Key Points
Association between clonal hematopoiesis and AID in patients with OA underscores the link between CH and inflammation.
High rate of CH precludes the use of hip bone–derived hematopoietic cells as healthy controls in experiments without prior sequencing.
Abstract
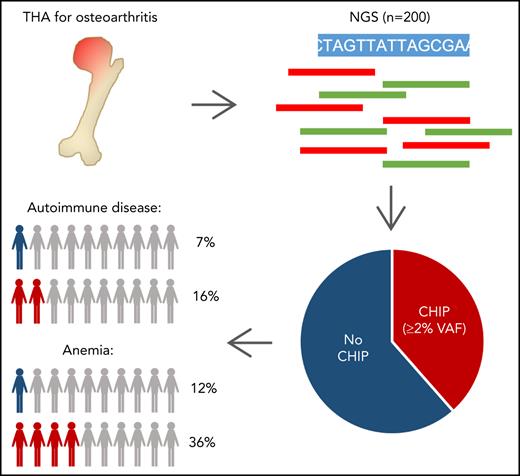
Clonal hematopoiesis (CH) is an age-related condition predisposing to blood cancer and cardiovascular disease (CVD). Murine models demonstrate CH-mediated altered immune function and proinflammation. Low-grade inflammation has been implicated in the pathogenesis of osteoarthritis (OA), the main indication for total hip arthroplasty (THA). THA-derived hip bones serve as a major source of healthy hematopoietic cells in experimental hematology. We prospectively investigated frequency and clinical associations of CH in 200 patients without known hematologic disease who were undergoing THA. Prevalence of CH was 50%, including 77 patients with CH of indeterminate potential (CHIP, defined as somatic variant allele frequencies [VAFs] ≥2%), and 23 patients harboring CH with lower mutation burden (VAF, 1% to 2%). Most commonly mutated genes were DNMT3A (29.5%), TET2 (15.0%), and ASXL1 (3.5%). CHIP is significantly associated with lower hemoglobin, higher mean corpuscular volume, previous or present malignant disease, and CVD. Strikingly, we observed a previously unreported association of CHIP with autoimmune diseases (AIDs; multivariable adjusted odds ratio, 6.6; 95% confidence interval, 1.7-30; P = .0081). These findings underscore the association between CH and inflammatory diseases. Our results have considerable relevance for managing patients with OA and AIDs or mild anemia and question the use of hip bone–derived cells as healthy experimental controls.
Introduction
Clonal hematopoiesis of indeterminate potential (CHIP) is defined as the presence of somatic mutations with a variant allele frequency (VAF) ≥2% in the peripheral blood of individuals without evidence of hematologic disease.1 Genome-wide or targeted sequencing revealed an ageing-associated increase of clonal hematopoiesis (CH) in healthy individuals.2-6 Clonal expansion of hematopoietic cells is often driven by somatic mutations in genes implicated in myeloid disease, most frequently DNMT3A, TET2, and ASXL1. CH is a premalignant state that predisposes to blood cancer, although the absolute risk of progression is low.6-8 CH carriers also have higher risks of atherosclerotic cardiovascular disease (CVD), death from coronary heart disease, and all-cause mortality.3,9 Leukocytes harboring somatic mutations exhibit altered immune properties, suggesting CH-mediated immune-cell dysfunction, with inflammasome-mediated endothelial injury driving atherosclerosis.10-12 Chronic low-grade inflammation also plays a critical role in the pathogenesis of osteoarthritis (OA),13 the main indication for total hip arthroplasty (THA). We prospectively studied CH in 200 THA patients without hematologic disease to characterize the spectrum of CH in this population and understand associations between CHIP and clinical parameters.
Study design
Bone marrow from femoral heads (n = 109) and peripheral blood (n = 91) were collected from 200 patients without known hematologic disease undergoing THA for OA between July 2017 and August 2020 within the German Cancer Consortium (DKTK CHOICE), after written informed consent (Table 1). The study was approved by the respective ethics committees in accordance with the Declaration of Helsinki. Detailed information about variant detection and data analysis are provided in the supplemental Data (available on the Blood Web site). In brief, targeted sequencing of 68 genes recurrently mutated in hematologic malignancies identified nonsynonymous variants with a VAF threshold of ≥1%. For correlative analyses of clinical parameters, only variants fulfilling the current CHIP definition (VAF ≥2%) were included. We tested for associations between CHIP and other variables by using univariable analyses (with adjustment for multiple testing) and by multivariable analysis considering covariables with univariable q < 0.1, as well as sex (for details, see supplemental Data).
Baseline characteristics of the total cohort
| Variable . | All patients . | VAF 1-2% . | CHIP (VAF ≥2%) . | Non-CHIP . | p-value (CHIP vs. non-CHIP) . | q value (CHIP vs. non-CHIP) . |
|---|---|---|---|---|---|---|
| n (%) | 200 | 23 (11.5) | 77 (38.5) | 123 (61.5) | ||
| Center | 0.12 | 0.28 | ||||
| TUM, Munich, n (%) | 109 (54.5) | 13 (56.5) | 47 (61.0) | 62 (50.4) | ||
| LMU, Munich, n (%) | 31 (15.5) | 5 (21.7) | 7 (9.1) | 24 (19.5) | ||
| TUD/UHL, n (%) | 60 (30.0) | 5 (21.7) | 23 (29.9) | 37 (30.1) | ||
| Indication for hip arthroplasty | 0.17 | 0.33 | ||||
| Primary (idiopathic) OA, n (%) | 149 (74.9) | 18 (78.3) | 61 (80.3) | 88 (71.5) | ||
| Secondary OA, n (%) | 50 (25.1) | 5 (21.7) | 15 (19.7) | 35 (28.5) | ||
| Age (y), median (range) | 71 (18-91) | 75 (53-85) | 74 (60-86) | 69 (18-91) | 5.4x10-6* | 9.1x10-5* |
| Male sex, n (%) | 66 (33.0) | 9 (39.1) | 24 (31.2) | 42 (34.1) | 0.66 | 0.81 |
| BMI, median (range) | 27.0 (16.5-51.0) | 26.7 (21.3-36.0) | 28.0 (19.3-37.0) | 27.0 (16.5-51.0) | 0.14 | 0.31 |
| Obesity (BMI ≥30), n (%) | 52 (26.1) | 5 (21.7) | 22 (28.9) | 30 (24.4) | ||
| Blood counts | ||||||
| Leukocytes (109/L), median (range) | 6.7 (3.6-13.8) | 6.8 (5.4-13.6) | 6.7 (5.1-13.8) | 6.7 (3.6-13.6) | 0.43 | 0.56 |
| Hemoglobin (g/dL), median (range) | 13.4 (8.0-18.3) | 13.9 (10.9-15.5) | 12.7 (8.0-18.3) | 13.7 (9.4-16.8) | 0.0020† | 0.017‡ |
| MCV (fL), median (range) | 90.0 (80.0-102.0) | 91.3 (86.0-95.4) | 91.9 (82.0-102.0) | 89.0 (80.0-97.7) | 0.0076† | 0.034‡ |
| Platelets (109/L), median (range) | 272.0 (112.0-563.0) | 274.0 (175.0-426.0) | 264.5 (112.0-563.0) | 275.0 (120.0-490.0) | 0.38 | 0.53 |
| Comorbidities, n (%) | 175 (87.9) | 20 (87.0) | 70 (92.1) | 105 (85.4) | ||
| Cardiovascular disease, n (%) | 132 (66.3) | 16 (69.6) | 59 (77.6) | 73 (59.3) | 0.0080† | 0.034‡ |
| Hypertension, n (%) | 122 (61.3) | 15 (65.2) | 57 (75.0) | 65 (52.8) | ||
| Coronary heart disease, n (%) | 30 (15.1) | 4 (17.4) | 15 (19.7) | 15 (12.2) | ||
| Myocardial infarction, n (%) | 22 (11.1) | 3 (13.0) | 12 (15.7) | 10 (8.1) | ||
| Stroke, n (%) | 13 (6.5) | 1 (4.3) | 6 (7.9) | 7 (5.7) | ||
| Cardiac arrhythmia, n (%) | 29 (14.6) | 2 (8.7) | 15 (19.7) | 14 (11.4) | ||
| Hypercholesterolemia, n (%) | 40 (20.1) | 5 (21.7) | 18 (23.7) | 22 (17.9) | 0.32 | 0.51 |
| Type 2 diabetes, n (%) | 19 (9.5) | 1 (4.3) | 7 (9.2) | 12 (9.8) | 0.90 | 0.90 |
| Hypothyroidism, n (%) | 38 (19.1) | 3 (13.0) | 15 (19.7) | 23 (18.7) | 0.86 | 0.90 |
| Autoimmune disease, n (%) | 20 (10.1) | 1 (4.3) | 12 (15.8) | 8 (6.5) | 0.034‡ | 0.097 |
| Malignant disease, n (%) | 26 (13.1) | 2 (8.7) | 15 (19.7) | 11 (8.9) | 0.028‡ | 0.096 |
| History of thrombosis/pulmonary embolism, n (%) | 12 (6.0) | 1 (4.3) | 3 (3.9) | 9 (7.3) | 0.33 | 0.51 |
| Anti-inflammatory drug use, n (%) | 66 (34.3) | 9 (39.1) | 26 (34.7) | 40 (32.5) | 0.76 | 0.86 |
| NSAID, n (%) | 64 (32.2) | 9 (39.1) | 25 (33.3) | 39 (31.7) | ||
| Steroids, n (%) | 4 (2.0) | 0 (0.0) | 0 (0.0) | 4 (3.3) | ||
| Other, n (%) | 7 (3.5) | 0 (0.0) | 2 (2.7) | 5 (4.1) |
| Variable . | All patients . | VAF 1-2% . | CHIP (VAF ≥2%) . | Non-CHIP . | p-value (CHIP vs. non-CHIP) . | q value (CHIP vs. non-CHIP) . |
|---|---|---|---|---|---|---|
| n (%) | 200 | 23 (11.5) | 77 (38.5) | 123 (61.5) | ||
| Center | 0.12 | 0.28 | ||||
| TUM, Munich, n (%) | 109 (54.5) | 13 (56.5) | 47 (61.0) | 62 (50.4) | ||
| LMU, Munich, n (%) | 31 (15.5) | 5 (21.7) | 7 (9.1) | 24 (19.5) | ||
| TUD/UHL, n (%) | 60 (30.0) | 5 (21.7) | 23 (29.9) | 37 (30.1) | ||
| Indication for hip arthroplasty | 0.17 | 0.33 | ||||
| Primary (idiopathic) OA, n (%) | 149 (74.9) | 18 (78.3) | 61 (80.3) | 88 (71.5) | ||
| Secondary OA, n (%) | 50 (25.1) | 5 (21.7) | 15 (19.7) | 35 (28.5) | ||
| Age (y), median (range) | 71 (18-91) | 75 (53-85) | 74 (60-86) | 69 (18-91) | 5.4x10-6* | 9.1x10-5* |
| Male sex, n (%) | 66 (33.0) | 9 (39.1) | 24 (31.2) | 42 (34.1) | 0.66 | 0.81 |
| BMI, median (range) | 27.0 (16.5-51.0) | 26.7 (21.3-36.0) | 28.0 (19.3-37.0) | 27.0 (16.5-51.0) | 0.14 | 0.31 |
| Obesity (BMI ≥30), n (%) | 52 (26.1) | 5 (21.7) | 22 (28.9) | 30 (24.4) | ||
| Blood counts | ||||||
| Leukocytes (109/L), median (range) | 6.7 (3.6-13.8) | 6.8 (5.4-13.6) | 6.7 (5.1-13.8) | 6.7 (3.6-13.6) | 0.43 | 0.56 |
| Hemoglobin (g/dL), median (range) | 13.4 (8.0-18.3) | 13.9 (10.9-15.5) | 12.7 (8.0-18.3) | 13.7 (9.4-16.8) | 0.0020† | 0.017‡ |
| MCV (fL), median (range) | 90.0 (80.0-102.0) | 91.3 (86.0-95.4) | 91.9 (82.0-102.0) | 89.0 (80.0-97.7) | 0.0076† | 0.034‡ |
| Platelets (109/L), median (range) | 272.0 (112.0-563.0) | 274.0 (175.0-426.0) | 264.5 (112.0-563.0) | 275.0 (120.0-490.0) | 0.38 | 0.53 |
| Comorbidities, n (%) | 175 (87.9) | 20 (87.0) | 70 (92.1) | 105 (85.4) | ||
| Cardiovascular disease, n (%) | 132 (66.3) | 16 (69.6) | 59 (77.6) | 73 (59.3) | 0.0080† | 0.034‡ |
| Hypertension, n (%) | 122 (61.3) | 15 (65.2) | 57 (75.0) | 65 (52.8) | ||
| Coronary heart disease, n (%) | 30 (15.1) | 4 (17.4) | 15 (19.7) | 15 (12.2) | ||
| Myocardial infarction, n (%) | 22 (11.1) | 3 (13.0) | 12 (15.7) | 10 (8.1) | ||
| Stroke, n (%) | 13 (6.5) | 1 (4.3) | 6 (7.9) | 7 (5.7) | ||
| Cardiac arrhythmia, n (%) | 29 (14.6) | 2 (8.7) | 15 (19.7) | 14 (11.4) | ||
| Hypercholesterolemia, n (%) | 40 (20.1) | 5 (21.7) | 18 (23.7) | 22 (17.9) | 0.32 | 0.51 |
| Type 2 diabetes, n (%) | 19 (9.5) | 1 (4.3) | 7 (9.2) | 12 (9.8) | 0.90 | 0.90 |
| Hypothyroidism, n (%) | 38 (19.1) | 3 (13.0) | 15 (19.7) | 23 (18.7) | 0.86 | 0.90 |
| Autoimmune disease, n (%) | 20 (10.1) | 1 (4.3) | 12 (15.8) | 8 (6.5) | 0.034‡ | 0.097 |
| Malignant disease, n (%) | 26 (13.1) | 2 (8.7) | 15 (19.7) | 11 (8.9) | 0.028‡ | 0.096 |
| History of thrombosis/pulmonary embolism, n (%) | 12 (6.0) | 1 (4.3) | 3 (3.9) | 9 (7.3) | 0.33 | 0.51 |
| Anti-inflammatory drug use, n (%) | 66 (34.3) | 9 (39.1) | 26 (34.7) | 40 (32.5) | 0.76 | 0.86 |
| NSAID, n (%) | 64 (32.2) | 9 (39.1) | 25 (33.3) | 39 (31.7) | ||
| Steroids, n (%) | 4 (2.0) | 0 (0.0) | 0 (0.0) | 4 (3.3) | ||
| Other, n (%) | 7 (3.5) | 0 (0.0) | 2 (2.7) | 5 (4.1) |
Data are presented as n (%) unless otherwise noted.
We applied a ×2 test for categorical variables and a Wilcoxon-Mann-Whitney test for continuous variables. q values (ie, false discovery rate-adjusted P values) were obtained using Benjamini-Hochberg procedure.
BMI, body mass index; MCV, mean corpuscular volume; NSAID, nonsteroidal anti-inflammatory drug.
P < .001.
P < .01.
P < 05.
Results and discussion
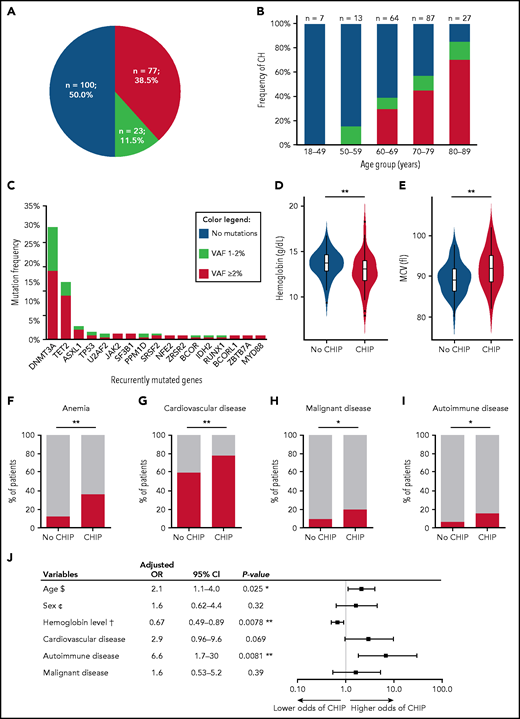
Our prospective study in a large, well-annotated patient cohort shows that somatic mutations typical of CH are very frequent in patients undergoing elective THA for OA. At least 1 variant was identified in 100 patients (50%; Figure 1A), with no imbalances regarding treatment center or sex (χ2 test P = .26 and P = .78, respectively; supplemental Figure 1A-B). CH became progressively more frequent with age (Figure 1B). Most patients (n = 56) had 1 variant, 32 harbored 2 variants, 10 carried 3 mutations, and 2 harbored 4 or 5 mutations. Number of variants per individual correlated with age (P = .031; Spearman’s rank correlation coefficient), whereas VAF (median, 2.7%; range, 1.0% to 32.7%) did not (P = .73) (supplemental Figure 2A-B). The prevalence of CH in our cohort was higher than previously reported in healthy individuals or specific patient groups, such as those with ischemic heart failure or CVD.3,9,14-16
Frequency, mutational spectrum and clinical correlates of CH in patients undergoing THA. (A) Occurrence of CH with VAF ≥1% in 200 patients. (B) Frequency of CH among different age groups (not showing 1 patient with missing information about age and 1 patient older than age 90 years). (C) Mutation frequency of recurrently mutated genes with VAF ≥1%. Correlation of CHIP with clinical parameters: representation of hemoglobin levels (D) and mean corpuscular volume (MCV) (E) levels according to the absence or presence of CHIP. Red bars showing the prevalence of anemia (hemoglobin <13 g/dL in men and <12 g/dL in women) (F), cardiovascular disease (G), previous or present malignant disease (H), and AID in patients with and without CHIP (I). Unadjusted P values were obtained using Wilcoxon-Mann-Whitney test for continuous variables (D-E) and χ2 test for categorical variables (F-I). (J) Multivariable logistic regression analysis ($) per 10-year increase, (¢) female vs male, and (†) per 1 g/dL increase. OR was adjusted for the effects of the other covariates. *P < .05; **P < .01. MCV, mean corpuscular volume.
Frequency, mutational spectrum and clinical correlates of CH in patients undergoing THA. (A) Occurrence of CH with VAF ≥1% in 200 patients. (B) Frequency of CH among different age groups (not showing 1 patient with missing information about age and 1 patient older than age 90 years). (C) Mutation frequency of recurrently mutated genes with VAF ≥1%. Correlation of CHIP with clinical parameters: representation of hemoglobin levels (D) and mean corpuscular volume (MCV) (E) levels according to the absence or presence of CHIP. Red bars showing the prevalence of anemia (hemoglobin <13 g/dL in men and <12 g/dL in women) (F), cardiovascular disease (G), previous or present malignant disease (H), and AID in patients with and without CHIP (I). Unadjusted P values were obtained using Wilcoxon-Mann-Whitney test for continuous variables (D-E) and χ2 test for categorical variables (F-I). (J) Multivariable logistic regression analysis ($) per 10-year increase, (¢) female vs male, and (†) per 1 g/dL increase. OR was adjusted for the effects of the other covariates. *P < .05; **P < .01. MCV, mean corpuscular volume.
Overall, we detected 158 variants with VAFs ≥1%, affecting 25 different genes, most frequently DNMT3A (29.5%), TET2 (15.0%), and ASXL1 (3.5%) (ie, DTA genes; Figure 1C; supplemental Figure 3A; supplemental Table 1), similar to previous reports. Comutation was most commonly observed between DNMT3A and TET2 (n = 8). Most patients (n = 61) had ≥1 DTA mutation with a VAF ≥2% or with VAFs between 1% and 2% (n = 25) (supplemental Figure 3B), but 14 patients carried exclusively non-DTA mutations.
We used a VAF threshold of ≥2% to compare clinical features of patients with variants fulfilling commonly accepted CHIP criteria (n = 77; 38.5%) with patients without such variants. CHIP carriers were older (median, age 74 vs 69 years for patients without CHIP; P = 5.4 × 10−6 [Wilcoxon-Mann-Whitney test ]; q = 9.1 × 10−6), had lower hemoglobin levels (median, 12.7 vs 13.7 g/dL; P = .0020; q = 0.017), and had higher mean corpuscular volume (median, 91.9 vs 89.0 fL; P = .0076; q = 0.034) (Figure 1D-E). Other blood cell counts showed no significant differences (Table 1). Hemoglobin levels were available for 125 of 200 patients, including 50 with CHIP. Although no patient in our study had a known hematologic disorder, 27 (21.6%) of 125 presented with subnormal hemoglobin values (Figure 1F). Of note, 18 (66.7%) of 27 patients with anemia (supplemental Figure 4) had detectable mutations with VAF ≥2% and can thus be classified as clonal cytopenia of uncertain significance, a higher proportion than recently reported in a large study of patients older than age 60 years with unexplained anemia.17 The prevalence of CHIP was lower in patients with normal hemoglobin levels (32.7%; P = .0014). Most anemic patients in our cohort had significant comorbidities or previous or present nonhematologic cancer, or they were receiving anticoagulants or immunosuppressive treatment for autoimmune disease (AID), all conditions that could predispose to anemia (supplemental Table 2). We observed an enrichment of SF3B1 and TP53 mutations in anemic CHIP patients compared with those with normal hemoglobin levels when, in concordance with a previous report,17 we applied a lower VAF threshold of ≥1% for these 2 genes (4 of 27 vs 2 of 98 patients, respectively; Fisher’s exact test P = .020; supplemental Table 2).
CHIP carriers were more likely to have CVD, as previously reported, and showed a trend toward higher frequency of previous or present malignant disease (Figure 1G-H; supplemental Table 3). Unexpectedly, CHIP carriers more commonly had AID (Figure 1I) comprising diverse autoimmune disorders (supplemental Table 4). Multivariable logistic regression confirmed significant associations between CHIP and older age (adjusted odds ratio [OR], 2.1; 95% confidence interval [CI], 1.1-4.0; P = .025), lower hemoglobin levels (adjusted OR, 0.67; 95% CI, 0.49-0.89; P = .0078), and AID (adjusted OR, 6.6; 95% CI, 1.7-30; P = .0081) (Figure 1J), but not with CVD or malignancy.
The association between CHIP and diverse AID is a novel finding. CHIP has been studied in patient cohorts with specific autoimmune conditions such as rheumatoid arthritis or antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), with reported prevalences of 17%18 and 30%,19 respectively. Arends et al19 found a higher prevalence of CHIP in AAV patients compared with age- and sex-matched controls, whereas a smaller retrospective study did not show enrichment of CHIP among patients with rheumatoid arthritis compared with published control cohorts.18 In contrast to these reports, our prospective analysis of THA patients was unbiased with respect to AID, CVD, and malignancy. Whether our findings can be generalized to individuals not undergoing THA remains to be studied.
THA is a common surgical procedure in older individuals, usually performed for advanced OA. Chronic low-grade inflammation is frequent in OA but a connection between OA and CH has not been reported. Inflammation can contribute to clonal expansion in the bone marrow. In turn, clonal TET2-mutated monocytes may have a proinflammatory phenotype characterized by production of cytokines, including interleukin-1β (IL-1β).20 Indirect evidence from the CANTOS trial suggests that inhibition of IL-1β may prevent worsening of joint destruction,21,22 indicating that chronic inflammation in progressive OA is partially mediated by IL-1β. This observation together with data presented here suggest a potential role for CH in OA pathogenesis. Unambiguous evidence of a specific association between OA and CH will require confirmation in a matched control cohort. The high sensitivity of our targeted sequencing assay, compared with earlier studies using genome-wide approaches, may provide an additional explanation for the high prevalence of CH in our cohort.
Recognition of CHIP is clinically relevant in allogeneic stem cell donors23,24 and autologous transplantation recipients25 and in the context of CVD. We cannot draw conclusions on a causal relationship between AID and CHIP. However, given the relationship between CHIP and inflammation, it is tempting to speculate whether CHIP contributes to clinical features of AID, and/or autoimmune-mediated inflammation reinforces outgrowth of CH clones. Further studies in this direction are needed to confirm this relationship, and CH should be considered as a confounder in studies evaluating anti-inflammatory therapies for OA or AID.
In summary, our prospective analysis of a large cohort of older THA patients that used a sensitive targeted sequencing assay revealed a high frequency of CH/CHIP and . Mutations were particularly frequent in patients with (mostly mild) anemia, indicating that clonal cytopenia of uncertain significance commonly contributes to pathogenesis of anemia in older people. Our data point to a potential, previously unappreciated role for CHIP in the pathogenesis of inflammatory and autoimmune disorders, supporting the concept of interplay between CH and systemic inflammation, which has possible implications for future therapies.
Finally, our results question the routine use of femoral heads as a source of healthy hematopoietic cells for use in basic research. Given the high CH prevalence in THA specimens, such experiments must include screening for somatic variants.
Acknowledgments
This work was supported by the German Cancer Consortium joint funding program (DKTK CHOICE (K. Spiekermann, L.C.H., U.P., K.H.M., and K.S.G.) as well as the Deutsche Forschungsgemeinschaft (SFB 1243, project A07 [K. Spiekermann], A06 [K.H.M.], and A09 [K.S.G.], SPP-2084 [L.C.H. and M.R.], SFB 1335 [F.B.]), the German Jose Carreras Leukämiestiftung (DJCLS R14/18 [K.S.G.], DJCLS 03R/2018 [U.P.]), and the Wilhelm Sander Stiftung (WSS/TUD/2018.123.1 [U.P.]).
K.S.G. received a grant (953407) from the European Research Council (ERC) under the European Union’s Horizon 2020 Marie Sklodowska-Curie Innovative Training Network. F.B. and C.M. received grants from the ERC under the European Union’s Horizon 2020 Research and Innovation Programme (682473 and 866411, respectively).
Authorship
Contribution: J.S.H. collected clinical patient characteristics, analyzed data, and drafted the manuscript; L.H. performed sequencing analyses, analyzed data, and drafted the manuscript; B.K. and M.R.-T. performed sequencing analyses; J.R., M.C.B., and M.v.d.G. performed sample processing and biobanking and assisted with data analysis; L.F., S.W., F.Z., K. Sockel, M. Solovey, M. Schneider, and A.S.K. collected clinical patient data; M.R., E.T., M.N., D.H., A.C.P., J.L., and A.R. provided femoral heads; J.S.H. and J.R. performed statistical tests with C.M. F.B., K. Spiekermann, L.C.H. and U.P. provided samples, conceptual advice, and critical comments; K.H.M. and K.S.G. designed the study, analyzed data, and wrote the manuscript; and all authors read and agreed with the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katharina S. Götze, Technical University of Munich (TUM), Klinikum Rechts der Isar, Ismaninger Str 22, Munich, 81675 Germany; e-mail: katharina.goetze@tum.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
J.S.H and L.H. contributed equally to this work.
K.H.M. and K.S.G. contributed equally to this work as senior co-authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal