Key Points
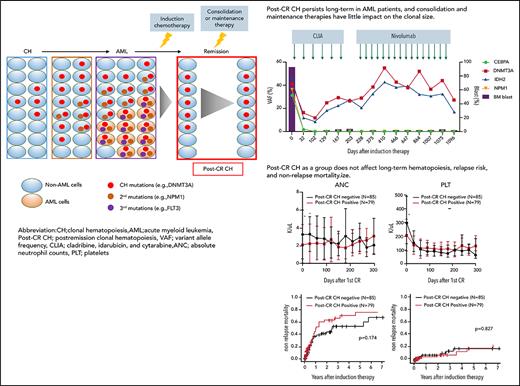
Postremission CH persists long term in AML patients, and consolidation and maintenance therapies have little impact on the clonal size.
Postremission CH as a group does not affect long-term hematopoiesis and relapse risk; however, the impact is heterogeneous among mutations.
Abstract
Although clonal hematopoiesis (CH) can precede the development of acute myeloid leukemia (AML), it can also persist after achieving remission. Long-term clonal dynamics and clinical implications of persistent CH are not well understood. Here, we studied the prevalence, dynamics, and clinical implications of postremission CH in 164 AML patients who attained complete remission after induction chemotherapies. Postremission CH was identified in 79 (48%) patients. Postremission CH persisted long term in 91% of the trackable patients despite treatment with various types of consolidation and maintenance therapies. Postremission CH was eradicated in 20 out of 21 (95%) patients who underwent allogeneic stem cell transplant. Although patients with postremission CH as a group had comparable hematopoiesis with those without it, patients with persistent TET2 mutations showed significant neutropenia long term. Postremission CH had little impact on relapse risk, nonrelapse mortality, and incidence of atherosclerotic cardiovascular disease, although the clinical impact of post-CR CH was heterogeneous among different mutations. These data suggest that although residual clonal hematopoietic stem cells are generally resistant to consolidation and maintenance therapies, they retain the ability to maintain normal hematopoiesis and have little impact on clinical outcomes. Larger study is needed to dissect the gene-specific heterogeneity.
Introduction
Clonal hematopoiesis (CH) is defined by the presence of a hematopoietic subpopulation carrying somatic mutations associated with hematologic malignancies1 and can precede the development of acute myeloid leukemia (AML). Because CH mutations are acquired early in hematopoietic stem cells (HSCs), they are detected in both nonleukemic and leukemic cells.2 We and others have previously shown that CH mutations, particularly DNMT3A, TET2, and ASXL1 (collectively referred to as DTA mutations), frequently persist following complete remission (CR)3-5 in AML. Moreover, CH mutations originally undetectable at diagnosis may expand through preferential selection after therapy.6 Although the persistence of CH mutations is not associated with increased risk of relapse in young AML patients treated with intensive chemotherapies,4 the clinical implications of persistent CH are not fully understood. Furthermore, data on the duration of CH persistence beyond the attainment of CR and whether the mode of postremission therapies (consolidation and maintenance) affect the dynamics of persistent CH are not known. Here, we sequenced multiple longitudinal samples from patients with AML after achieving remission, analyzed the dynamics of persistent CH, and investigated its clinical implications.
Study design
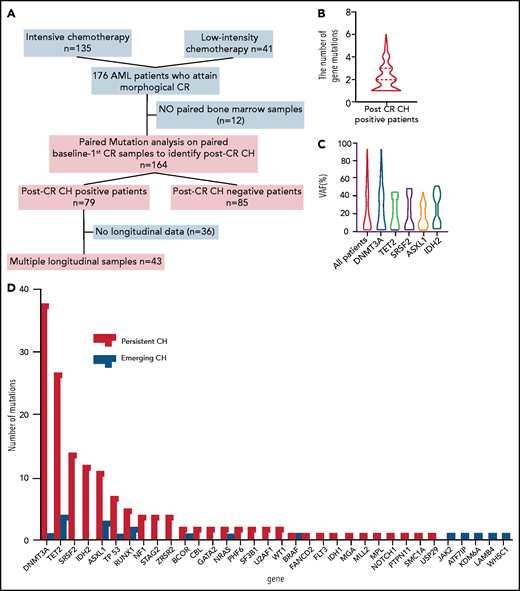
We studied 164 patients with AML who attained morphological CR following induction chemotherapies (Figure 1A). The study was approved by the institutional IRB and conducted in accordance with the Declaration of Helsinki. Bone marrow samples collected at baseline, initial CR (median day 31 [interquartile range (IQR) 27-41]), and subsequent timepoints during CR were sequenced by targeted sequencing of 295 genes (median 484× [IQR 262-590]; supplemental Table 1, available on the Blood Web site) as previously described (supplemental methods).3 Variants with >2.5% variant allele frequency (VAF) at CR were defined as post-CR CH, following the cutoff of molecular minimal residual disease in the previous studies.3-5 Post-CR CH was also stratified by the degree of VAF (low VAF: first quartile; intermediate VAF: second to third quartile; high VAF: fourth quartile). Two types of post-CR CH were defined: (1) persistent preleukemic CH; the variants were detected in the original AML samples and persisted after CR; and (2) emerging CH; the variants were not detected in the original AML samples but emerged after attaining CR. Statistical methods are described in the supplemental methods.
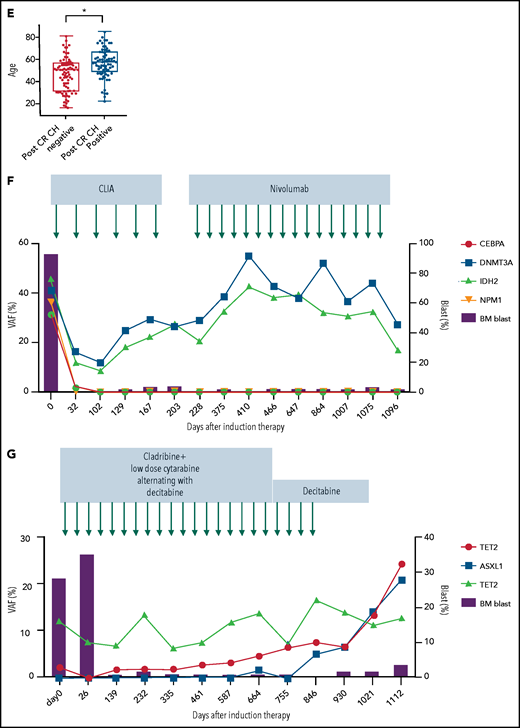
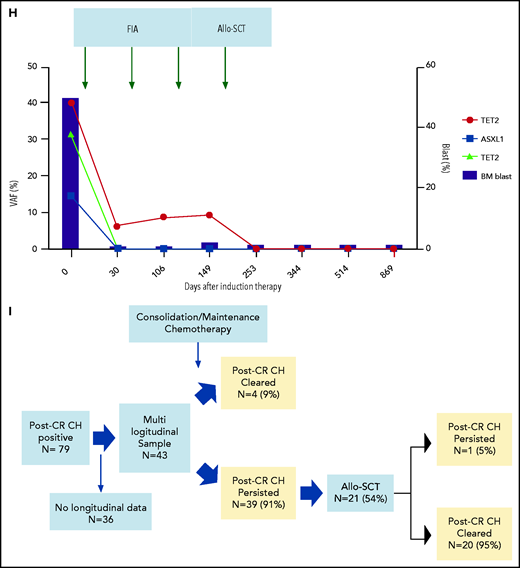
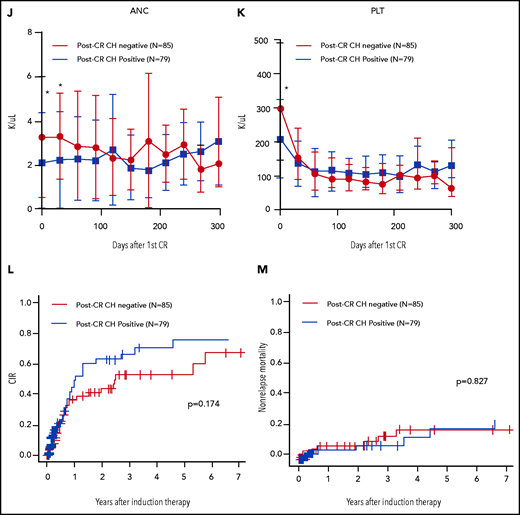
Characteristics, dynamics, and clinical significance of postremission CH in AML. (A) CONSORT diagram for the study cohort. (B) Violin plot showing the distribution of the number of gene mutations detected as post-CR CH. (C) Violin plots showing VAFs of post-CR CH mutations in all patients and based on the mutated genes. (D) Bar graph showing the number of mutations detected as persistent preleukemic CH (red bar) or emerging CH (blue bar). (E) Box plots comparing the age of patients with or without post-CR CH (*P < .001). (F-G) Representative cases with clonal dynamics of persistent preleukemic CH (F), emerging CH (G), and post-CR CH cleared by allo-SCT (H). (I) The summary of the clonal trajectories of post-CR CH in the 43 patients in whom we were able to track the long-term clonal dynamics. (J-K) The long-term trend of peripheral blood counts in patients with (red line) and without post-CR CH (black line). Patients with post-CR CH had significantly lower absolute neutrophil counts (ANC) and platelets (PLT) at the beginning (day 0 corresponds to the first attainment of CR), but later the difference became insignificant. *P < .001. (L) Cumulative incidence of relapse in post-CR CH-patients (black line) and in post-CR CH+ patients (red line). (M) Nonrelapse mortality in post-CR CH−patients (black line) and in post-CR CH+ patients (red line). Data were censored at the time of allo-SCT in first CR. BM, bone marrow; CLIA, cladribine, idarubicin, and cytarabine; FAI, fludarabine, idarubicin, and cytarabine.
Characteristics, dynamics, and clinical significance of postremission CH in AML. (A) CONSORT diagram for the study cohort. (B) Violin plot showing the distribution of the number of gene mutations detected as post-CR CH. (C) Violin plots showing VAFs of post-CR CH mutations in all patients and based on the mutated genes. (D) Bar graph showing the number of mutations detected as persistent preleukemic CH (red bar) or emerging CH (blue bar). (E) Box plots comparing the age of patients with or without post-CR CH (*P < .001). (F-G) Representative cases with clonal dynamics of persistent preleukemic CH (F), emerging CH (G), and post-CR CH cleared by allo-SCT (H). (I) The summary of the clonal trajectories of post-CR CH in the 43 patients in whom we were able to track the long-term clonal dynamics. (J-K) The long-term trend of peripheral blood counts in patients with (red line) and without post-CR CH (black line). Patients with post-CR CH had significantly lower absolute neutrophil counts (ANC) and platelets (PLT) at the beginning (day 0 corresponds to the first attainment of CR), but later the difference became insignificant. *P < .001. (L) Cumulative incidence of relapse in post-CR CH-patients (black line) and in post-CR CH+ patients (red line). (M) Nonrelapse mortality in post-CR CH−patients (black line) and in post-CR CH+ patients (red line). Data were censored at the time of allo-SCT in first CR. BM, bone marrow; CLIA, cladribine, idarubicin, and cytarabine; FAI, fludarabine, idarubicin, and cytarabine.
Results and discussion
Table 1 and supplemental Table 3 describe the clinical characteristics of 164 AML patients. Following induction chemotherapies, 79 patients (48%) had post-CR CH, among which 66 (40%) had persistent preleukemic CH alone, 2 (1%) had emerging CH alone, and 11 (7%) had both. The median number of gene mutations in post-CR CH was 2 (range, 1-6; Figure 1B) per patient. The number of mutations at baseline did not significantly affect the number of post-CR CH mutations (supplemental Figure 1).
Clinical characteristics of 164 AML patients studied
| Characteristics . | All patients, (N = 164) . | No post-CR CH, (N = 85) (% or range) . | Post-CR CH, (N = 79) (% or range) . | P* . |
|---|---|---|---|---|
| Sex | ||||
| Female | 80 (49) | 38 (45) | 42 (53) | .348 |
| Age | 53 | 47 | 57 | <.001 |
| (17-85) | (17-81) | (23-85) | ||
| AML subtype | ||||
| De novo | 129 (79) | 71 (84) | 58 (73) | .162 |
| Secondary | 14 (9) | 4 (5) | 10 (13) | |
| Therapy-related | 21 (13) | 10 (12) | 11 (14) | |
| ELN classification | ||||
| Favorable | 36 (22) | 21 (25) | 15 (19) | .806 |
| Intermediate | 65 (40) | 34 (40) | 31 (39) | |
| Adverse | 61 (37) | 29 (34) | 32 (41) | |
| Induction therapy | ||||
| High intensity | 126 (77) | 74 (87) | 52 (66) | .04 |
| Low intensity | 38 (23) | 11 (13) | 27 (34) | |
| Allo-SCT in −1st CR | ||||
| No | 101 (62) | 56 (66) | 45 (57) | .246 |
| Yes | 63 (38) | 29 (34) | 34 (43) |
| Characteristics . | All patients, (N = 164) . | No post-CR CH, (N = 85) (% or range) . | Post-CR CH, (N = 79) (% or range) . | P* . |
|---|---|---|---|---|
| Sex | ||||
| Female | 80 (49) | 38 (45) | 42 (53) | .348 |
| Age | 53 | 47 | 57 | <.001 |
| (17-85) | (17-81) | (23-85) | ||
| AML subtype | ||||
| De novo | 129 (79) | 71 (84) | 58 (73) | .162 |
| Secondary | 14 (9) | 4 (5) | 10 (13) | |
| Therapy-related | 21 (13) | 10 (12) | 11 (14) | |
| ELN classification | ||||
| Favorable | 36 (22) | 21 (25) | 15 (19) | .806 |
| Intermediate | 65 (40) | 34 (40) | 31 (39) | |
| Adverse | 61 (37) | 29 (34) | 32 (41) | |
| Induction therapy | ||||
| High intensity | 126 (77) | 74 (87) | 52 (66) | .04 |
| Low intensity | 38 (23) | 11 (13) | 27 (34) | |
| Allo-SCT in −1st CR | ||||
| No | 101 (62) | 56 (66) | 45 (57) | .246 |
| Yes | 63 (38) | 29 (34) | 34 (43) |
Clinical characteristics were compared between patients with and without post-CR CH.
Differences in characteristics were tested by Fisher exact test.
The median VAF of post-CR CH was 14% (IQR: 2.5% to 33%; Figure 1C). The most frequently mutated genes for persistent preleukemic CH were DNMT3A (N = 38 [23%]), followed by TET2 (N = 28 [17%]), SRSF2 (N = 14, [9%]), IDH2 (N = 12 [7%]), and ASXL1 (N = 11 [7%]). The mutational profile of emerging CH was different from that of persistent preleukemic CH, in which DNMT3A mutations were rare, whereas signaling mutations (JAK2, BRAF, NRAS) were involved (Figure 1D). As expected, post-CR CH was associated with older age (Figure 1E). Although the sequencing platforms are different, overrepresentation of SRSF2 and IDH2 mutations and relatively higher VAF were notable in post-CR CH compared with age-related CH in the general population (supplemental Figure 2).7
Among 79 patients with post-CR CH, 43 patients had multiple postremission samples available for sequencing (median 5 timepoints per patient, median 467 days of follow-up [IQR 290-827 days]), enabling us to analyze the long-term clonal dynamics and the impact of consolidation or maintenance chemotherapies on post-CR CH. Various types of postremission chemotherapies (some under clinical trials) were used, which included cytarabine-based chemotherapies (N = 29), hypomethylating agents (HMA, N = 12), venetoclax (N = 1), lenalidomide (N = 3), and nivolumab (N = 1; supplemental Table 4). Overall, post-CR CH persisted in 39 of 43 (91%) patients during and after postremission chemotherapies (Figure 1F-I). In 4 (9%) patients, we observed clearance of post-CR CH following high-dose cytarabine regimen (supplemental Figure 3). Although consolidation or maintenance therapies had little impact on post-CR CH, 20 out of 21 (95%) patients who underwent allogeneic stem cell transplant (allo-SCT) had a clearance of post-CR CH regardless of the conditioning intensity (Figure 1H-I; supplemental Table 5; supplemental Figures 4-6). These data suggest that induction or consolidation chemotherapies spare HSCs with CH mutations, whereas conditioning chemotherapies that are meant to ablate HSCs, combined with cell competition of donor cells after transplant, might prevent the rise of CH stem cells. This is broadly consistent with prior findings demonstrating that chemoresistance of DNMT3A-mutant HSCs can be overcome by the higher dose of chemotherapies.8
We then evaluated the impact of post-CR CH in hematopoiesis. Consistent with the previous report,9 patients with post-CR CH had significantly lower neutrophil and platelet count recovery at the first remission (Figure 1J-K). However, this difference became insignificant over time, suggesting that HSCs with CH mutations have comparable long-term capacity in hematopoiesis with wild-type HSCs. Notably, patients with persistent TET2 mutations showed prolonged neutropenia, suggesting that the gene-specific impact of post-CR CH in hematopoiesis may be heterogeneous (supplemental Figures 7-13). None of the other hematologic parameters, including red cell distribution width, demonstrated the difference between patients with or without post-CR CH (supplemental Figures 7-13).
Last, we investigated the correlation between post-CR CH and clinical outcomes. Consistent with the prior studies,3,4 post-CR CH as a group did not affect the relapse risk in AML (Figure 1L). However, subgroup analyses stratified by the genes, the number of mutations, and VAF showed that ASXL1 or TP53 mutations, >1 mutation, and high VAF were associated with increased risk of relapse (supplemental Figures 14-15). We also investigated the association with nonrelapse mortality and the incidence of atherosclerotic cardiovascular disease (ASCVD) after remission. However, we did not observe a significant difference in the incidence of nonrelapse mortality and ASCVD (only 1 patient developed ASCVD) based on the post-CR CH status, regardless of genes, VAF, and the number of mutations (Figure 1M; supplemental Figures 16-18).
Because some post-CR CH using 2.5% VAF cutoff might include molecular minimal residual disease , we also repeated the analyses by considering post-CR CH with intermediate or high VAF only. Intermediate or high VAF post-CR CH was found in 59 patients (36%) with an overall similar mutational landscape (supplemental Figure 19). All other correlations were similar to those of VAF 2.5% cutoff (supplemental Figures 19-20).
In summary, we analyzed the clonal dynamics and clinical implications of post-CR CH in AML. Our data show that post-CR CH persists long term, and it is resistant to various types of consolidation and maintenance therapies except for allo-SCT. Although the biology and chemosensitivity of post-CR CH are likely different from those of preleukemic CH, the lack of changes in VAF during the postremission therapies highlights the challenges of targeting CH by currently available therapeutics, which is consistent with some of the preclinical models.8,10
Overall, postremission CH as a group had little clinical significance. Aside from the mild deficiency in the initial recovery of neutrophil and platelet counts, patients with post-CR CH had comparable long-term hematopoiesis, risk of relapse, and nonrelapse mortality and morbidity to those without it. Although the small sample size might confound these findings in this study, more research is needed to fully understand the long-term clinical consequences and gene-specific impact of post-CR CH.
Acknowledgments
This study was supported, in part by the Cancer Prevention and Research Institute of Texas grant R120501 (P.A.F.), the Welch Foundation grant G-0040 (P.A.F.), the University of Texas System STARS Award grant PS100149 (P.A.F.), Physician Scientist Program at Anderson (K.T.), Lyda Hill Foundation (P.A.F.), the Charif Souki Cancer Research Fund (H.M.K.), the Anderson Cancer Center Leukemia SPORE–National Institutes of Health (NIH), National Cancer Institute (NCI) grant P50 CA100632 (H.M.K. and K.T.), the Anderson Cancer Center Support Grant NIH/NCI P30 CA016672, S10 shared instrumentation grant for NovaSeq 6000 NIH 1S10OD024977-01, Japan Society for the Promotion of Science (T.T. and K.M.), Sabin Family Foundation Fellowship (K.T.), and generous philanthropic contributions from Anderson’s Moon Shot Program (K.T., G.G.-M., and H.M.K.).
Authorship
Contribution: K.T. designed the study and led the research team; T.T., K.M., S.L., F.W., K.F., Y.S., and K.T. collected and analyzed data; J.M., N.D., N.P., C.D.D., M.Y., T.M.K., F.R., M.Y.K., H.M.K., and K.T. collected samples and treated the patients; K.T., C.G., and L.L. performed DNA sequencing; K.T., F.W., and K.F. performed bioinformatics analysis; K.T. and T.T. wrote the manuscript; and all authors reviewed and approved the manuscript .
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Koichi Takahashi, Department of Leukemia, UT MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: ktakahashi@mdanderson.org.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
T.T. and K.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal