice, is a general prognostic parameter but does not predict a survival benefit from sHDCT/ASCT vs continuous LEN/DEX. Even in the subgroup of 75 patients with TTP1 beyond 48 months, PFS and OS was not significantly different between trial arms. Importantly, this is well beyond the 18- and 36-month TTP1 cutoffs recommended by guidelines1,3 as a selection criterion for sHDCT/ASCT. We thus expect these updated results to affect clinical practice in the field.
As opposed to when the ReLApsE trial was planned, LEN/DEX is no longer standard treatment in resource-rich countries1,2; after 1 to 3 prior lines of treatment, patients are commonly LEN-refractory because of near universal use of LEN maintenance after frontline HDCT/ASCT7 and as part of combination regimens. However, in the absence of a PFS benefit of sHDCT/ASCT over LEN/DEX, a benefit over even more effective, state-of-the-art LEN-based8,9 or LEN-free10,11 triplet regimens and chimeric antigen receptor (CAR) T cells12 is unlikely.
Although no subgroups benefited from sHDCT/ASCT in the ReLApsE trial, the long follow-up allowed us to identify factors associated with exceptional PFS (≥5 years) despite less than state-of-the-art treatment. Beyond TTP1, these were factors related to disease biology, primarily absence of high-risk cytogenetic lesions and normal lactate dehydrogenase (supplemental Table 5), which were also associated with survival in multivariate analyses (supplemental Table 1). Retrospectively, the subgroup (n = 25) selected by these factors achieved median PFS of 41.7 months as compared with 15.9 months for the remainder (n = 153) of the trial population (P = .007; supplemental Figure 7). Conversely, patients with high-risk features have the highest medical need and benefit most from advanced treatment options as has been shown for functional high-risk patients with CAR T cells.13 These factors may thus inform treatment stratification in resource-limited settings (including limited slot availability in countries in which CAR T cells are approved in second line) and in avoiding rare but severe side effects of CAR T-cell therapy in low-risk patients.
Our data do not preclude sHDCT/ASCT for patients who actively wish to pursue such a strategy typically after above-average tolerability and TTP1 in the context of frontline transplant. Although fixed duration treatment would be attractive in terms of quality of life, these patients should be counseled that, unlike with CAR T cells, treatment continuation/maintenance treatment is necessary after sHDCT/ASCT to achieve median PFS beyond 1 to 1.5 years.4,14,15
The true impact on OS of receiving sHDCT/ASCT (as opposed to never receiving sHDCT/ASCT) is more difficult to assess because the ReLApsE trial was not powered to detect an OS difference. Moreover, 29% of patients in the transplant arm did not receive the planned sHDCT/ASCT on trial and 35% of patients in the control arm received sHDCT/ASCT as a further line of treatment after disease progression. We addressed these factors in landmark analyses from the time of sHDCT/ASCT and in sensitivity analyses to account for crossover, which did not indicate a major effect of sHDCT/ASCT on OS. Importantly, any potential effect needs to be balanced against the transient (∼6 months) negative impact on quality of life associated with sHDCT/ASCT16 and the availability of effective treatment modalities such as CAR T cells and triplet regimens for RRMM.
To the best of our knowledge, no RCTs are being conducted or planned to evaluate sHDCT/ASCT against any of the current standard regimens for RRMM, thus further underlining the significance of our results.
Although we address the question of sHDCT/ASCT after previous frontline HDCT/ASCT, the number of patients without the latter is insufficient to draw conclusions. Importantly, our data do not address the issue of deferring transplant to relapse in favor of transplant-free frontline regimens.
In conclusion, the GMMG ReLApsE trial does not support sHDCT/ASCT at relapse after previous frontline HDCT/ASCT regardless of TTP1.
Acknowledgments
The trial was designed and conducted by the German Myeloma Multicenter Group (GMMG). The authors thank all investigators, study nurses, and all members of the study teams at the participating trial sites, the research staff, the laboratory teams, the coordination centers for clinical trials in Heidelberg and Leipzig, and the participating patients and their families for their contributions.
This work was supported by the Dietmar Hopp Foundation, as well as by Celgene, Chugai, and Amgen.
Authorship
Contribution: H.G. designed the trial; H.G., M.-A.B., J.S., and T.H. analyzed the data; H.G., M.-A.B., J.S., M.S.R., S.S., M.M., E.K.M., A.J., P.B., M.G., S.K., B.G., P.R., U.G., R.F., M.H., I.v.M., H.W.L., C.S., I.-W.B., H.J.S., R.N., B.B., and K.C.W. collected data; M.-A.B. wrote the manuscript; and all authors revised and approved the manuscript.
Conflict-of-interest disclosure: M.-A.B. reports consultancy with Takeda and Novartis; received honoraria from Takeda, Novartis, Sanofi, and Stemline; received travel support from Celgene, Amgen, Janssen, and Sanofi; and received research funding from Novartis and Sanofi. M.S.R. reports consultancy with, honoraria from, and membership on an entity's board of directors or advisory committees of, Janssen; AbbVie, GlaxoSmithKline (GSK), Bristol Myers Squibb (BMS), Sanofi and Amgen; and received research funding from Janssen, BMS, Sanofi, and Heidelberg Pharma. M.M. reports honoraria from Amgen, Takeda, BMS, Janssen, Stemline, and Roche. E.K.M. reports a consulting or advisory role with Amgen, BMS/Celgene, GSK, Janssen-Cilag, Oncopeptides, Pfizer, Sanofi, Stemline, and Takeda; reports honoraria from Amgen, BMS/Celgene, GSK, Janssen-Cilag, Oncopeptides, Pfizer, Sanofi, Stemline, and Takeda; reports research funding from BMS/Celgene, GSK, Janssen-Cilag, Sanofi, and Takeda; and reports travel accommodations and expenses from BMS/Celgene, GSK, Janssen-Cilag, Sanofi, Stemline, and Takeda. S.K. reports honoraria from Amgen. B.G. reports consultancy/advisory board membership with Roche, Kite, and Novartis; reports grants from, or contracts with, Roche, Kite, and Novartis; received honoraria from Roche, Kite, and Novartis; and received travel support from Roche, Kite, and Novartis. U.G. reports stock and other ownership interests in BioNTech; reports honoraria from Boehringer Ingelheim, Amgen, AstraZeneca, BMS, Merck Sharp & Dohme (MSD) Oncology, Sanofi, Fujifilm, Novartis, Cellrion, and Ipsen; reports consultancy/advisory board membership with Amgen and MSD Oncology; received research funding from Ipsen, MacroGenics; and reports travel support from Boehringer Ingelheim, and GSK. R.F. reports honoraria from Janssen, Amgen, GSK, Takeda, and BMS. M.H. reports consultancy with Novartis, BMS/Celgene, Gilead, Pfizer, Incyte, Sanofi/Aventis, Roche, Amgen, Sobi, and Janssen; and reports honoraria from Novartis, Sobi, Gilead, and Falk Foundation. I.v.M. reports consultancy with Takeda, Pfizer, Sanofi, and BMS. C.S. reports consultancy/advisory board membership with BMS, GSK, Janssen, Pfizer, Roche, and Takeda; reports honoraria from Amgen, BMS, GSK, Janssen, MSD, Novartis, Roche, Sanofi, and Takeda; reports research funding from Janssen and Takeda; and received travel support from BMS, Janssen, Sanofi Aventis, and Takeda. H.J.S. reports consultancy/advisory board membership with Amgen, AstraZeneca, BMS/Celgene, Genzyme, GSK, Janssen-Cilag, Oncopeptides, Pfizer, Sanofi, and Stemline; reports honoraria from AbbVie, Amgen, AstraZeneca, BMS/Celgene, Genzyme, GSK, Janssen-Cilag, Oncopeptides, Pfizer, Roche, Sanofi, Stemline, and Takeda; and received travel support from Amgen, BMS/Celgene, Janssen-Cilag, and Sanofi. B.B. reports honoraria from Janssen-Cilag, Amgen, Sanofi, GSK, and Pfizer; and travel support from Janssen-Cilag, Amgen, and Sanofi. K.C.W. reports consultancy/advisory board membership with AbbVie, Amgen, Adaptive Biotech, BMS/Celgene, BeiGene, Janssen, GSK, Karyopharm, Oncopeptides, Pfizer, Regeneron, Roche Pharma, Sanofi, Takeda, and Menarini; reports honoraria from AbbVie, Amgen, Adaptive Biotech, AstraZeneca, BMS/Celgene, BeiGene, Janssen, GSK, Karyopharm, Novartis, Oncopeptides, Pfizer, Roche Pharma, Sanofi, Stemline, Takeda, and Menarini; and received research funding from AbbVie, Amgen, BMS/Celgene, Janssen, GSK, Pfizer, Sanofi, and Takeda. H.G. reports consultancy/advisory board membership with Amgen, BMS, Janssen, Sanofi, and Adaptive Biotechnology; received honoraria from Amgen, BMS, Chugai, GSK, Janssen, Novartis, Sanofi, and Pfizer; received research funding from Amgen, BMS, Celgene, GlycoMimetics Inc, GSK, Heidelberg Pharma, Hoffmann-La Roche, Karyopharm, Janssen, Incyte Corporation, Millenium Pharmaceuticals Inc, Molecular Partners, Merck Sharp and Dohme, MorphoSys AG, Pfizer, Sanofi, Takeda, and Novartis; received travel support from Amgen, BMS, GSK, Janssen, Novartis, Sanofi, and Pfizer; reports grants from Amgen, Array Biopharma/Pfizer, BMS/Celgene, Chugai, Dietmar Hopp Foundation, Janssen, Johns Hopkins University, Mundipharma GmbH, and Sanofi. The remaining authors declare no competing financial interests.
A list of the German-Speaking Myeloma Multicenter Group trial sites and investigators involved in the ReLApsE trial appears in “Appendix.”
Correspondence: Hartmut Goldschmidt, Department of Internal Medicine V, University Hospital Heidelberg, INF 410, 69120 Heidelberg, Germany; email: hartmut.goldschmidt@med.uni-heidelberg.de.
Appendix
The members of the German-Speaking Myeloma Multicenter Group (GMMG) trial sites and investigators involved in the ReLApsE trial are Hartmut Goldschmidt, Heidelberg Myeloma Center and GMMG Study Group, Department of Medicine V, Heidelberg University Hospital and Medical Faculty Heidelberg, Heidelberg, Germany; Britta Besemer and Katja C. Weisel, Department of Hematology, Hematology, Oncology, Immunology and Rheumatology, University of Tuebingen, Tuebingen, Germany; Richard Noppeney, Department of Hematology, University Hospital Essen, Essen, Germany; Hans J. Salwender, Asklepios Tumorzentrum Hamburg, AK Altona, Hamburg, Germany; Igor-Wolfgang Blau and Axel Nogai, Internal Medicine III, Charité Campus Benjamin Franklin, Berlin, Germany; Christof Scheid, Department I of Internal Medicine, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf, University of Cologne, Cologne, Germany; Hans W. Lindemann, Department of Hematology and Oncology, Kath. Krankenhaus Hagen gem. GmbH, Hagen, Germany; Ivana von Metzler and Hans Martin, Department of Hematology and Oncology, Goethe University, Frankfurt, Germany; Mathias Haenel, Department of Hematology, Oncology and Stem Cell Transplantation, Klinikum Chemnitz GmbH, Chemnitz, Germany; Roland Fenk, Department of Hematology, Oncology and Clinical Immunology, University of Duesseldorf, Duesseldorf, Germany; Ullrich Graeven, Department of Hematology, Oncology and Gastroenterology, Kliniken Maria Hilf GmbH, Mönchengladbach, Germany; Peter Reimer, Department of Hematology, Oncology and Stem Cell Transplantation, Evangelische Kliniken Essen-Mitte, Essen, Germany; Bertram Glass and Martin Schmidt-Hieber, Department of Hematology and Cellular Therapy, Helios Hospital Berlin-Buch, Berlin, Germany; Stefan Klein, Department of Hematology and Oncology, University Hospital Mannheim, Mannheim, Germany; Martin Goerner, Department of Hematology, Oncology and Palliative Care, Community Hospital Bielefeld, Bielefeld, Germany; Peter Brossart and Ingo Schmidt-Wolf, Department of Oncology, Hematology, Immuno-Oncology and Rheumatology, Med. Klinik und Poliklinik III, University Hospital Bonn, Bonn, Germany.
References
Author notes
The primary analysis of the GMMG ReLApsE trial has previously been published (available at https://doi.org/10.1038/s41375-020-0948-0).
Data are available on request from the corresponding author, Hartmut Goldschmidt (hartmut.goldschmidt@med.uni-heidelberg.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal