Key Points
In a randomized, placebo-controlled, phase 1/2 study in patients with SCD, voxelotor (500-1000 mg per day) was well tolerated.
All patients receiving voxelotor for ≥28 days demonstrated hematologic improvements, suggesting disease-modifying activity in SCD.
Abstract
New treatments directly targeting polymerization of sickle hemoglobin (HbS), the proximate event in the pathophysiology of sickle cell disease (SCD), are needed to address the severe morbidity and early mortality associated with the disease. Voxelotor (GBT440) is a first-in-class oral therapy specifically developed to treat SCD by modulating the affinity of hemoglobin (Hb) for oxygen, thus inhibiting HbS polymerization and downstream adverse effects of hemolytic anemia and vaso-occlusion. GBT440-001 was a phase 1/2 randomized, double-blind, placebo-controlled, single and multiple ascending dose study of voxelotor in adult healthy volunteers and patients with SCD, followed by a single-arm, open-label extension study. This report describes results of voxelotor (500-1000 mg per day) in patients with sickle cell anemia. The study evaluated the safety, tolerability, pharmacokinetic, and pharmacodynamic properties of voxelotor and established proof of concept by improving clinical measures of anemia, hemolysis, and sickling. Thirty-eight patients with SCD received 28 days of voxelotor 500, 700, or 1000 mg per day or placebo; 16 patients received 90 days of voxelotor 700 or 900 mg per day or placebo. Four patients from the 90-day cohort were subsequently enrolled in an extension study and treated with voxelotor 900 mg per day for 6 months. All patients who received multiple doses of voxelotor for ≥28 days experienced hematologic improvements including increased Hb and reduction in hemolysis and percentage of sickled red cells, supporting the potential of voxelotor to serve as a disease-modifying therapy for SCD. Voxelotor was well tolerated with no treatment-related serious adverse events and no evidence of tissue hypoxia. These trials were registered at www.clinicaltrials.gov as #NCT02285088 and #NCT03041909.
Introduction
Sickle cell disease (SCD) is an autosomal-recessive disorder caused by a mutation of the β-globin gene, HBB:c.20A>T (p.Glu7Val), leading to the production of sickle hemoglobin (HbS).1 Upon deoxygenation, HbS polymerizes, deforming red blood cells (RBCs) into a sickle shape and causing permanent cell membrane damage.1-3 These damaged RBCs block capillaries and undergo hemolysis, causing anemia, tissue ischemia, painful vaso-occlusive crisis (VOC), vascular injury, and end-organ damage. This leads to fatigue, reduced quality of life, and early death.1,3 SCD affects ∼100 000 people in the United States and millions worldwide, most of whom are in sub-Saharan Africa.4-6 New therapeutic options for SCD have evolved very slowly and the treatment of SCD remains a serious, unmet medical need.7
There are currently no licensed therapies that were designed to directly target the molecular mechanism of HbS polymerization. Because oxygenated HbS cannot polymerize, it is expected that modifying HbS to increase the proportion of oxygenated to deoxygenated HbS in RBCs would modify disease severity.8,9 At the time of this study, the only approved therapy for SCD was hydroxyurea, which is indicated to reduce the frequency of painful crises and the need for blood transfusions in patients with recurrent moderate to severe sickle cell crises.10 Voxelotor (previously called GBT440) is a first-in-class oral therapy developed to treat SCD by modulating the affinity of hemoglobin (Hb) for oxygen. Voxelotor forms a reversible covalent bond with the N-terminal valine of the α chain of Hb, resulting in an allosteric modification of Hb,9 which increases oxygen affinity.11 By increasing the oxygen affinity of Hb, voxelotor decreases the concentration of deoxygenated HbS, the HbS conformation that forms polymers. The therapeutic rationale of reducing the concentration of polymerizing HbS is supported in part by the observation that individuals who are compound heterozygotes for HbS and a deletional form of hereditary persistence of fetal Hb who maintain 20% to 30% of nonpolymerizing fetal Hb in a pancellular distribution do not have clinical manifestations of SCD12-16 ; this suggests that targeting 20% to 30% Hb occupancy with voxelotor may be beneficial.
Preclinical studies with purified HbS demonstrate that voxelotor-modified HbS is as effective as fetal Hb in delaying HbS polymerization. In vitro studies using blood from SCD patients as well as in vivo animal studies indicate that voxelotor has high specificity for binding to Hb, has a half-life supporting once-daily dosing, increases Hb-oxygen affinity, reduces sickling, improves sickle RBC deformability, reduces blood viscosity, prolongs RBC half-life, and exhibits a linear pharmacokinetic (PK)/pharmacodynamic (PD) relationship.11,17
The GBT440-001 (NCT02285088) phase 1/2 study was designed to evaluate the safety and tolerability of single and multiple doses of voxelotor administered to healthy volunteers and SCD patients. Secondary objectives included characterization of the PK of voxelotor in plasma and whole blood; the PD effect of increasing Hb-oxygen affinity; the PK-PD relationship; proof of concept on improving sickling, anemia, and hemolysis; and an evaluation of voxelotor effect on cardiovascular parameters at rest and during exercise. Herein, we present results of voxelotor treatment, administered as multiple doses in patients with SCD.
Methods
Patients and methods
This was a phase 1/2, randomized, double-blind, placebo-controlled study of voxelotor in healthy volunteers and patients with SCD. Data from healthy volunteers and SCD patients treated with single doses and from healthy volunteers treated with multiple doses are reported separately. After characterizing the PK/PD and safety in at least 1 multiple-dose cohort of healthy volunteers and in 1 single-dose cohort of SCD patients, screening for the multiple-dose investigation for 28 days in SCD patients was initiated. The study was conducted in accordance with Good Clinical Practice guidelines, and in conformity with the ethical principles of the Declaration of Helsinki, and was compliant with all relevant country-specific laws and regulations in the United Kingdom and United States. The study protocol and all other appropriate study-related information were reviewed and approved by an independent ethics committee.
Patient selection
Patients with SCD were referred by 7 clinics in the United Kingdom to the Quintiles Drug Research Unit at Guy’s Hospital in London. Key inclusion criteria included patients with SCD (sickle cell anemia [HbSS] or HbS/β0 thalassemia), aged 18 to 60 years, and >50 kg at screening. Women of childbearing potential and all men were required to use contraception. For patients receiving hydroxyurea, the dose must have been stable for ≥3 months prior to screening, with no anticipated need for dose adjustment during the study. Key exclusion criteria included Hb levels <6.0 g/dL or >10.4 g/dL, or transfusion or hospitalization within 30 days of screening. Other exclusion criteria included alanine aminotransferase or alkaline phosphatase >3 times the upper limit of normal or aspartate aminotransferase >4 times the upper limit of normal, or moderate or severe renal dysfunction (defined as calculated modification of diet in renal disease [MDRD] estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2, appropriately corrected for race and sex). All patients provided written informed consent prior to the commencement of any study-related procedures.
Study design
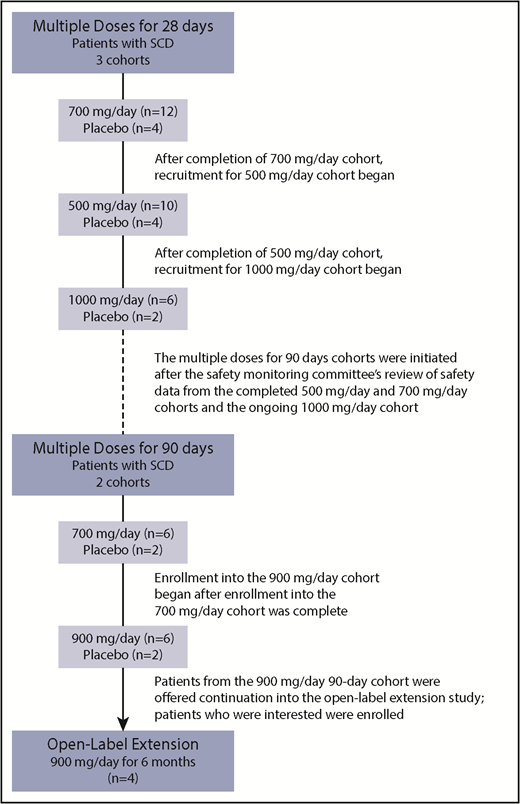
Voxelotor was administered as multiple doses (500, 700, or 1000 mg) for 28 days and multiple doses (700 or 900 mg) for 90 days (Figure 1). Patients from the 90-day, 900-mg cohort were offered continuation with voxelotor; patients who were interested enrolled into a separate, open-label extension study to provide 6 months’ cumulative treatment with voxelotor 900 mg (NCT03041909). The highest dose of 900 mg selected for the 90-day cohort was based on the availability of 300-mg capsules, which allowed for a more convenient study drug administration of 3 × 300-mg capsules vs 9 × 100-mg capsules.
Voxelotor was administered orally as 100-mg or 300-mg capsules with matching placebo capsules. The capsule strength of 300 mg was introduced for the 90-day, 900-mg cohort. Prior to the availability of the 300-mg capsules, all patients received the 100-mg capsules. Eight patients were enrolled in each dose cohort and were randomized 6:2 to receive voxelotor or matching placebo. Two dose cohorts (500 mg and 700 mg for 28 days) were expanded to include up to a total of 16 patients to better characterize the safety of and effect of voxelotor on anemia and hemolysis. The starting dose of voxelotor for the first SCD cohort was 700 mg administered for 28 days, based on achieving a target of 20% Hb modification. The 700-mg (n = 16), 500-mg (n = 14), and 1000-mg cohorts recruited sequentially upon completion of the previous cohort.
A safety monitoring committee (SMC) consisting of the principal investigator, sponsor medical monitor, and an independent safety physician was responsible for safety and tolerability decisions for dose escalation to the next cohort. If ≥2 patients in a given cohort receiving voxelotor experienced a dose-limiting toxicity (DLT) and no patients receiving placebo had the same or similar DLT, dose escalation was to be stopped. DLT was defined as any moderate, grade ≥2 adverse event (AE) or any worsening by ≥1 grade of a preexisting condition in patients with SCD that was related to study drug. Additionally, the SMC reviewed safety data from the 90-day cohort when the first patient reached days 28, 60, and 90 to approve continued dosing in the cohort through 90 days.
Enrollment in the 90-day cohorts was initiated following the SMCs’ review of safety data from all completed (500 mg and 700 mg) and ongoing (1000 mg) multiple-dose, 28-day cohorts (Figure 1). The starting dose for the first 90-day cohort was 700 mg. Enrollment in the 900-mg cohort was initiated after all 8 patients (6 voxelotor and 2 placebo) were enrolled in the 700-mg cohort.
Assessments
Efficacy was assessed by standard clinical hematology laboratory measures (Hb, reticulocytes, unconjugated bilirubin, dense RBCs, and lactate dehydrogenase [LDH]) and percentage of sickled red cells. For patients enrolled in the 28-day cohorts, hematology laboratory measures were collected at screening and baseline and on days 4, 8, 15, 22, and 28. For the patients enrolled in the 90-day cohorts, hematology laboratory measures were collected at screening and baseline; on days 4, 15, 22, 28, 44, 60, 75, and 90; and then monthly for the patients enrolled in the extension study. Assessments were also collected 30 days following the last dose of study treatment in all patients. The percentage of sickled red cells was analyzed from blood smears by 2 independent blinded readers. Six independent microscopy fields were randomly selected and imaged at ×40 magnification per smear. Greater than 500 RBCs from 3 or more different fields were counted per blood smear. Elongated crescent-shaped RBCs with tapering of opposite ends that culminated in a point were counted as sickled.18 Elliptical RBCs with smooth rounded edges were counted as normal.18 Results were reported as percentage of sickled red cells, calculated as the number of sickled red cells divided by the total number of RBCs, multiplied by 100.
Cardiopulmonary exercise testing (CPET) was performed in the 90-day dosing cohorts with bicycle ergometer and ventilatory gas analysis to assess the adequacy of tissue oxygen delivery during exercise stress and to ensure that the voxelotor-related increase in Hb-oxygen affinity did not impair oxygen delivery. CPET assessments were collected at baseline and on days 28 and 91.
Safety was assessed in all patients who received at least 1 dose of study drug. Safety assessments included symptoms inquiry, physical examinations, vital signs (blood pressure, heart rate, temperature), standard clinical laboratory tests (chemistry panel, complete hematology panel, urinalysis, pregnancy tests), 12-lead electrocardiogram, and AE reporting from the time of study drug administration to 30 days after the last dose of study drug. Erythropoietin was collected as a biomarker related to tissue oxygen delivery. In the 90-day cohort, functional exercise capacity was evaluated using bicycle CPET at baseline and after 90 days of dosing. CPET was conducted as per guidelines developed by the American Thoracic Society/American College of Chest Physicians.
PK and PD
Serial whole blood samples were collected to determine voxelotor concentrations in whole blood, plasma, and RBCs and Hb-oxygen affinity. A validated liquid chromatography–tandem mass spectrometry method was used to analyze samples. Analytical ranges were 10 to 10 000 ng/mL for blood and 5 to 5000 ng/mL for plasma samples. Voxelotor concentration in RBCs was calculated from:
RBC Conc = (Blood Conc − [(1 − Hematocrit) × PlasmaConc])/Hematocrit
Oxygen equilibrium curves (OECs) and changes in Hb-oxygen affinity were measured using a TCS Hemox Analyzer. Clinical samples were collected in sodium citrate vacutainers and kept at 4°C until analyzed (within 12-43 hours after collection) according to the method previously described11 to obtain p20 and p50 values (partial pressure of O2 at which Hb is 20% or 50% saturated with O2). Δ p20 and Δ p50 values were determined by subtracting the day −1 p20 or p50 value from the sample p20 or p50 value. Due to the biphasic nature of the OECs upon Hb modification with voxelotor, the p20 value is more sensitive than the p50 value and, therefore, was used to calculate percentage Hb modification with voxelotor.19
Statistical analyses
Demographic data were summarized by treatment. Efficacy, safety, and PK/PD results were summarized using descriptive statistics by treatment. No formal power calculations were performed for the sample size. PK parameters including maximum concentration (Cmax) and area under the concentration-time curve over 24-hour dose intervals at steady-state (AUC0-24) were derived using noncompartmental methods with Phoenix WinNonlin version 6.4 (Certara, Princeton, NJ).
Clinical trial data sharing
Summary participant data underlying the results reported herein, after deidentification, will be shared.
Results
Patients
Data obtained from healthy volunteers and SCD patients treated with single doses and healthy volunteers treated with multiple doses for 15 days will be reported in a separate publication. Here, we report results on SCD patients who received multiple doses.
Overall, baseline demographics and clinical characteristics of SCD were generally well balanced across treatment groups; in this stable adult population, most patients were not taking hydroxyurea, and most patients had either 0 or 1 VOC that required hospitalization in the prior year (Table 1). All patients had the HbSS genotype. For the 28-day cohorts, 38 patients were randomized. The cohorts were 500 mg of voxelotor (n = 10), 700 mg of voxelotor (n = 12), 1000 mg of voxelotor (n = 6), or placebo (n = 10). For the 90-day cohorts, 16 patients were randomized to receive 700 mg per day voxelotor (n = 6), 900 mg per day voxelotor (n = 6), or placebo (n = 4). In addition, 4 patients from the 900-mg cohort of the randomized study received voxelotor 900 mg in the extension study for a cumulative treatment duration of 6 months, including 1 patient who received placebo in the randomized study and received 900 mg per day voxelotor for 6 months in the extension study. There was complete compliance with study drug administration in 91% of patients based on the daily diary and pill counts for the entire dosing period; there were few missed doses due to noncompliance reasons, with 5 patients missing 1 to 4 doses over the course of the study. Compliance while outside of the research facility was facilitated by the instruction to complete the diary on a daily basis with frequent telephone reminders and the high self-motivation of the patients.
Patient demographics and baseline characteristics
| Voxelotor/placebo, mg/d . | Multiple dose for 28 d . | Multiple dose for 90 d . | |||||
|---|---|---|---|---|---|---|---|
| Placebo . | 500 . | 700 . | 1000 . | Placebo . | 700 . | 900 . | |
| N | 10 | 10 | 12 | 6 | 4 | 6 | 6 |
| Median age (range), y | 38 (21-53) | 29 (20-48) | 29 (20-56) | 40 (25-47) | 28 (18-48) | 41 (29-53) | 37 (25-42) |
| Male sex, n (%) | 6 (60) | 8 (80) | 4 (33) | 2 (33) | 3 (75) | 4 (67) | 3 (50) |
| Median BMI (range), kg/m2 | 23.5 (19.6-30.5) | 21.4 (17.2-27.5) | 23.0 (17.8-34.4) | 22.4 (20.2-26.1) | 18.8 (17.2-28.4) | 26.9 (21.9-35.3) | 22.8 (20.0-27.3) |
| Median baseline Hb (range), g/dL | 8.1 (7.2-10.0) | 7.9 (7.0-9.7) | 9.1 (7.5-9.8) | 8.2 (7.5-8.4) | 7.9 (7.2-9.3) | 8.3 (7.1-9.7) | 9.0 (7.6-9.7) |
| Hospitalizations due to painful crisis in the previous 12 mo, median (range) | 1 (0-7) | 0 (0-1) | 1 (0-7) | 1 (0-4) | 1 (0-2) | 0 (0-2) | 0 (0-1) |
| Patients with 0 events in previous 12 mo, n | 5 | 7 | 5 | 3 | 2 | 4 | 5 |
| Blood transfusions in previous 12 mo, median (range) | 0 (0-1) | 0 (0) | 0 (0-4) | 0 (0-5) | 0 (0) | 0 (0) | 0 (0-1) |
| Current use of HU, n (%) | 3 (30) | 1 (10) | 3 (25) | 2 (33) | 1 (25) | 0 (0) | 2 (33) |
| Voxelotor/placebo, mg/d . | Multiple dose for 28 d . | Multiple dose for 90 d . | |||||
|---|---|---|---|---|---|---|---|
| Placebo . | 500 . | 700 . | 1000 . | Placebo . | 700 . | 900 . | |
| N | 10 | 10 | 12 | 6 | 4 | 6 | 6 |
| Median age (range), y | 38 (21-53) | 29 (20-48) | 29 (20-56) | 40 (25-47) | 28 (18-48) | 41 (29-53) | 37 (25-42) |
| Male sex, n (%) | 6 (60) | 8 (80) | 4 (33) | 2 (33) | 3 (75) | 4 (67) | 3 (50) |
| Median BMI (range), kg/m2 | 23.5 (19.6-30.5) | 21.4 (17.2-27.5) | 23.0 (17.8-34.4) | 22.4 (20.2-26.1) | 18.8 (17.2-28.4) | 26.9 (21.9-35.3) | 22.8 (20.0-27.3) |
| Median baseline Hb (range), g/dL | 8.1 (7.2-10.0) | 7.9 (7.0-9.7) | 9.1 (7.5-9.8) | 8.2 (7.5-8.4) | 7.9 (7.2-9.3) | 8.3 (7.1-9.7) | 9.0 (7.6-9.7) |
| Hospitalizations due to painful crisis in the previous 12 mo, median (range) | 1 (0-7) | 0 (0-1) | 1 (0-7) | 1 (0-4) | 1 (0-2) | 0 (0-2) | 0 (0-1) |
| Patients with 0 events in previous 12 mo, n | 5 | 7 | 5 | 3 | 2 | 4 | 5 |
| Blood transfusions in previous 12 mo, median (range) | 0 (0-1) | 0 (0) | 0 (0-4) | 0 (0-5) | 0 (0) | 0 (0) | 0 (0-1) |
| Current use of HU, n (%) | 3 (30) | 1 (10) | 3 (25) | 2 (33) | 1 (25) | 0 (0) | 2 (33) |
BMI, body mass index; HU, hydroxyurea.
Efficacy
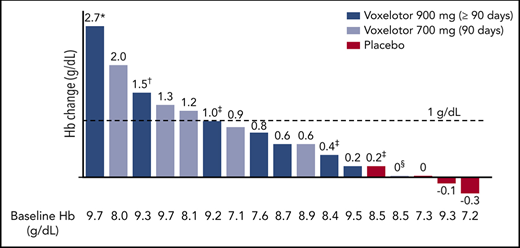
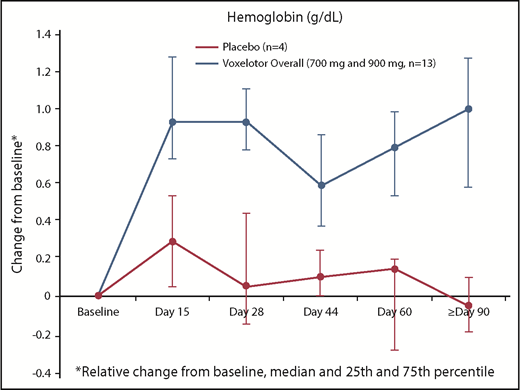
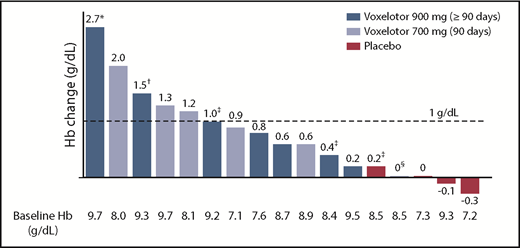
By 2 weeks of treatment, all doses of voxelotor resulted in an increase in median Hb levels and/or reduction in clinical laboratory markers of hemolysis (Tables 2 and 3; Figure 2). Long-term dosing with 900 mg showed these improvements were durable through 6 months of treatment, with a median increase in Hb of ∼1 g/dL (Table 3). Furthermore, nearly half of these patients (46%) achieved an increase in Hb of ≥1 g/dL from baseline (Figure 3). Improvements in Hb were observed in patients regardless of hydroxyurea use (Figure 3). Statistically significant improvements in unconjugated bilirubin and reticulocyte counts were observed with dosing at 90 days to 6 months, favoring voxelotor over placebo; LDH showed greater variability (Table 3). Additionally, improvements were observed for dense RBCs favoring voxelotor over placebo; however, the difference was not statistically significant. All treatment doses demonstrated reductions in the percentage of sickled red cells, including a statistically significant difference for patients treated long-term; a reduction of 73% and 79% from baseline was demonstrated at 700 mg and 900 mg, respectively, compared with an increase of ∼7% for placebo (Table 3; Figure 4). No patients had a previous history of splenectomy. For the 90-day dosing cohorts, a dose response effect was not observed; the PK parameters for these 2 dose levels were very similar. Therefore, efficacy results are summarized together for 700 mg and 900 mg, which show an improvement over placebo.
Change in hemolysis measures from baseline to day 28
| Change from baseline to day 28 . | Multiple dose for 28 d . | |||
|---|---|---|---|---|
| Voxelotor/placebo, mg/d | Placebo | 500 | 700 | 1000 |
| N | 10 | 10 | 12 | 6 |
| Hb, median (25th, 75th percentile), g/dL | −0.1 (−0.4, 0.4) | 0.4 (0.1, 0.7) | 0.7 (0.5, 1.0) | 0 (−0.4, 0.3) |
| Unconjugated bilirubin, median (25th, 75th percentile), % change | −3.6 (−25.9, 6.7) | −30.6 (−48.9, −15.4) | −42.6 (−44.4, −23.8) | −56.3 (−57.8, −47.1) |
| Percentage of reticulocytes, median (25th, 75th percentile), % change | 9.0 (−1.7, 13.7) | −31.2 (−48.9, −20.8) | −37.0 (−52.6, −4.5) | −49.9 (−64.3, −34.4) |
| LDH, median (25th, 75th percentile), % change | −6.6 (−16.8, −2.9) | −19.9 (−39.0, 6.2) | −12.0 (−30.2, −5.7) | −12.4 (−20.2, −12.1) |
| Sickled red cells, median (25th, 75th percentile), % change | 12.9 (−13.6, 12.9) | −56.4 (−70.2, −26.2) | −45.9 (−93.0, −6.0) | −45.7 (−57.9, 5.9) |
| Change from baseline to day 28 . | Multiple dose for 28 d . | |||
|---|---|---|---|---|
| Voxelotor/placebo, mg/d | Placebo | 500 | 700 | 1000 |
| N | 10 | 10 | 12 | 6 |
| Hb, median (25th, 75th percentile), g/dL | −0.1 (−0.4, 0.4) | 0.4 (0.1, 0.7) | 0.7 (0.5, 1.0) | 0 (−0.4, 0.3) |
| Unconjugated bilirubin, median (25th, 75th percentile), % change | −3.6 (−25.9, 6.7) | −30.6 (−48.9, −15.4) | −42.6 (−44.4, −23.8) | −56.3 (−57.8, −47.1) |
| Percentage of reticulocytes, median (25th, 75th percentile), % change | 9.0 (−1.7, 13.7) | −31.2 (−48.9, −20.8) | −37.0 (−52.6, −4.5) | −49.9 (−64.3, −34.4) |
| LDH, median (25th, 75th percentile), % change | −6.6 (−16.8, −2.9) | −19.9 (−39.0, 6.2) | −12.0 (−30.2, −5.7) | −12.4 (−20.2, −12.1) |
| Sickled red cells, median (25th, 75th percentile), % change | 12.9 (−13.6, 12.9) | −56.4 (−70.2, −26.2) | −45.9 (−93.0, −6.0) | −45.7 (−57.9, 5.9) |
Change in hemolysis measures from baseline to 90 days or more
| Change from baseline to end of treatment . | Dosing duration ≥90 d . | P* for pooled voxelotor vs placebo . | |||
|---|---|---|---|---|---|
| Voxelotor/placebo, mg/d | 700† | 900‡ | 700/900 | Placebo | |
| N | 6 | 7§ | 13 | 4 | |
| Hb, median (25th, 75th percentile), g/dL | 1.1 (0.6, 1.3) | 0.8 (0.5, 1.3) | 1.0 (0.6, 1.3) | −0.1 (−0.2, 0.1) | <.05 |
| Unconjugated bilirubin, median (25th, 75th percentile), % change | −37.2 (−43.4, −23.7) | −42.9 (−58.4, −30.5) | −39.7 (−49.9, −28.8) | 14.8 (1.8, 18.5) | <.05 |
| Percentage of reticulocytes, median (25th, 75th percentile), % change | −21.0 (−32.9, −18.1) | −18.9 (−35.4, −6.2) | −18.9 (−32.9, −15.0) | 8.9 (2.5, 25.5) | <.05 |
| LDH, median (25th, 75th percentile), % change | 0.8 (−14.7, 1.1) | −47.7 (−63.4, −12.5) | −12.9 (−47.7, 0.9) | 0.5 (−0.7, 7.2) | NS |
| Dense RBC, median (25th, 75th percentile), % change | −35.5|| (−63.1, 18.1) | −21.0¶ (−60.5, 11.3) | −31.4# (−62.5, 11.3) | 3.8** (−21.3, 4.3) | NS |
| Sickled red cell, median (25th, 75th percentile), % change | −72.6 (−79.0, −60.6) | −79.2¶ (−91.3, −57.7) | −74.0†† (−88.6, −57.0) | 6.9 (3.9, 10.3) | <.05 |
| Change from baseline to end of treatment . | Dosing duration ≥90 d . | P* for pooled voxelotor vs placebo . | |||
|---|---|---|---|---|---|
| Voxelotor/placebo, mg/d | 700† | 900‡ | 700/900 | Placebo | |
| N | 6 | 7§ | 13 | 4 | |
| Hb, median (25th, 75th percentile), g/dL | 1.1 (0.6, 1.3) | 0.8 (0.5, 1.3) | 1.0 (0.6, 1.3) | −0.1 (−0.2, 0.1) | <.05 |
| Unconjugated bilirubin, median (25th, 75th percentile), % change | −37.2 (−43.4, −23.7) | −42.9 (−58.4, −30.5) | −39.7 (−49.9, −28.8) | 14.8 (1.8, 18.5) | <.05 |
| Percentage of reticulocytes, median (25th, 75th percentile), % change | −21.0 (−32.9, −18.1) | −18.9 (−35.4, −6.2) | −18.9 (−32.9, −15.0) | 8.9 (2.5, 25.5) | <.05 |
| LDH, median (25th, 75th percentile), % change | 0.8 (−14.7, 1.1) | −47.7 (−63.4, −12.5) | −12.9 (−47.7, 0.9) | 0.5 (−0.7, 7.2) | NS |
| Dense RBC, median (25th, 75th percentile), % change | −35.5|| (−63.1, 18.1) | −21.0¶ (−60.5, 11.3) | −31.4# (−62.5, 11.3) | 3.8** (−21.3, 4.3) | NS |
| Sickled red cell, median (25th, 75th percentile), % change | −72.6 (−79.0, −60.6) | −79.2¶ (−91.3, −57.7) | −74.0†† (−88.6, −57.0) | 6.9 (3.9, 10.3) | <.05 |
NS, not significant.
Wilcoxon rank-sum test.
Ninety days of dosing.
Ninety days to 6 months of dosing (2 patients had 90 days of dosing, 1 patient had 118 days of dosing, and 4 patients had 6 months of dosing).
Includes 1 patient who received placebo in GBT440-001 and voxelotor 900 mg for 6 months in GBT440-024.
n = 4.
n = 6.
n = 10.
n = 3.
n = 12.
Hb change from baseline to last observation (≥90 days): responder analysis for ≥1 g/dL. *Day 15 presented due to a protocol-specified dose reduction on day 17 (because of a 2.7 g/dL increase in Hb). †Day 150 presented because this is the last time point collected for Hb while the patient was receiving study drug. ‡Concurrent hydroxyurea. §Documented nonadherence with study drug regimen.
Hb change from baseline to last observation (≥90 days): responder analysis for ≥1 g/dL. *Day 15 presented due to a protocol-specified dose reduction on day 17 (because of a 2.7 g/dL increase in Hb). †Day 150 presented because this is the last time point collected for Hb while the patient was receiving study drug. ‡Concurrent hydroxyurea. §Documented nonadherence with study drug regimen.
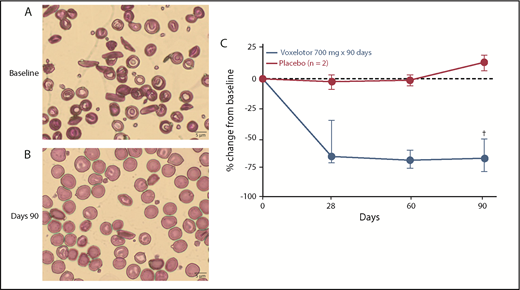
Sickled red cells. Change from (A) baseline (Wright-Giemsa stain) to (B) day 90 (Wright-Giemsa stain) in SCD patients. (C) Percentage of sickled red cells. Relative change from baseline, median, and 25th and 75th percentile; baseline irreversibly sickled cell (ISC) counts ranged from 3.1% to 17.2%. †Represents 5 of 6 subjects at day 90 (D90).
Sickled red cells. Change from (A) baseline (Wright-Giemsa stain) to (B) day 90 (Wright-Giemsa stain) in SCD patients. (C) Percentage of sickled red cells. Relative change from baseline, median, and 25th and 75th percentile; baseline irreversibly sickled cell (ISC) counts ranged from 3.1% to 17.2%. †Represents 5 of 6 subjects at day 90 (D90).
Interestingly, with 28-day dosing, a time dependency was observed in the effect on Hb but not on other measures of hemolysis: an initial rapid rise in Hb at all doses was evident by day 15, with a transient attenuation of effect on Hb between days 22 and 28. This transient attenuation of effect likely accounts for the absence of Hb increase in the 1000-mg cohort at the 28-day time point (Table 2). With longer-duration dosing for up to 6 months at 700 mg and 900 mg, a sustained and durable increase in Hb is evident (Figure 2).
Safety and tolerability
Overall, voxelotor was well tolerated at doses up to and including 1000 mg per day for 28 days and 900 mg per day for 6 months. A maximum tolerated dose was not identified. Treatment-emergent AEs (TEAEs) reported in ≥10% of patients are presented in Table 4. The majority of TEAEs were grade 1 or 2. Treatment-related TEAEs included headache, diarrhea, and rash. TEAEs of headache occurred in patients receiving voxelotor or placebo (Table 4). There were no treatment-related, grade ≥3 TEAEs. In voxelotor-treated patients, all VOC events occurred off-treatment (ie, after the last dose or during a dose hold) and were considered to be related to the underlying SCD. In the 4 patients who received voxelotor for 6 months, 1 patient experienced a grade 3 VOC 30 days after the last dose of study drug; this patient had a history of 2 VOCs in the prior year. Four patients had dose reductions due to TEAEs: grade 1 abdominal discomfort and nausea (n = 1; 700 mg), grade 1 increased hepatic enzyme (n = 2; 700 mg), and grade 2 papular pruritic rash (n = 1; 900 mg); the rash did not recur with dose escalation back to 900 mg. One patient at 900 mg who had a 2.7 g/dL increase in Hb at day 15 had a dose reduction on day 17 to 700 mg and further reduction to 600 mg on day 22 (Figure 3); this reduction was protocol-defined based on an Hb increase of >2.0 g/dL over baseline. One patient (1000 mg) discontinued study treatment due to a grade 2 rash. Twelve serious AEs (SAEs) were reported for 12 patients who received multiple doses of study drug. The majority of SAEs were grade 3 VOC. None of the SAEs were considered related to treatment. There were no deaths reported.
TEAEs occurring in 10% or more of patients
| . | Voxelotor/placebo, mg/d . | Pooled placebo . | |||||
|---|---|---|---|---|---|---|---|
| 500 . | 700 . | 700 . | 900 . | 1000 . | All . | ||
| Days | 28 | 28 | 90 | 90 d to 6 mo | 28 | 28-90 | |
| N | 10 | 12 | 6 | 7* | 6 | 41* | 14 |
| Headache, n (%) | 4 (40) | 5 (42) | 1 (17) | 2 (29) | 4 (67) | 16 (39) | 8 (57) |
| Back pain, n (%) | 2 (20) | 3 (25) | 0 | 2 (29) | 2 (33) | 9 (22) | 2 (14) |
| Pain, n (%) | 1 (10) | 4 (33) | 0 | 2 (29) | 1 (17) | 8 (20) | 4 (29) |
| Pain in extremity, n (%) | 1 (10) | 1 (8) | 1 (17) | 2 (29) | 0 | 5 (12) | 0 |
| Diarrhea,† n (%) | 0 | 2 (17) | 0 | 2 (14) | 2 (33) | 6 (15) | 0 |
| Cough, n (%) | 0 | 2 (17) | 2 (33) | 0 | 1 (17) | 5 (12) | 0 |
| Rash,‡ n (%) | 0 | 0 | 1 (17) | 1 (14) | 3 (50) | 5 (12) | 1 (7) |
| Sickle cell anemia with crisis,§ n (%) | 2 (20) | 2 (17) | 2 (33) | 2 (29) | 1 (17) | 9 (22) | 1 (7) |
| . | Voxelotor/placebo, mg/d . | Pooled placebo . | |||||
|---|---|---|---|---|---|---|---|
| 500 . | 700 . | 700 . | 900 . | 1000 . | All . | ||
| Days | 28 | 28 | 90 | 90 d to 6 mo | 28 | 28-90 | |
| N | 10 | 12 | 6 | 7* | 6 | 41* | 14 |
| Headache, n (%) | 4 (40) | 5 (42) | 1 (17) | 2 (29) | 4 (67) | 16 (39) | 8 (57) |
| Back pain, n (%) | 2 (20) | 3 (25) | 0 | 2 (29) | 2 (33) | 9 (22) | 2 (14) |
| Pain, n (%) | 1 (10) | 4 (33) | 0 | 2 (29) | 1 (17) | 8 (20) | 4 (29) |
| Pain in extremity, n (%) | 1 (10) | 1 (8) | 1 (17) | 2 (29) | 0 | 5 (12) | 0 |
| Diarrhea,† n (%) | 0 | 2 (17) | 0 | 2 (14) | 2 (33) | 6 (15) | 0 |
| Cough, n (%) | 0 | 2 (17) | 2 (33) | 0 | 1 (17) | 5 (12) | 0 |
| Rash,‡ n (%) | 0 | 0 | 1 (17) | 1 (14) | 3 (50) | 5 (12) | 1 (7) |
| Sickle cell anemia with crisis,§ n (%) | 2 (20) | 2 (17) | 2 (33) | 2 (29) | 1 (17) | 9 (22) | 1 (7) |
Includes 1 patient who received placebo in GBT440-001 and transitioned to voxelotor 900 mg in the extension study.
Grade 1; all resolved with continued dosing.
Two patients had treatment-related rashes (preferred terms of rash and rash papular): 1 in the voxelotor 1000-mg group and 1 in the voxelotor 900-mg group. Other TEAEs of rash were not consistent with drug rashes.
Also referred to as VOC. Events occurred off-treatment (during posttreatment follow-up; n = 9) or after a dose hold/dose reduction (voxelotor 900-mg group; n = 1).
CPET showed that the change in oxygen consumption (VO2 max) from baseline to maximal exercise and ventilatory threshold were similar between patients receiving voxelotor or placebo for 90 days (Table 5). In addition, there was no evidence of voxelotor-related decrease in workload, increase in erythropoietin levels, or increase in heart rate at rest or during peak exercise (Table 5).
Peak exercise oxygen uptake, ventilatory threshold, and erythropoietin with voxelotor treatment
| Voxelotor/placebo . | Placebo . | 700 mg/d . | 900 mg/d . | Voxelotor-treated pooled . | P* for pooled voxelotor vs placebo . |
|---|---|---|---|---|---|
| N | 4 | 6 | 6 | 12 | |
| Peak exercise oxygen uptake VO2max, mL/min/kg | |||||
| Median baseline (range) | 23.3 (15.7-23.8) | 16.7 (8.2-19.6) | 21.2 (17.1-23.4) | 18.9 (8.2-23.4) | |
| Median change at day 91 (range) | −2.4 (−7.5 to 0.3) | 1.0 (−6.6 to 2.1) | −1.9 (−5.0 to −0.4) | −0.4 (−6.6 to 2.1) | NS |
| Ventilatory threshold oxygen uptake, mL/min/kg | |||||
| Median baseline (range) | 11.9 (11.7-12.7) | 9.4 (6.4-12.4) | 12.8 (11.5-16.3) | 12.0 (6.4-16.3) | |
| Median change at day 91 (range) | −1.4 (−5.5 to 2.3) | 1.6 (−0.1 to 4.7) | −1.4 (−1.8 to 1.4) | 0.6 (−1.8 to 4.7) | NS |
| Erythropoietin | |||||
| Median baseline mU/mL (IQR 25%:75%) | 112.2 (46.5, 183.0) | 91.2 (77.6, 125.0) | 113.5 (42.4, 150.0) | 104.4 (68.6, 137.5) | |
| % Median change to day 90 (IQR 25%:75%) | −43.5 (−79.3, 19.7) | 0.4 (−32.9, 27.6) | −7.2 (−11.5, −4.7) | −5.2 (−22.2, 14.8) | NS |
| Vital signs: heart rate at rest | |||||
| Median baseline, beats per minute (range) | 83 (75-93) | 81 (65-101) | 87 (71-99) | 81 (65-101) | |
| Median change to day 91 (range) | 17 (17) | 11 (6-38) | 3 (0-5) | 6 (0-38) | NC |
| Vital signs: heart rate at peak exercise | |||||
| Median baseline, beats per minute (range) | 162 (160-162) | 150 (116-173) | 171 (146-184) | 164 (116-184) | |
| Median change to day 91 (range) | −12 (−28 to 4) | −2 (−20 to 16) | 1 (−7 to 8) | −2 (−20 to 16) | NC |
| Voxelotor/placebo . | Placebo . | 700 mg/d . | 900 mg/d . | Voxelotor-treated pooled . | P* for pooled voxelotor vs placebo . |
|---|---|---|---|---|---|
| N | 4 | 6 | 6 | 12 | |
| Peak exercise oxygen uptake VO2max, mL/min/kg | |||||
| Median baseline (range) | 23.3 (15.7-23.8) | 16.7 (8.2-19.6) | 21.2 (17.1-23.4) | 18.9 (8.2-23.4) | |
| Median change at day 91 (range) | −2.4 (−7.5 to 0.3) | 1.0 (−6.6 to 2.1) | −1.9 (−5.0 to −0.4) | −0.4 (−6.6 to 2.1) | NS |
| Ventilatory threshold oxygen uptake, mL/min/kg | |||||
| Median baseline (range) | 11.9 (11.7-12.7) | 9.4 (6.4-12.4) | 12.8 (11.5-16.3) | 12.0 (6.4-16.3) | |
| Median change at day 91 (range) | −1.4 (−5.5 to 2.3) | 1.6 (−0.1 to 4.7) | −1.4 (−1.8 to 1.4) | 0.6 (−1.8 to 4.7) | NS |
| Erythropoietin | |||||
| Median baseline mU/mL (IQR 25%:75%) | 112.2 (46.5, 183.0) | 91.2 (77.6, 125.0) | 113.5 (42.4, 150.0) | 104.4 (68.6, 137.5) | |
| % Median change to day 90 (IQR 25%:75%) | −43.5 (−79.3, 19.7) | 0.4 (−32.9, 27.6) | −7.2 (−11.5, −4.7) | −5.2 (−22.2, 14.8) | NS |
| Vital signs: heart rate at rest | |||||
| Median baseline, beats per minute (range) | 83 (75-93) | 81 (65-101) | 87 (71-99) | 81 (65-101) | |
| Median change to day 91 (range) | 17 (17) | 11 (6-38) | 3 (0-5) | 6 (0-38) | NC |
| Vital signs: heart rate at peak exercise | |||||
| Median baseline, beats per minute (range) | 162 (160-162) | 150 (116-173) | 171 (146-184) | 164 (116-184) | |
| Median change to day 91 (range) | −12 (−28 to 4) | −2 (−20 to 16) | 1 (−7 to 8) | −2 (−20 to 16) | NC |
CPET (peak exercise and ventilatory threshold) not available in all patients. CPET data available for n = 3 (placebo), n = 5 (900 mg), n = 11 (voxelotor-treated pooled).
IQR, interquartile range; NC, not calculated; NS, not significant.
Two-sample Student t test.
PK and PD
PK and PD parameters of voxelotor derived from plasma and RBC concentration time profiles following multiple doses (500-1000 mg) for 28 days and ≥90 days are shown in Table 6. Following multiple daily dosing, the Cmax and AUC increased proportionally with dose. The Cmax and AUC0-24 at steady state at 1000 mg, the highest dose tested, were 483 µg/mL and 10 100 h × µg/mL, respectively. The accumulation was ∼3.5-fold compared with day 1 exposure, which is as expected from the single-dose PK.
Mean (plus or minus SD) PK and PD parameters in SCD patients
| . | Dosing duration 28 d* . | Dosing duration ≥90 d† . | ||||
|---|---|---|---|---|---|---|
| Voxelotor/placebo, mg/d | Placebo | 500 | 700 | 1000 | 700 | 900 |
| RBC PK parameters | ||||||
| Cmax, μg/mL | — | 271 ± 102 | 329 ± 129 | 483 ± 239 | 242 ± 99.7 | 336 ± 61.8 |
| AUC0-24, h × μg/mL | — | 5050 ± 1320 | 7560 ± 2210 | 10 100 ± 4790 | 5200 ± 2300 | 7250 ± 1430 |
| Plasma PK parameters | ||||||
| Cmax, μg/mL | — | 3.6 ± 0.8 | 5.2 ± 1.9 | 8.1 ± 2.8 | 4.9 ± 2.0 | 6.3 ± 1.9 |
| AUC0-24, h × μg/mL | — | 60 ± 12.9 | 95.7 ± 28.2 | 151 ± 49.7 | 83.1 ± 38.8 | 128 ± 27.1 |
| PD parameters (obtained from whole blood) | ||||||
| p50, mm Hg | 34.3 ± 3.3 | 30.9 ± 2.1 | 29.6 ± 2.6 | 28.0 ± 3.2 | ND | ND |
| p20, mm Hg | 18.4 ± 1.6 | 15.1 ± 1.8 | 13.9 ± 3.1 | 10.0 ± 3.2 | ND | ND |
| % Hb Mod, % | −1.4 ± 4.4 | 10.6 ± 7.2 | 14.7 ± 9.6 | 27.0 ± 11.6 | 12.7 ± 4.4‡ | 19.8 ± 4.5‡ |
| . | Dosing duration 28 d* . | Dosing duration ≥90 d† . | ||||
|---|---|---|---|---|---|---|
| Voxelotor/placebo, mg/d | Placebo | 500 | 700 | 1000 | 700 | 900 |
| RBC PK parameters | ||||||
| Cmax, μg/mL | — | 271 ± 102 | 329 ± 129 | 483 ± 239 | 242 ± 99.7 | 336 ± 61.8 |
| AUC0-24, h × μg/mL | — | 5050 ± 1320 | 7560 ± 2210 | 10 100 ± 4790 | 5200 ± 2300 | 7250 ± 1430 |
| Plasma PK parameters | ||||||
| Cmax, μg/mL | — | 3.6 ± 0.8 | 5.2 ± 1.9 | 8.1 ± 2.8 | 4.9 ± 2.0 | 6.3 ± 1.9 |
| AUC0-24, h × μg/mL | — | 60 ± 12.9 | 95.7 ± 28.2 | 151 ± 49.7 | 83.1 ± 38.8 | 128 ± 27.1 |
| PD parameters (obtained from whole blood) | ||||||
| p50, mm Hg | 34.3 ± 3.3 | 30.9 ± 2.1 | 29.6 ± 2.6 | 28.0 ± 3.2 | ND | ND |
| p20, mm Hg | 18.4 ± 1.6 | 15.1 ± 1.8 | 13.9 ± 3.1 | 10.0 ± 3.2 | ND | ND |
| % Hb Mod, % | −1.4 ± 4.4 | 10.6 ± 7.2 | 14.7 ± 9.6 | 27.0 ± 11.6 | 12.7 ± 4.4‡ | 19.8 ± 4.5‡ |
—, not applicable; AUC0-24 indicates area under the concentration-time curve from time 0 to 24 hours; Cmax, maximum blood or plasma concentration; % Hb Mod, percentage hemoglobin modification; ND, not determined; p20 and p50, partial pressure of O2 at which Hb is 20% or 50% saturated with O2; SD, standard deviation.
Parameters on day 28 (at 6 hours postdose for PD).
Parameters on day 90 (at 6 hours postdose for PD).
Percentage Hb occupancy derived from voxelotor RBC concentrations at 6 hours postdose on day 90.
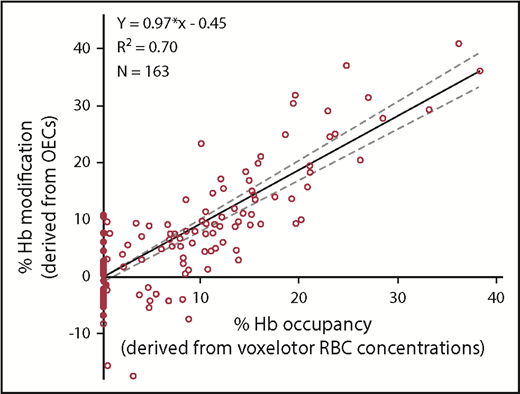
The PD effect of voxelotor was measured by changes in Hb-oxygen affinity. Voxelotor treatment resulted in a concentration-dependent decrease in p20 and p50, indicating an increase in Hb-oxygen affinity (Figure 5; Table 6). Patients receiving 900 mg and 1000 mg achieved a mean percentage Hb modification near the 20% to 30% predicted therapeutic window.11 The PD effect was highly correlated to PK (RBC voxelotor concentration, R2 = 0.70; Figure 6).
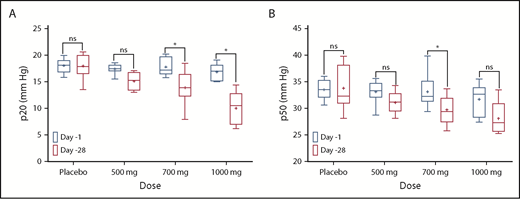
A summary of the p20 and p50 values observed in SCD patients after 28 days of dosing. (A) p20 values; (B) p50 values. *Sidak multiple comparisons tests were used to measure statistical significance. ns, not significant.
A summary of the p20 and p50 values observed in SCD patients after 28 days of dosing. (A) p20 values; (B) p50 values. *Sidak multiple comparisons tests were used to measure statistical significance. ns, not significant.
Linear correlation. Linear correlation observed between the percentage of Hb modification (derived from OECs) and the percentage of Hb occupancy (derived from voxelotor RBC concentrations) in time-matched samples from SCD patients.
Linear correlation. Linear correlation observed between the percentage of Hb modification (derived from OECs) and the percentage of Hb occupancy (derived from voxelotor RBC concentrations) in time-matched samples from SCD patients.
Discussion
These results show that multiple doses of voxelotor in SCD patients from 500 to 1000 mg daily resulted in dose-dependent drug exposure and PD effects, with a dose-dependent increase in Hb-oxygen affinity.
Patients treated with voxelotor for ≥90 days showed a 1.0 g/dL median increase in Hb and a substantial and durable reduction in hemolysis and peripheral blood sickled red cells, with ∼40% reduction in unconjugated bilirubin, 20% reduction in reticulocytes, 30% reduction in dense RBCs, and >70% reduction in sickled red cells. The median Hb concentration increased rapidly after initiating dosing with voxelotor treatment, starting as early as day 4. These improvements were sustained with long-term dosing for ≥90 days and almost half of the patients achieved an improvement in Hb concentration of ≥1 g/dL. Fluctuations in Hb levels over the first 8 weeks may reflect the time needed to achieve a new steady state. Although the number of patients receiving concurrent hydroxyurea in this study was small, improvements were observed regardless of concurrent hydroxyurea use, and the benefit of voxelotor on hematologic parameters appears to be additive to hydroxyurea.
Overall, these data in a limited number of patients indicate that voxelotor treatment leads to a rapid, substantial, and durable reduction in hemolysis. These findings are consistent with an inhibition of intracellular erythrocyte HbS polymerization as a potential mechanism responsible for the reduction in hemolysis in SCD.
The improvement in hemolytic anemia with voxelotor treatment is promising because chronic hemolytic anemia has increasingly become recognized as a critical driver of SCD pathophysiology via both chronic anemia and a systemic hemolysis-related vasculopathy. Chronic hemolytic anemia is an independent and powerful predictor of chronic organ damage, including stroke, silent infarcts, renal failure, and pulmonary hypertension, as well as early mortality in SCD.20 Notably, low Hb is a powerful predictor of stroke; for every 1 g/dL reduction in Hb in the Cooperative Study, the risk of ischemic stroke increased by nearly twofold.21 By improving hemolytic anemia, voxelotor has the potential to improve long-term clinical outcomes and potentially survival.
This current study also provides preliminary evidence that voxelotor is generally safe and well tolerated. In these patients with SCD treated with voxelotor for 28 days to ≥90 days, all treatment-related AEs were grade 1 or 2 and did not require treatment discontinuation (except for 1 patient with a rash). This study was not sufficiently powered or long enough in duration to assess the effect of voxelotor on the incidence of VOC. However, there were no sickle cell crises during treatment; all VOC events in voxelotor-treated patients occurred in the 28-day follow-up period after study drug was stopped or after a dose hold. There were no treatment-related grade 3 events, no treatment-related SAEs, and no events indicative of systemic drug hypersensitivity reactions. There were no dose- or exposure-related safety signals or concerns that would limit further exploration of 900 mg or higher doses. Overall, voxelotor was well tolerated for ≥90 days of treatment, with a low incidence of treatment-related TEAEs (headache, diarrhea, rash), and most TEAEs were consistent with SCD. However, safety data in more patients beyond 90 days are needed to assess the long-term safety profile of voxelotor.
Individuals with α thalassemia have less hemolysis and higher Hb, which is associated with an increased risk for VOC due to increased blood viscosity of sickled RBCs.22 Furthermore, in clinical studies of senicapoc, an experimental Gardos channel inhibitor targeting an improvement in RBC hydration in SCD patients, an Hb increase led to an increase in VOC, possibly through increased blood viscosity.23 Voxelotor’s mechanism of action differs from senicapoc. Voxelotor acts upstream by inhibiting HbS polymerization, which is expected to improve RBC function and, therefore, not lead to an increase in viscosity. In contrast, senicapoc acts downstream of HbS polymerization by inhibiting the Gardos channel to prevent dehydration of already damaged sickled RBCs. Data from patients treated with senicapoc show that no significant reduction in intracellular Hb concentration occurred, and thus it is unlikely to affect HbS polymerization.24
To fully evaluate the potential benefit/risk profile of voxelotor, it was important to assess whether the mechanism of action of increased oxygen affinity would create safety concerns. In this current study, there were no clinical signs of tissue hypoxia in patients treated with voxelotor (eg, electrocardiogram changes, resting tachycardia) and no increase in erythropoietin levels compared with placebo. Furthermore, CPET was performed with quantitation of exercise capacity and oxygen consumption during maximal exercise stress. These results showed no evidence of compromised tissue oxygen delivery in patients treated with voxelotor up to 1000 mg for 28 days and up to 900 mg for 90 days. In addition, reticulocytes were significantly reduced with voxelotor dosing, which is consistent with improved RBC survival, improved anemia, and improved oxygen delivery to tissues. Reassuringly, regarding oxygen delivery, voxelotor treatment increased Hb-oxygen affinity modestly, with p50s achieved in the normal range. This study therefore demonstrates that Hb-oxygen affinity modulation to a therapeutic range may be safely achieved.
As expected, the PK and PD were highly correlated and the PD effects demonstrated that voxelotor doses ≥900 mg are likely to achieve percentage Hb modification within the expected therapeutic range of 20% to 30%. Voxelotor PK in whole blood and plasma showed a remarkable partitioning into the RBC compartment (∼99% of the voxelotor in blood is within the RBCs), which provides evidence of specificity of binding to Hb and limits the potential for off-target binding and resulting safety concerns.
In conclusion, this study suggests a favorable benefit-risk profile of voxelotor for the treatment of SCD. Voxelotor was well tolerated at doses up to 1000 mg for 28 days and 900 mg up to 6 months. The linear, dose-proportional PK and long half-life of voxelotor support once-daily dosing. Voxelotor demonstrated rapid, sustained, and clinically meaningful improvement in anemia, sickling, and clinical laboratory markers of hemolysis, supporting the potential for voxelotor to serve as a disease-modifying therapy for SCD, which is being further investigated in patients with SCD in the ongoing phase 3 HOPE study (NCT03036813).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the patients, families, caregivers, research nurses, study coordinators, and support staff; Theresa Thuener and Carla Washington (Global Blood Therapeutics, Inc, South San Francisco, CA) and Gabriella Passacquale (IQVIA Drug Research Unit at Guy’s Hospital, London, United Kingdom); and clinical subinvestigators who contributed to this study. The latter stages of the study were carried out in the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London.
This work was supported by Global Blood Therapeutics, Inc (South San Francisco, CA). Medical writing support was provided by Valerie Hilliard (ApotheCom, San Francisco, CA), funded by Global Blood Therapeutics, Inc.
Authorship
Contribution: J.H., C.J.H., P.T., D.M.L., J.P., and M.A. helped design the study for patients with SCD, referred patients with SCD, and reviewed and provided input into the manuscript writing; T.M. was the principal investigator, responsible for study conduct under International Conference on Harmonisation Good Clinical Practice guidelines, and reviewed and provided input into this publication; D.D.G. was the independent safety physician for the safety committee, and reviewed and provided input into the manuscript writing; K.D. performed experiments to measure sickled red cells, discussed and interpreted data, and contributed to manuscript writing; A.H. helped design and plan study, oversaw PK sample analysis, performed PK/PD data analysis, suggested dose-escalation levels between cohorts, and contributed to manuscript writing; M.P. designed and implemented the in vitro model for Hb modification, trained the staff for OEC determinations, troubleshooted and analyzed PD data, prepared figures, discussed and interpreted data, and contributed to manuscript writing; V.S. performed experiments to develop and implement the in vitro Hb modification model, trained the staff at the clinical site for OEC determinations, and troubleshooted and analyzed PD data; S.D. was the sponsor’s biostatistician, reviewed and interpreted data, and contributed to manuscript writing; N.L. provided input to the study design, reviewed study data, and contributed to manuscript writing; M.T. provided input to the study design, reviewed and interpreted data, and contributed to manuscript writing; and J.L.-G. was the sponsor’s principal investigator, was a member of the safety committee, designed the study, and reviewed and provided input into manuscript writing.
Conflict-of-interest disclosure: J.H. is a consultant/advisor for Novartis; Global Blood Therapeutics, Inc; Bluebird Bio; and Terumo BCT. P.T. is a consultant/advisor for Apopharma; Bluebird Bio; Global Blood Therapeutics, Inc; Novartis; and Terumo. D.M.L. is a consultant/advisor for Agios, Cerus, and Novartis. J.P. received research funding from Novartis; was a consultant/advisor for Novartis, Shire, and Celgene; and received honoraria from Novartis and Celgene. M.A. is a consultant for, and receives honoraria from, Novartis. T.M. is an employee of, and owns shares of, IQVIA. D.D.G. is an independent consultant. K.D., A.H., M.P., V.S., S.D., M.T., and J.L.-G. are employees of, and have equity ownership in, Global Blood Therapeutics, Inc. N.L. is a consultant clinical site liaison for Global Blood Therapeutics, Inc. C.J.H. declares no competing financial interests.
Correspondence: Jo Howard, Guy’s Hospital, Great Maze Pond, London SE1 9RT, United Kingdom; e-mail: jo.howard@gstt.nhs.uk.