Key Points
Ivosidenib or enasidenib combined with induction and consolidation chemotherapy were both well tolerated in newly diagnosed mIDH1/2 AML.
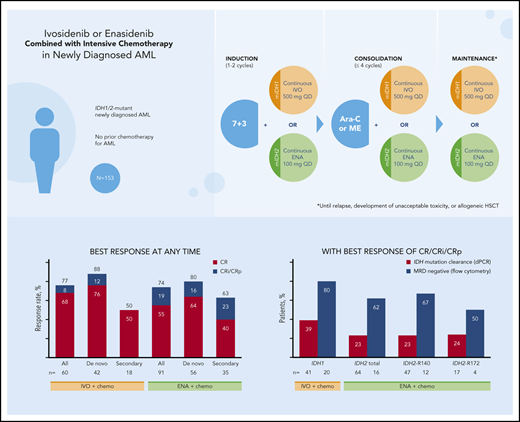
CR/CRi/CRp rates: 77% (ivosidenib) and 74% (enasidenib); 39% and 23% of patients had mIDH1/2 clearance by digital polymerase chain reaction.
Abstract
Ivosidenib (AG-120) and enasidenib (AG-221) are targeted oral inhibitors of the mutant isocitrate dehydrogenase (mIDH) 1 and 2 enzymes, respectively. Given their effectiveness as single agents in mIDH1/2 relapsed or refractory acute myeloid leukemia (AML), this phase 1 study evaluated the safety and efficacy of ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed mIDH1/2 AML. Ivosidenib 500 mg once daily and enasidenib 100 mg once daily were well tolerated in this setting, with safety profiles generally consistent with those of induction and consolidation chemotherapy alone. The frequency of IDH differentiation syndrome was low, as expected given the concurrent administration of cytotoxic chemotherapy. In patients receiving ivosidenib, the frequency and grades of QT interval prolongation were similar to those observed with ivosidenib monotherapy. Increases in total bilirubin were more frequently observed in patients treated with enasidenib, consistent with this inhibitor’s known potential to inhibit UGT1A1, but did not appear to have significant clinical consequences. In patients receiving ivosidenib (n = 60) or enasidenib (n = 91), end-of-induction complete remission (CR) rates were 55% and 47%, respectively, and CR/CR with incomplete neutrophil or platelet recovery (CR/CRi/CRp) rates were 72% and 63%, respectively. In patients with a best overall response of CR/CRi/CRp, 16/41 (39%) receiving ivosidenib had IDH1 mutation clearance and 15/64 (23%) receiving enasidenib had IDH2 mutation clearance by digital polymerase chain reaction; furthermore, 16/20 (80%) and 10/16 (63%), respectively, became negative for measurable residual disease by multiparameter flow cytometry. This trial was registered at www.clinicaltrials.gov as #NCT02632708.
Introduction
Intensive induction chemotherapy with cytarabine and an anthracycline (“7 + 3”) remains the most effective treatment for adults with newly diagnosed acute myeloid leukemia (AML) who can withstand its toxicities. Recent modifications to this backbone have improved event-free survival and overall survival in defined subsets of patients.1-3
Mutations in the isocitrate dehydrogenase (IDH) 1 or 2 genes are seen in ∼20% of patients with AML.4-7 Mutant IDH (mIDH) proteins catalyze the production of D-2-hydroxyglutarate (2-HG),4,8 resulting in DNA and histone hypermethylation, with consequent changes in gene expression and impaired cellular differentiation.9-11 Ivosidenib and enasidenib are targeted, oral, small-molecule inhibitors of the mIDH1 and mIDH2 enzymes, respectively, approved by the US Food and Drug Administration as monotherapies for adults with relapsed or refractory (R/R) AML and a susceptible IDH1 or IDH2 mutation.12,13 In a phase 1 study, ivosidenib 500 mg once daily resulted in an overall response rate of 41.6% and a median response duration of 6.5 months in patients with mIDH1 R/R AML.14 Similarly, the overall response rate in patients with mIDH2 R/R AML receiving enasidenib 100 mg once daily was 40.3%, with a median response duration of 5.8 months.15 We hypothesized that combining ivosidenib or enasidenib with intensive induction and consolidation chemotherapy would improve outcomes for patients with newly diagnosed mIDH1/2 AML.
Methods
Study design
This phase 1, multicenter, open-label study enrolled patients with mIDH1 or mIDH2 newly diagnosed AML. Induction therapy consisted of continuous ivosidenib 500 mg once daily (mIDH1) or enasidenib 100 mg once daily (mIDH2) in combination with cytarabine (200 mg/m2 per day for 7 days) and either daunorubicin (60 mg/m2 per day for 3 days) or idarubicin (12 mg/m2 per day for 3 days). A second cycle of induction was permitted according to institutional practice.
Six patients were initially enrolled to each of the 4 induction treatment arms. If ≤2 of the 6 patients initially enrolled in a treatment arm experienced a dose-limiting toxicity (DLT) at the standard dose of either mIDH inhibitor, then an additional 6 patients were to be evaluated at that dose. If ≤3 of the expanded cohort of 12 patients experienced a DLT, then that dose was to be declared suitable for further evaluation. However, if ≥3 of the initial 6 patients or ≥4 of the first 12 patients experienced a DLT, a lower dose was to be administered. After the safety of the combination regimens had been determined for each of the 4 induction therapy cohorts, ∼30 patients in total were to be enrolled into each cohort to better characterize the safety profile of these regimens. This larger number of patients in each induction cohort also ensured that an adequate number would proceed to the consolidation phase of treatment, so that the safety profile of the mIDH inhibitors in combination with consolidation therapy could be evaluated. Owing to an early concern regarding delayed recovery of blood counts in patients receiving enasidenib with induction chemotherapy, 2 additional cohorts (for the daunorubicin- and idarubicin-based regimens) were treated with enasidenib 100 mg once daily beginning on day 8 (instead of day 1) of the first cycle of induction therapy.
Patients achieving at least a partial remission at the end of induction could receive consolidation therapy (up to 4 cycles of intermediate- or high-dose cytarabine, or 1 cycle of mitoxantrone/etoposide), while continuing to receive ivosidenib or enasidenib continuously once daily. The safety of each consolidation therapy regimen was evaluated after the first 6 evaluable patients had completed ≥28 days of consolidation treatment or discontinued owing to toxicity.
Patients remaining in remission at the end of consolidation could receive maintenance treatment with ivosidenib or enasidenib monotherapy daily until relapse, development of an unacceptable toxicity, or allogeneic hematopoietic stem cell transplantation (HSCT). Patients appropriate for HSCT could proceed to HSCT at any point; resumption of mIDH inhibitor therapy was not allowed after HSCT. See supplemental Appendix on the Blood Web site for further details on treatment.
The study was conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice, and the protocol was approved by human investigation committees at participating sites. Written informed consent was provided by all patients before screening and enrollment.
The study sponsor analyzed the data and conducted the statistical analyses. All authors had access to the primary clinical trial data on request.
Patients
Patients ≥18 years of age with newly diagnosed AML (de novo or secondary) were eligible if they had an Eastern Cooperative Oncology Group performance status score of 0 to 2 and a documented IDH1 and/or IDH2 mutation by local laboratory testing. Secondary AML was defined as AML arising after myelodysplastic syndrome or another hematologic disorder, or AML arising after exposure to genotoxic injury (ie, radiation and/or chemotherapy). Patients were ineligible if they had received any prior chemotherapy for AML except hydroxyurea, but previous treatment of myelodysplastic syndrome or another antecedent hematologic disorder, including hypomethylating agents, was permitted if the last dose was ≥14 days before study treatment initiation.
Assessments
The primary objective was to assess the safety and tolerability of the combination regimens. Treatment-emergent adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Analyses of time to hematologic recovery were conducted for the induction phase (supplemental Appendix).
Secondary objectives included investigator assessment of clinical responses using the modified 2003 International Working Group response criteria for AML, and overall survival (further details in supplemental Appendix).
Pharmacokinetics and pharmacodynamics
Analyses were conducted as reported in the supplemental Appendix.
Translational analyses
Retrospective confirmation of IDH mutation and analysis of co-occurring mutations at baseline were performed by next-generation sequencing (NGS) using the 95-gene Rapid Heme Panel (detection sensitivity, 5%).16 Samples from baseline and specified on-study time points were analyzed for mutation evolution using the Personalis ACE Cancer Panel (Menlo Park, CA)17 for ivosidenib-treated patients, and the Archer VariantPlex Core Myeloid Panel (Boulder, CO) for enasidenib-treated patients; analyses were limited to the 33 genes represented on both platforms (supplemental Table 1), and a 2% limit of detection for variant allele frequency (VAF) was applied to both datasets. Heatmaps were produced in R, version 3.6.0. Longitudinal mIDH1/2 VAF was assessed using the OncoBEAM BEAMing digital polymerase chain reaction (dPCR) assay (lower limit of detection, 0.02% to 0.04%; Sysmex Inostics Inc., Hamburg, Germany).18 Mutation clearance was defined as a reduction in mIDH1/2 VAF that was below the limit of detection for ≥1 on-treatment time point on or after day 21 of induction therapy in patients who had detectable mIDH1/2 at baseline. Measurable residual disease (MRD) was assessed centrally by multiparameter flow cytometry for a subset of patients. Further detail on all exploratory assessments is provided in the supplemental Appendix.
Statistical analysis
The data cutoff date was 13 December 2018. The safety sets included all patients who received at least 1 dose of ivosidenib, enasidenib, or chemotherapy during the relevant study period (ie, induction, consolidation, and maintenance). All efficacy analyses were performed using the full analysis set (FAS), comprising all enrolled patients who received at least 1 dose of ivosidenib or enasidenib. Only response assessments occurring on or after day 21 were used to determine the best response. The proportion of patients in each best overall response category was evaluated using the Clopper-Pearson method for the overall treatment period (induction, consolidation, and maintenance; excludes post-HSCT response) and for the de novo AML and secondary AML subgroups. Median overall survival and 12-month overall survival rates were estimated using the Kaplan-Meier method, along with associated 95% confidence intervals based on log-log transformation. See supplemental Appendix for additional statistical methods.
Results
Patient characteristics and disposition
Sixty patients were dosed in the ivosidenib plus induction chemotherapy cohorts and 93 in the enasidenib plus induction chemotherapy cohorts from January 2016 to July 2018. Baseline characteristics are provided in Table 1 and patient disposition in Figure 1.
Baseline characteristics
| Characteristic . | Ivosidenib 500 mg + chemotherapy, n = 60 . | Enasidenib 100 mg + chemotherapy, n = 93 . |
|---|---|---|
| Age, median (range), y | 62.5 (24-76) | 63.0 (27-77) |
| Age category, n (%), y | ||
| <60 | 21 (35) | 34 (37) |
| ≥60 | 39 (65) | 59 (63) |
| Men, n (%) | 30 (50) | 52 (56) |
| Type of AML, n (%) | ||
| De novo | 42 (70) | 58 (62) |
| Secondary | 18 (30) | 35 (38) |
| Prior hypomethylating agent, n (%)* | 4 (22) | 17 (49) |
| IDH1 mutation type, n (%)† | ||
| R132 | 58 (97) | 2 (2) |
| Other or unknown | 2 (3) | 1 (1) |
| IDH2 mutation type, n (%)† | ||
| R140 | 1 (2) | 66 (71) |
| R172 | 1 (2) | 25 (27) |
| Other or unknown | 0 | 2 (2) |
| Cytogenetic risk status by investigator, n (%) | ||
| Favorable | 0 | 2 (2) |
| Intermediate | 42 (70) | 64 (69) |
| Poor | 13 (22) | 20 (22) |
| Unknown | 5 (8) | 7 (8) |
| Characteristic . | Ivosidenib 500 mg + chemotherapy, n = 60 . | Enasidenib 100 mg + chemotherapy, n = 93 . |
|---|---|---|
| Age, median (range), y | 62.5 (24-76) | 63.0 (27-77) |
| Age category, n (%), y | ||
| <60 | 21 (35) | 34 (37) |
| ≥60 | 39 (65) | 59 (63) |
| Men, n (%) | 30 (50) | 52 (56) |
| Type of AML, n (%) | ||
| De novo | 42 (70) | 58 (62) |
| Secondary | 18 (30) | 35 (38) |
| Prior hypomethylating agent, n (%)* | 4 (22) | 17 (49) |
| IDH1 mutation type, n (%)† | ||
| R132 | 58 (97) | 2 (2) |
| Other or unknown | 2 (3) | 1 (1) |
| IDH2 mutation type, n (%)† | ||
| R140 | 1 (2) | 66 (71) |
| R172 | 1 (2) | 25 (27) |
| Other or unknown | 0 | 2 (2) |
| Cytogenetic risk status by investigator, n (%) | ||
| Favorable | 0 | 2 (2) |
| Intermediate | 42 (70) | 64 (69) |
| Poor | 13 (22) | 20 (22) |
| Unknown | 5 (8) | 7 (8) |
For patients with secondary AML only.
Patients with dual IDH1 and IDH2 mutations were assigned to ivosidenib or enasidenib on the basis of the IDH mutation with the higher allele burden.
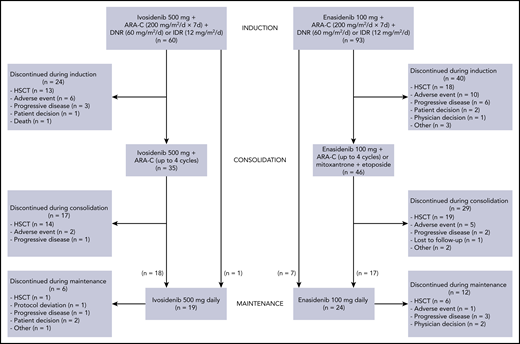
Disposition of the study population. ARA-C, cytarabine; DNR, daunorubicin; IDR, idarubicin.
Disposition of the study population. ARA-C, cytarabine; DNR, daunorubicin; IDR, idarubicin.
In the ivosidenib cohorts, the median age was 62.5 years and 70% of patients had de novo AML. Of the 60 patients in the ivosidenib induction cohorts, 9 (15%) received 2 induction cycles, 35 (58%) went on to receive consolidation, and 19 (32%) received ivosidenib maintenance therapy. At the data cutoff date, 47 (78%) ivosidenib-treated patients had discontinued study treatment, most commonly for HSCT (28 patients), whereas 13 patients were continuing to receive ivosidenib as maintenance therapy.
In the enasidenib cohorts, the median age was 63.0 years and 62% of patients had de novo AML. Of the 93 patients in the enasidenib induction cohorts, 2 who were assigned to receive enasidenib starting on day 8 had an ongoing adverse event or died on day 8 and, as a result, never received a dose of enasidenib. These 2 patients are included in the induction safety analysis set but not in the FAS used for efficacy analyses. Twenty-two patients (24%) received 2 induction cycles, 46 patients (49%) received consolidation, and 24 (26%) received enasidenib maintenance therapy. At the data cutoff date, 81 enasidenib-treated patients (87%) had discontinued study treatment, most commonly for HSCT (43 patients), whereas 12 patients continued to receive enasidenib as maintenance therapy.
Safety
During the initial safety evaluation of the combination therapies, the sole DLT occurred during induction in a 64-year-old man with de novo AML in the enasidenib plus daunorubicin/cytarabine cohort. He experienced persistent grade 4 thrombocytopenia in the absence of residual leukemia on day 42 of induction cycle 1. No patients in the ivosidenib cohorts experienced a DLT. Ivosidenib and enasidenib were well tolerated at their starting doses of 500 and 100 mg each day; therefore, these doses were chosen for evaluation in additional patients.
The toxicities observed during induction and consolidation therapy with the combinations were similar to the toxicities seen with “7 + 3” and cytarabine alone (Table 2; supplemental Tables 2 and 3). Only 2 patients in the enasidenib cohorts and none in the ivosidenib cohorts received mitoxantrone/etoposide as consolidation therapy; hence, the safety of this combination consolidation regimen could not be formally evaluated.
Nonhematologic TEAEs of any grade reported in >20% of patients in any treatment group, and the corresponding frequencies of grade ≥3 events, during the induction and consolidation periods, regardless of attribution
| TEAE, n (%) . | Induction period . | Consolidation period . | ||||||
|---|---|---|---|---|---|---|---|---|
| Ivosidenib 500 mg + chemotherapy, n = 60 . | Enasidenib 100 mg + chemotherapy, n = 93 . | Ivosidenib 500 mg + chemotherapy, n = 35 . | Enasidenib 100 mg + chemotherapy, n = 46 . | |||||
| All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | |
| Any TEAE | 60 (100.0) | 58 (96.7) | 92 (98.9) | 87 (93.5) | 35 (100.0) | 34 (97.1) | 45 (97.8) | 41 (89.1) |
| Diarrhea | 43 (71.7) | 1 (1.7) | 55 (59.1) | 5 (5.4) | 7 (20.0) | 0 | 17 (37.0) | 0 |
| Nausea | 33 (55.0) | 0 | 50 (53.8) | 2 (2.2) | 11 (31.4) | 0 | 15 (32.6) | 1 (2.2) |
| Rash* | 33 (55.0) | 3 (5.0) | 51 (54.8) | 13 (14.0) | 12 (34.3) | 1 (2.9) | 13 (28.3) | 1 (2.2) |
| Decreased appetite | 32 (53.3) | 5 (8.3) | 31 (33.3) | 3 (3.2) | 4 (11.4) | 0 | 11 (23.9) | 1 (2.2) |
| Vomiting | 21 (35.0) | 0 | 31 (33.3) | 1 (1.1) | 9 (25.7) | 1 (2.9) | 10 (21.7) | 0 |
| Stomatitis | 20 (33.3) | 3 (5.0) | 23 (24.7) | 4 (4.3) | 4 (11.4) | 0 | 9 (19.6) | 4 (8.7) |
| Fatigue | 19 (31.7) | 0 | 24 (25.8) | 2 (2.2) | 5 (14.3) | 0 | 9 (19.6) | 2 (4.3) |
| Hypokalemia | 17 (28.3) | 7 (11.7) | 29 (31.2) | 9 (9.7) | 3 (8.6) | 1 (2.9) | 11 (23.9) | 4 (8.7) |
| Pyrexia | 16 (26.7) | 4 (6.7) | 31 (33.3) | 2 (2.2) | 8 (22.9) | 1 (2.9) | 13 (28.3) | 0 |
| Constipation | 14 (23.3) | 0 | 25 (26.9) | 0 | 6 (17.1) | 0 | 13 (28.3) | 0 |
| Hypophosphatemia | 14 (23.3) | 10 (16.7) | 20 (21.5) | 12 (12.9) | 0 | 0 | 7 (15.2) | 4 (8.7) |
| Edema peripheral | 14 (23.3) | 0 | 37 (39.8) | 0 | 2 (5.7) | 0 | 13 (28.3) | 0 |
| Abdominal pain | 13 (21.7) | 2 (3.3) | 19 (20.4) | 0 | 4 (11.4) | 0 | 8 (17.4) | 1 (2.2) |
| Aspartate aminotransferase increased | 13 (21.7) | 4 (6.7) | 18 (19.4) | 4 (4.3) | 1 (2.9) | 0 | 6 (13.0) | 2 (4.3) |
| Electrocardiogram QT prolonged† | 16 (26.7) | 6 (10.0) | 11 (11.8) | 7 (7.5) | 3 (8.6) | 1 (2.9) | 7 (15.2) | 3 (6.5) |
| Alanine aminotransferase increased | 11 (18.3) | 4 (6.7) | 22 (23.7) | 5 (5.4) | 2 (5.7) | 1 (2.9) | 6 (13.0) | 2 (4.3) |
| Cough | 11 (18.3) | 0 | 22 (23.7) | 1 (1.1) | 3 (8.6) | 0 | 9 (19.6) | 0 |
| Headache | 10 (16.7) | 0 | 29 (31.2) | 0 | 6 (17.1) | 1 (2.9) | 8 (17.4) | 0 |
| Hypoalbuminemia | 10 (16.7) | 3 (5.0) | 21 (22.6) | 5 (5.4) | 1 (2.9) | 1 (2.9) | 3 (6.5) | 0 |
| Blood bilirubin increased‡ | 11 (18.3) | 4 (6.7) | 46 (49.5) | 15 (16.1) | 2 (5.7) | 1 (2.9) | 13 (28.3) | 5 (10.9) |
| Hypocalcemia | 9 (15.0) | 3 (5.0) | 24 (25.8) | 6 (6.5) | 2 (5.7) | 1 (2.9) | 6 (13.0) | 2 (4.3) |
| TEAE, n (%) . | Induction period . | Consolidation period . | ||||||
|---|---|---|---|---|---|---|---|---|
| Ivosidenib 500 mg + chemotherapy, n = 60 . | Enasidenib 100 mg + chemotherapy, n = 93 . | Ivosidenib 500 mg + chemotherapy, n = 35 . | Enasidenib 100 mg + chemotherapy, n = 46 . | |||||
| All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | |
| Any TEAE | 60 (100.0) | 58 (96.7) | 92 (98.9) | 87 (93.5) | 35 (100.0) | 34 (97.1) | 45 (97.8) | 41 (89.1) |
| Diarrhea | 43 (71.7) | 1 (1.7) | 55 (59.1) | 5 (5.4) | 7 (20.0) | 0 | 17 (37.0) | 0 |
| Nausea | 33 (55.0) | 0 | 50 (53.8) | 2 (2.2) | 11 (31.4) | 0 | 15 (32.6) | 1 (2.2) |
| Rash* | 33 (55.0) | 3 (5.0) | 51 (54.8) | 13 (14.0) | 12 (34.3) | 1 (2.9) | 13 (28.3) | 1 (2.2) |
| Decreased appetite | 32 (53.3) | 5 (8.3) | 31 (33.3) | 3 (3.2) | 4 (11.4) | 0 | 11 (23.9) | 1 (2.2) |
| Vomiting | 21 (35.0) | 0 | 31 (33.3) | 1 (1.1) | 9 (25.7) | 1 (2.9) | 10 (21.7) | 0 |
| Stomatitis | 20 (33.3) | 3 (5.0) | 23 (24.7) | 4 (4.3) | 4 (11.4) | 0 | 9 (19.6) | 4 (8.7) |
| Fatigue | 19 (31.7) | 0 | 24 (25.8) | 2 (2.2) | 5 (14.3) | 0 | 9 (19.6) | 2 (4.3) |
| Hypokalemia | 17 (28.3) | 7 (11.7) | 29 (31.2) | 9 (9.7) | 3 (8.6) | 1 (2.9) | 11 (23.9) | 4 (8.7) |
| Pyrexia | 16 (26.7) | 4 (6.7) | 31 (33.3) | 2 (2.2) | 8 (22.9) | 1 (2.9) | 13 (28.3) | 0 |
| Constipation | 14 (23.3) | 0 | 25 (26.9) | 0 | 6 (17.1) | 0 | 13 (28.3) | 0 |
| Hypophosphatemia | 14 (23.3) | 10 (16.7) | 20 (21.5) | 12 (12.9) | 0 | 0 | 7 (15.2) | 4 (8.7) |
| Edema peripheral | 14 (23.3) | 0 | 37 (39.8) | 0 | 2 (5.7) | 0 | 13 (28.3) | 0 |
| Abdominal pain | 13 (21.7) | 2 (3.3) | 19 (20.4) | 0 | 4 (11.4) | 0 | 8 (17.4) | 1 (2.2) |
| Aspartate aminotransferase increased | 13 (21.7) | 4 (6.7) | 18 (19.4) | 4 (4.3) | 1 (2.9) | 0 | 6 (13.0) | 2 (4.3) |
| Electrocardiogram QT prolonged† | 16 (26.7) | 6 (10.0) | 11 (11.8) | 7 (7.5) | 3 (8.6) | 1 (2.9) | 7 (15.2) | 3 (6.5) |
| Alanine aminotransferase increased | 11 (18.3) | 4 (6.7) | 22 (23.7) | 5 (5.4) | 2 (5.7) | 1 (2.9) | 6 (13.0) | 2 (4.3) |
| Cough | 11 (18.3) | 0 | 22 (23.7) | 1 (1.1) | 3 (8.6) | 0 | 9 (19.6) | 0 |
| Headache | 10 (16.7) | 0 | 29 (31.2) | 0 | 6 (17.1) | 1 (2.9) | 8 (17.4) | 0 |
| Hypoalbuminemia | 10 (16.7) | 3 (5.0) | 21 (22.6) | 5 (5.4) | 1 (2.9) | 1 (2.9) | 3 (6.5) | 0 |
| Blood bilirubin increased‡ | 11 (18.3) | 4 (6.7) | 46 (49.5) | 15 (16.1) | 2 (5.7) | 1 (2.9) | 13 (28.3) | 5 (10.9) |
| Hypocalcemia | 9 (15.0) | 3 (5.0) | 24 (25.8) | 6 (6.5) | 2 (5.7) | 1 (2.9) | 6 (13.0) | 2 (4.3) |
TEAE, treatment-emergent adverse event.
Rash includes preferred terms rash, rash maculopapular, rash pruritic, rash erythematous, rash macular, dermatitis, dermatitis acneiform, dermatitis allergic, dermatitis bullous, exfoliative rash, skin ulcer, drug eruption, and urticaria.
Electrocardiogram QT prolonged includes ventricular tachycardia, ventricular arrhythmia, cardiac arrest, cardiorespiratory arrest, electrocardiogram QT prolonged, multiple organ dysfunction syndrome, and syncope.
Blood bilirubin increased includes preferred terms of increased blood bilirubin and hyperbilirubinemia.
The 30- and 60-day mortality rates were 5% and 10%, respectively, in the ivosidenib cohorts, and 5% and 9%, respectively, in the enasidenib cohorts. There were 13 on-study deaths (including the survival follow-up period) in the ivosidenib-treated cohorts, of which 7 (54%) occurred during treatment (occurring within 28 days of the last dose). Of 31 on-study deaths (including survival follow-up) in the enasidenib-treated cohort, 13 (42%) occurred during treatment. None of the deaths were related to ivosidenib or enasidenib; the majority were attributed to disease progression or complications of the underlying disease, such as respiratory failure, lung infection, intracranial hemorrhage, or sepsis.
The use of ivosidenib and enasidenib in combination with induction chemotherapy did not affect the time to recovery of the absolute neutrophil count (ANC) or platelet count. In the ivosidenib cohorts, the median times to recovery of the ANC (>500/µL) and platelet count (>50 000/µL) during induction were both 28 days (supplemental Table 4). Early in the study there was concern regarding delayed recovery of platelets and neutrophils in patients who received enasidenib with induction chemotherapy. Therefore, a cohort of 25 patients received an alternative dosing schedule in which enasidenib dosing started on day 8, after completion of the administration of “7 + 3,” instead of at the beginning. Although a comparison of the time to blood count recovery between the cohorts starting enasidenib on day 1 vs day 8 was limited by the small number of patients who started enasidenib on day 8, the time to count recovery was generally similar between the 2 groups (supplemental Table 5). Overall, when combining the enasidenib day 1 and day 8 cohorts, the median times to ANC and platelet count recovery during the induction phase were 34 and 29 days, respectively (supplemental Table 4).
IDH differentiation syndrome (IDH-DS) was reported in 2 patients (3.3%) receiving ivosidenib and 2 patients (2.2%) receiving enasidenib. All 4 cases occurred during induction, starting between days 29 and 48. Three of the 4 cases were grade ≥3 in severity. Ultimately, IDH-DS was reported as resolved in 3 of the 4 patients and all 3 went on to achieve a complete remission (CR) or CR with incomplete platelet recovery (CRp). The fourth patient died of a lung infection and IDH-DS was reported as ongoing at the time of death.
In the ivosidenib cohorts, QT interval prolongation was observed in 16 patients (26.7%) during induction and was grade ≥3 in 6 of these patients (10%). During the consolidation phase, QT prolongation was observed in 3 patients (8.6%) in the ivosidenib cohorts and was grade ≥3 in 1 patient (2.9%). In the enasidenib cohorts, QT prolongation during induction was reported in 11 patients (11.8%) and was grade ≥3 in 7 patients (7.5%), whereas during the consolidation period, QT prolongation was reported in 7 patients (15.2%) and was grade ≥3 in 3 patients (6.5%).
In patients treated with enasidenib, increased blood bilirubin was reported during induction in 46 patients (49.5%) and was grade ≥3 in 15 patients (16%). During consolidation, increased blood bilirubin was reported in 13 patients (28.3%) and was grade ≥3 in 5 patients (11%). In patients treated with ivosidenib, increased blood bilirubin was reported during induction in 11 patients (18.3%) and was grade ≥3 in 4 patients (6.7%). During consolidation, increased blood bilirubin was reported in 2 patients (5.7%) and was grade ≥3 in 1 patient (2.9%). Further analysis of these data showed that in patients treated with enasidenib, grade 1 and 2 increases in blood bilirubin were primarily due to increases in unconjugated bilirubin. When grade ≥3 increases in blood bilirubin occurred in enasidenib-treated patients, they consisted of increases in both the conjugated and unconjugated forms, but with a trend toward greater proportional increases in unconjugated bilirubin than in patients treated with ivosidenib. This is consistent with enasidenib’s inhibition of UGT1A1.
Efficacy
Response
In the ivosidenib-treated cohorts, rates of CR and the combined measure of CR plus CR with incomplete neutrophil recovery (CRi) or CRp (CR/CRi/CRp) at the end of the induction period were 55% and 72%, respectively, and were 68% and 77%, respectively, when evaluated at any time on study. In the enasidenib-treated cohorts, CR and CR/CRi/CRp rates were 47% and 63%, respectively, at the end of the induction period, and were 55% and 74%, respectively, when evaluated at any time on study (supplemental Figure 1).
In the ivosidenib-treated cohorts, CR and CR/CRi/CRp rates at any time during the study were higher in patients with de novo AML than in those with secondary AML (Table 3). Similar findings were observed in the enasidenib-treated cohorts. Post hoc analyses of best overall response in patients with de novo AML with or without myelodysplastic syndrome-related cytogenetic abnormalities, in secondary AML with or without prior HMA treatment, and by IDH2 mutation type were conducted but proved to be inconclusive because of the limited number of patients in these subgroups (supplemental Tables 6-8).
Best overall responses at any time during the study in the FAS
| Responsecategory . | Ivosidenib 500 mg + chemotherapy, n (%) . | Enasidenib 100 mg + chemotherapy, n (%) . | ||||
|---|---|---|---|---|---|---|
| All,N = 60 . | De novo AML,n = 42 . | Secondary AML,n = 18 . | All,N = 91* . | De novo AML,n = 56 . | Secondary AML,n = 35 . | |
| CR/CRi/CRp | 46 (77) | 37 (88) | 9 (50) | 67 (74) | 45 (80) | 22 (63) |
| CR | 41 (68) | 32 (76) | 9 (50) | 50 (55) | 36 (64) | 14 (40) |
| CRi/CRp | 5 (8) | 5 (12) | — | 17 (19) | 9 (16) | 8 (23) |
| MLFS | 4 (7) | 3 (7) | 1 (6) | 10 (11) | 5 (9) | 5 (14) |
| PR | 2 (3) | — | 2 (11) | 2 (2) | 1 (2) | 1 (3) |
| Treatment failure† | 8 (13) | 2 (5) | 6 (33) | 12 (13) | 5 (9) | 7 (20) |
| Responsecategory . | Ivosidenib 500 mg + chemotherapy, n (%) . | Enasidenib 100 mg + chemotherapy, n (%) . | ||||
|---|---|---|---|---|---|---|
| All,N = 60 . | De novo AML,n = 42 . | Secondary AML,n = 18 . | All,N = 91* . | De novo AML,n = 56 . | Secondary AML,n = 35 . | |
| CR/CRi/CRp | 46 (77) | 37 (88) | 9 (50) | 67 (74) | 45 (80) | 22 (63) |
| CR | 41 (68) | 32 (76) | 9 (50) | 50 (55) | 36 (64) | 14 (40) |
| CRi/CRp | 5 (8) | 5 (12) | — | 17 (19) | 9 (16) | 8 (23) |
| MLFS | 4 (7) | 3 (7) | 1 (6) | 10 (11) | 5 (9) | 5 (14) |
| PR | 2 (3) | — | 2 (11) | 2 (2) | 1 (2) | 1 (3) |
| Treatment failure† | 8 (13) | 2 (5) | 6 (33) | 12 (13) | 5 (9) | 7 (20) |
MLFS, morphologic leukemia-free state.
Two patients assigned to receive enasidenib starting on day 8 had an ongoing adverse event or died on day 8 and thus never received enasidenib; these 2 patients were not included in the FAS used for efficacy analyses.
Treatment failure = stable disease + progressive disease + discontinuation before response assessment on or after induction day 21 + discontinuation with best response of not evaluable.
Overall survival
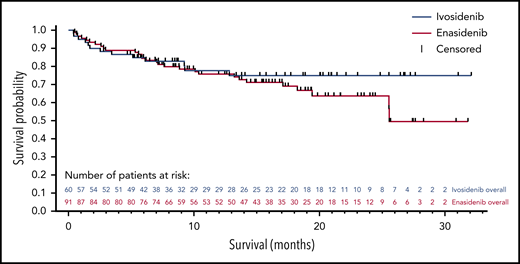
After a median follow-up period of 9.3 months (range, 0.4-32.1 months), median overall survival was not reached in the ivosidenib-treated cohorts; the 12-month survival probability after induction day 1 was 78% (Figure 2). When patients were censored at the time of HSCT, the 12-month survival probability was 74%. In the enasidenib-treated cohorts, median overall survival was 25.6 months (95% confidence intervals, 25.5 and not calculable) after a median follow-up period of 14.5 months (range, 0.5-31.8), and the 12-month survival probability was 76% (Figure 2). When patients were censored at time of HSCT, the 12-month survival probability was 67%. Event-free survival analyses were conducted but were limited by the relatively high number of patients who were censored when they discontinued treatment to proceed to HSCT (supplemental Appendix).
Overall survival in the FAS set. Patients not censored at the time of HSCT.
Pharmacokinetic and pharmacodynamic data
Pharmacokinetic/pharmacodynamic parameters observed in this study were consistent with findings from previous studies in which ivosidenib and enasidenib were administered as single agents19,20 and appeared to be similar across daunorubicin and idarubicin induction cohorts. Ivosidenib and enasidenib were rapidly absorbed, with peak plasma concentrations at 4 hours following single (data not shown) and multiple doses (supplemental Table 9). Exposure at steady state following 14 days of daily dosing was higher than after a single dose, with moderate accumulation (ranging from 1.7- to 2.4-fold) for ivosidenib and high accumulation (ranging from 6.3- to 8.3-fold) for enasidenib.
Plasma 2-HG concentrations were elevated at baseline and decreased after single and multiple doses of ivosidenib or enasidenib (supplemental Table 9). After multiple doses, mean trough plasma 2-HG concentrations were reduced by up to 99%, to levels observed in healthy participants, and were maintained throughout continued ivosidenib or enasidenib dosing. However, in contrast to the decreases in 2-HG observed in single-agent studies of these mIDH inhibitors, the decreases observed in this study likely also reflect the antileukemic activity of the standard chemotherapeutic agents.
Baseline mutation profiling and clinical response
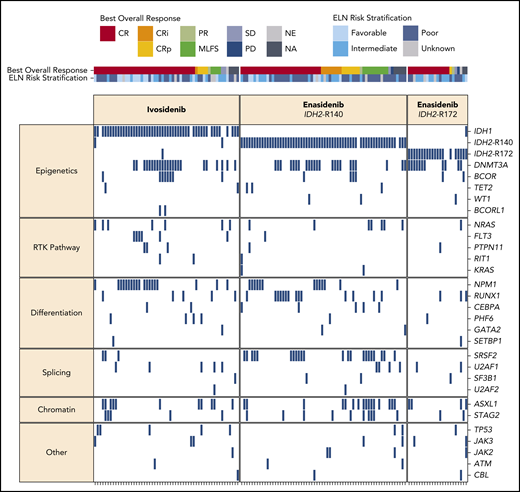
In the ivosidenib-treated cohorts, the most frequent baseline co-mutations were DNMT3A (41%), NPM1 (34%), ASXL1 (20%), and BCOR (14%), and in the enasidenib cohorts were DNMT3A (39%), SRSF2 (25%), ASXL1 (23%), and RUNX1 (20%) (supplemental Figure 2). Figure 3 summarizes the known or likely oncogenic variants detected at baseline, with patients organized by best overall response. In the ivosidenib-treated cohort, there were no co-mutations associated with a higher likelihood of CR/CRi/CRp. In the enasidenib-treated cohort, mutations in ASXL1, NRAS, U2AF1, and TP53 were associated with a lack of CR/CRi/CRp, whereas DNMT3A mutations were marginally associated with CR/CRi/CRp (supplemental Table 10).
Baseline mutational landscape and best overall clinical responses. Each column represents an individual patient, organized by best overall response. Genes (rows) are grouped by biological pathway. A blue box indicates the detection of a known or likely oncogenic variant in at least 1 sample type (peripheral blood and/or bone marrow). NA, not assessed; NE, not evaluable; PD, progressive disease; RTK, receptor tyrosine kinase; SD, stable disease.
Baseline mutational landscape and best overall clinical responses. Each column represents an individual patient, organized by best overall response. Genes (rows) are grouped by biological pathway. A blue box indicates the detection of a known or likely oncogenic variant in at least 1 sample type (peripheral blood and/or bone marrow). NA, not assessed; NE, not evaluable; PD, progressive disease; RTK, receptor tyrosine kinase; SD, stable disease.
IDH1/2 mutation clearance and MRD assessment
In ivosidenib-treated patients with a best response of CR/CRi/CRp and who had samples available for analysis, 16 of 41 (39%) had mIDH1 clearance. In enasidenib-treated patients with a best response of CR/CRi/CRp and who had samples available for analysis, 15 of 64 (23%) had mIDH2 clearance from bone marrow mononuclear cells by dPCR (Table 4).
IDH mutation clearance and MRD status in patients with a best response of CR/CRi/CRp
| Treatment . | n . | IDH mutation clearance, n (%) . | n . | MRD−, n (%) . |
|---|---|---|---|---|
| Ivosidenib + chemotherapy | 41 | 16 (39) | 20 | 16 (80) |
| Enasidenib + chemotherapy | ||||
| Total | 64 | 15 (23) | 16 | 10 (62) |
| R140 | 47 | 11 (23) | 12 | 8 (67) |
| R172 | 17 | 4 (24) | 4 | 2 (50) |
| Treatment . | n . | IDH mutation clearance, n (%) . | n . | MRD−, n (%) . |
|---|---|---|---|---|
| Ivosidenib + chemotherapy | 41 | 16 (39) | 20 | 16 (80) |
| Enasidenib + chemotherapy | ||||
| Total | 64 | 15 (23) | 16 | 10 (62) |
| R140 | 47 | 11 (23) | 12 | 8 (67) |
| R172 | 17 | 4 (24) | 4 | 2 (50) |
MRD negativity was assessed using multiparameter flow cytometry in a subset of patients that was not identical to that in which mIDH clearance was assessed. Among ivosidenib-treated patients with a best response of CR/CRi/CRp, 16 of 20 (80%) were MRD−, whereas among enasidenib-treated patients with a best response of CR/CRi/CRp, 10 of 16 (63%) were MRD− (Table 4). Supplemental Table 11 shows mutation clearance and MRD− rates for all response categories and by IDH2 mutation type. Longitudinal mutation clearance at the per-patient level is shown in supplemental Figures 3 and 4.
In the subset of patients with a best overall response of CR/CRi/CRp who had data for both mIDH1/2 clearance by dPCR and MRD by multiparameter flow cytometry, 6 of 6 (100%) ivosidenib-treated patients and 1 of 2 (50%) enasidenib-treated patients who cleared their IDH1/2 mutation also achieved MRD−. However, 9 of 15 (60%) ivosidenib-treated patients and 9 of 10 (90%) enasidenib-treated patients who achieved elimination of MRD had detectable mIDH1/2, suggesting that dPCR for mIDH clearance is more sensitive for determining the presence of underlying disease than assessment of MRD using multiparameter flow cytometry (supplemental Table 12).
Co-mutation clearance at end of induction
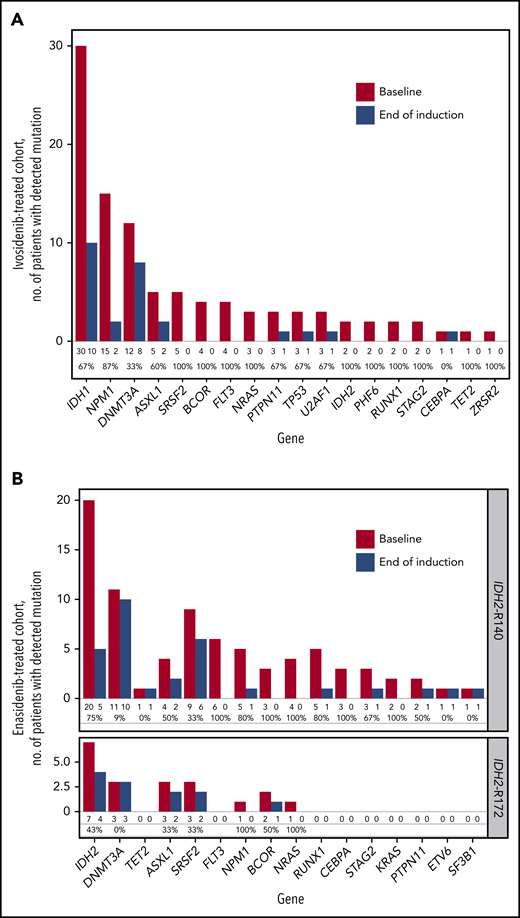
We performed targeted NGS profiling at baseline and the end of induction in patients with a best of response of CR/CRi/CRp (ivosidenib n = 31, enasidenib n = 28) to monitor co-occurring mutation clearance at the 2% VAF level. Figure 4 shows a per-gene overview of the number of patients with a detectable mutation at baseline vs the end of induction. The frequency of mutation clearance at the end of induction varied considerably by gene. Mutations in DNMT3A, TET2, and/or ASXL1 (DTA mutations) are common in patients with age-related clonal hematopoiesis, and DTA mutations have been shown frequently to persist after chemotherapy, with no significant impact on prognosis.21,22 We observed that aside from DTA mutations, most co-occurring mutations were cleared by the combination of ivosidenib or enasidenib and induction chemotherapy.
Paired sample analysis of mutations by NGS at screening and end of induction. (A) Ivosidenib-treated patients with a best response of CR/CRi/CRp only, n = 31, Personalis ACE Cancer Panel. (B) Enasidenib-treated patients with a best response of CR/CRi/CRp only, n = 28, Archer VariantPlex Core Myeloid panel. Values under each bar denote the number of patients with a mutation detected at baseline and end of induction, and the mutation clearance rate (%) for each gene.
Paired sample analysis of mutations by NGS at screening and end of induction. (A) Ivosidenib-treated patients with a best response of CR/CRi/CRp only, n = 31, Personalis ACE Cancer Panel. (B) Enasidenib-treated patients with a best response of CR/CRi/CRp only, n = 28, Archer VariantPlex Core Myeloid panel. Values under each bar denote the number of patients with a mutation detected at baseline and end of induction, and the mutation clearance rate (%) for each gene.
Supplemental Table 13 summarizes mutation clearance (NGS; sensitivity 2% VAF) at the end of induction. In ivosidenib-treated patients with a response of CR/CRi/CRp, the rates of clearance of mIDH1 and non-DTA co-mutations were 67% and 58%, respectively. In enasidenib-treated patients with a response of CR/CRi/CRp, the rate of clearance for mIDH2-R140 was 75%, whereas for mIDH2-R172 it was 43%. The rate of non-DTA mutation clearance was the same in patients with mIDH2-R140 (43%) and those with mIDH2-R172 (43%).
Mutation profiling at relapse
Of the 4 ivosidenib-treated and 8 enasidenib-treated patients who relapsed, mutational profiling at relapse was available for 3 patients from the ivosidenib cohorts and 1 patient from the enasidenib cohorts. Co-mutations at relapse were the same as those at screening, except in 1 ivosidenib-treated patient in whom a GNAS mutation (L46del) emerged, and in another ivosidenib-treated patient in whom a KDM5C F1376fs mutation emerged and mIDH1 was not detectable.
Discussion
Because ivosidenib and enasidenib have activity as single agents in R/R mIDH1/2 AML,14,15 we assessed their combination with intensive induction and consolidation chemotherapy in patients with newly diagnosed mIDH1/2 AML. Ivosidenib 500 mg once daily and enasidenib 100 mg once daily were well tolerated when combined with induction and consolidation therapy, with safety profiles similar to those seen with induction and consolidation chemotherapy alone. The addition of ivosidenib or enasidenib, regardless of start date, did not affect the time needed for hematologic recovery after induction chemotherapy when compared with historical references in relatively similar patient populations.23,24 Therefore, future studies will initiate treatment with the IDH inhibitors concurrently with the start of induction therapy. Thirty- and 60-day mortality rates in this older patient population were comparable with those observed in patients with newly diagnosed AML given intensive chemotherapy. IDH-DS has been observed in ∼10% of patients treated with ivosidenib or enasidenib as single agents.14,15 Because ivosidenib and enasidenib were administered in combination with cytotoxic chemotherapy in this study, rates of IDH-DS were low, as expected. QT interval prolongation has been associated with ivosidenib monotherapy,14 and in the present study the frequency and grades of QT prolongation were similar to those observed with ivosidenib monotherapy in R/R AML.14 However, ivosidenib’s independent contribution to QT prolongation in this study could not be determined, given that the use of supportive medications known to prolong the QT interval (eg, quinolones, azole antifungals, 5-HT3 antagonists) was permitted. Increased blood bilirubin was more frequently observed in the enasidenib cohorts, consistent with enasidenib’s known inhibition of the UGT1A1 enzyme,15 but it did not appear to have significant clinical consequences.
In previous studies, CR rates in patients with newly diagnosed or previously treated mIDH1/2 AML who received induction and consolidation chemotherapy ranged from 38% to 65%, although definitions of CR varied across the studies.5,25,26 The end-of-induction CR and CR/CRi/CRp rates observed in our study were 55% and 72%, respectively, in the ivosidenib-treated patients, and 47% and 63%, respectively, in the enasidenib-treated patients. The best overall CR and CR/CRi/CRp rates were 68% and 77%, respectively, in ivosidenib-treated patients, and 55% and 74%, respectively, in enasidenib-treated patients. These response rates are encouraging, particularly in this group of patients, which included a substantial proportion with secondary AML (∼30%) and a majority ≥60 years of age. However, because this was a phase 1 study with no comparator group, and owing to the limited data available for older patients with newly diagnosed mIDH AML treated with intensive induction and consolidation therapy, the interpretation of our remission rates is challenging. A randomized, controlled trial is needed to adequately compare response rates for each mIDH inhibitor combination with intensive chemotherapy alone.
To better understand the depth of clinical responses in the present study, mIDH clearance (by highly sensitive dPCR) and the elimination of MRD (by multiparameter flow cytometry) were assessed. These analyses were limited in that samples were not regularly collected after the end of induction and thus are not time-matched across patients. Additionally, the subset of patients with samples available for mIDH clearance analysis was not the same as the subset with samples available for MRD analysis. Nevertheless, among patients who achieved CR/CRi/CRp, ivosidenib given with intensive induction and consolidation chemotherapy was associated with mIDH1 clearance in 39% of patients (16 of 41) and with elimination of MRD in 80% of patients (16 of 20). Enasidenib given with the same chemotherapy was associated with mIDH2 clearance in 23% of patients (15 of 64) and with the elimination of MRD in 63% of patients (10 of 16). In the small group of patients for whom results were available both for mIDH1/2 clearance and for MRD, clearance of mIDH1/2 was often associated with elimination of MRD. However, a substantial proportion of patients who demonstrated persistence of mIDH1/2 achieved elimination of MRD, suggesting that mIDH clearance as assessed by dPCR is a more sensitive measure for detecting the presence of the underlying disease than assessing MRD using multiparameter flow cytometry. Comparisons of mutation clearance rates with those of other studies cannot be made because of the limited number of samples collected for this assessment in our study and the irregular timing of sample collection after cycle 1 day 28; moreover, the cutoff used for determination of mutation clearance varies between studies.
In patients with mIDH R/R AML, the presence of co-mutations in receptor tyrosine kinase pathway genes such as NRAS, KRAS, and FLT3 has been associated with resistance to single-agent therapy with inhibitors of mIDH.14,27,28 Our longitudinal molecular profiling in a subset of patients using NGS (cutoff 2%) of mutations at baseline, and at the time of best response, showed that mutations in FLT3 and RAS were cleared after induction chemotherapy, suggesting that the combination of an mIDH inhibitor with chemotherapy in the first-line setting may overcome one of the most common mechanisms of resistance to single-agent inhibitors of mIDH1/2. Although mutational profiling data were available for only 4 of the patients who relapsed, the data suggest that relapse can occur both with and without the emergence of new mutations.
In this study, ivosidenib-treated and enasidenib-treated patients had 12-month survival probabilities of >75%, which is promising given that survival rates have historically been low, especially for older patients with AML,29,30 although improvements have been noted in the past few decades, particularly in younger patients.31 Survival follow-up is ongoing to determine whether these encouraging rates will be maintained over a longer period.
In summary, the combination of ivosidenib or enasidenib with intensive induction and consolidation therapy was well tolerated in patients with newly diagnosed mIDH1 or mIDH2 AML, and the initial clinical activity was encouraging. The benefit of adding ivosidenib or enasidenib to induction and consolidation chemotherapy followed by single-agent maintenance therapy for patients with newly diagnosed mIDH AML is being further evaluated in an ongoing randomized phase 3 trial.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Investigators interested in data sharing and collaboration should contact datasharing@agios.com.
The online version of this article contains a data supplement.
Acknowledgments
Editorial assistance was provided to the authors by Helen Varley, CMPP, Excel Medical Affairs, Horsham, United Kingdom, and supported by Agios.
This work was supported by Agios Pharmaceuticals, Inc., in collaboration with Bristol-Myers Squibb. Agios provided financial support for the study and participated in the design, study conduct, and analysis and interpretation of data, as well as the writing, review, and approval of the manuscript.
Authorship
Contribution: E.M.S., C.D.D., A.T.F., M.R.S., R.M.S., H.D., B.L., G.J.O., S.N., L.H., C.A., M.C., and M.S.T. designed the study; E.M.S., C.D.D., A.T.F., A.S.M., K.W.P., M.R.S., A.S.S., R.M.S., E.S.W., C.S.S., H.D., D.A.P., J.K.M., O.O., B.L., G.J.O., P.A.P., M.R., H.M.K., and M.S.T. participated in patient enrollment and care of patients; E.M.S., C.D.D., A.T.F., A.S.M., K.W.P., M.R.S., A.S.S., R.M.S., E.S.W., C.S.S., H.D., D.A.P., J.K.M., O.O., B.L., G.J.O., P.A.P., M.R., M.G.F., F.L., A.F., S.N., B.F., S.C., H.W., B.W., L.H., C.A., M.C., H.M.K., and M.S.T. participated in data collection, analysis, and interpretation; E.M.S., C.D.D., B.F., S.C., H.W., L.H., C.A., and M.C. oversaw drafting of the manuscript; and all authors participated in manuscript development and final approval of the submitted version.
Conflict-of-interest disclosure: E.M.S. is a stockholder in/has ownership of Auron Therapeutics; is a consultant/advisor to AbbVie, Agios, Astellas, Bayer, BioLineRx, Celgene, Daiichi Sankyo, Genentech, Novartis, Pfizer, PTC Therapeutics, and Syros; received research funding from Agios, Amgen, Bayer, Celgene, and Syros; and received travel expenses from AbbVie, Astellas, BioTheryX, Celgene, Daiichi Sankyo, Novartis, Syndax, and Syros. C.D.D. is a consultant/advisor to AbbVie, Agios, Celgene, Daiichi Sankyo, Jazz, MedImmune, and Notable Labs; and received research funding from AbbVie, Agios, Calithera, Celgene, and Daiichi Sankyo. A.T.F. is a consultant/advisor to Agios, AbbVie, Amgen, Astellas, Boston Biomedical, Celgene (Bristol-Myers Squibb), Daiichi Sankyo, Jazz, Takeda, and Trovagene and received research funding from Agios and Celgene (Bristol-Myers Squibb). A.S.M. is a consultant/advisor to AbbVie, Agios, Astellas, Jazz, and PTC Therapeutics. K.W.P. is an advisor to Agios, AbbVie, Astellas, Boston Biomedical, and Celgene/Bristol-Myers Squibb and received institutional research funding from AbbVie, Agios, Astellas, and Takeda. M.R.S. received research support from Astex, Incyte, Sunesis, Takeda, and TG Therapeutics; is a stockholder in/has ownership of Karyopharm; is a consultant/advisor to AbbVie, Bristol-Myers Squibb, Celgene, Merck, Ryvu, Sierra Oncology, and TG Therapeutics; and has patents with/received royalties from Boehringer Ingelheim. A.S.S. is on a speakers’ bureau for Amgen, Celgene, and Stemline and is an advisor to Amgen. R.M.S. is on a steering committee for Celgene; is an advisor to AbbVie, Actinium, Agios, Amgen, Astellas, BioLineRx, Celgene, Daiichi Sankyo, ElevateBio, GEMoaB, Janssen, MacroGenics, Novartis, Syndax, Takeda, and Trovagene; received clinical research funding from AbbVie, Agios, and Syndax; and is on data safety monitoring boards for Argenx, Celgene, and Takeda. E.S.W. is an advisor to Jazz, Pfizer, and Shionogi. H.D. is a consultant to/received honoraria from AbbVie, Agios, Amgen, Astellas, Astex, Celgene, Helsinn, Janssen, Jazz, Novartis, Oxford Biomedicals, and Roche and received institutional clinical research funding from Amgen, AROG, Bristol-Myers Squibb, Celgene, Jazz, Pfizer, and Sunesis. D.A.P. is a consultant to AbbVie, Agios, Amgen, Celgene, Celyad, Daiichi Sankyo, Forty Seven, Gilead, Pfizer, and Takeda; and received research funding from AbbVie and Pfizer. J.K.M. is on a speakers’ bureau for Amgen, Celgene, Jazz, and Takeda; received honoraria from Celgene; is a consultant to Jazz, Pfizer, and Takeda; and is a stockholder in/has ownership in COTA. O.O. is an advisor to AbbVie, Celgene, and Impact Biomedicines and received institutional clinical research funding from AbbVie, Agios, AstraZeneca, Celgene, Daiichi Sankyo, Incyte, NS Pharma, and OncoTherapy Science. B.L. is an advisor to AbbVie, Agios, AIMM Therapeutics, Astellas, Astex, Celgene, Clear Creek Bio, F. Hoffman-La Roche, Frame, GEMoaB, and Oxford Biomedical. G.J.O. is a consultant to AbbVie, Agios, Amgen, Astellas, Celgene, Daiichi Sankyo, Jazz, Novartis, Pfizer, and Roche and received research funding from BD, Celgene, Genentech, Johnson & Johnson, and Novartis. P.A.P. received honoraria from DAVA Oncology and France Foundation and is a member of an entity's board of directors/advisory committees/speakers’ bureau for Celgene. M.R. is involved with the provision of services for Auron Therapeutics, Celgene, and Physicians' Education Resource and is a stockholder in/has ownership of Auron Therapeutics. M.G.F. is an employee of and stockholder in/has ownership of Bristol-Myers Squibb. F.L. is an employee of and stockholder in/has ownership of Celgene International. A.F. is an employee of Bristol-Myers Squibb. S.N., S.C., C.A., and M.C. are employees of and stockholders in/have ownership of Agios. B.F., H.W., and L.H. were employees of and stockholders in/had ownership of Agios at the time of the study. B.W. is an employee of, stockholder in/has ownership of, and received patents/royalties from Agios. H.M.K. received research funding from AbbVie, Agios, Amgen, ARIAD, Astex, Bristol-Myers Squibb, Cyclacel, Daiichi Sankyo, ImmunoGen, Jazz, Novartis, and Pfizer and received honoraria from AbbVie, Actinium, Agios, Amgen, Pfizer, and Takeda. M.S.T. received patents/royalties from UpToDate; is a consultant/member of an entity's board of directors/advisor to AbbVie, BioLineRx, Daiichi Sankyo, Delta-Fly Pharma, Jazz, KAHR, Nohla, Oncolyze, Orsenix, Rigel, Roche, and Tetraphase; and received research funding from AbbVie, ADC Therapeutics, BioSight, Cellerant, and Orsenix. C.S.S. declares no competing financial interests.
The current affiliation for K.W.P. is Penn Medicine, Philadelphia, PA.
Correspondence: Eytan M. Stein, Leukemia Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 East 74th St, New York, NY 10021; e-mail: steine@mskcc.org.
REFERENCES
Author notes
E.M.S. and C.D.D. are joint lead authors.
H.M.K. and M.S.T. are joint lead senior authors.

Comments
Enasidenib might affect the sensitivity and prognosis of acute myelogenous leukemia patients to cytarabine by inhibiting UGT1A1
Enasidenib is found to inhibit the activity of phase II drug metabolism enzyme UGT1A1, which is reported to be responsible for the detoxification of cytarabine through glucuronidation.2,3 Interestingly, we found in our previous study that the UGT1A1*6 and *28 polymorphisms which result in decreased UGT1A1 activity could increase sensitivity of AML patients to cytarabine-based chemotherapy, and improve their prognosis.4 Moreover, EM Stein et al. also showed the addition of enasidenib, regardless of start date, did not affect the time required for the recovery of neutrophil or platelet count after induction chemotherapy.1 Besides, Díaz-Santa J et al. observed that the duration of neutropenia and thrombocytopenia in AML patients after induction chemotherapy was not affected by UGT1A1 genotypes. However, after consolidation chemotherapy, AML patients with homozygous UGT1A1*28 mutation had a longer neutropenia and higher mortality rate,3 indicating a possible link between low UGT1A1 glucuronidation activity and fatal toxic events.
In view of this, we propose that enasidenib may increase the sensitivity of cytarabine by inhibiting UGT1A1. Whereas, under high-dose cytarabine chemotherapy regimens, this low-glucuronidation mode may result in increased cytarabine exposure, which in turn leads to increased risk for myelosuppression, prolonged recovery time, and poor prognosis. Therefore, the time and dosage of enasidenib in consolidation therapy phase of AML patients deserve further discussion.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Reference
[1]Stein EM, DiNardo CD, Fathi AT, et al. Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: a phase 1 study. Blood. 2021;137(13): 1792-1803.
[2] Zahreddine HA, Culjkovic-Kraljacic B, Gasiorek J, et al. GLI1-inducible glucuronidation targets a broad spectrum of drugs. ACS Chem Biol. 2019;14(3): 348-355.
[3] Díaz-Santa J, Rodríguez-Romanos R, Osca G, et al. UGT1A1 genotype influences clinical outcome in patients with intermediate-risk acute myeloid leukemia treated with cytarabine-based chemotherapy. Leukemia. 2020;34(11): 2925-2933.
[4] Chen P, Zhu KW, Zhang DY, et al. Influence of UGT1A1 polymorphisms on the outcome of acute myeloid leukemia patients treated with cytarabine-base regimens. J Transl Med. 2018;16(1): 1-9.