TO THE EDITOR:
Pediatric acute lymphoblastic leukemia (ALL) is characterized by recurrent preleukemic chromosomal translocations that emerge frequently in utero.1 The most common translocation t(12;21) occurs in 25% of B-cell precursor ALL and results in the formation of the chimeric transcription factor ETV6-RUNX1. Secondary oncogenic hits acquired postnatally are necessary to develop overt leukemia.2,3 Therefore, the number of newborns harboring a preleukemic translocation is expected to equal or exceed the incidence of the corresponding leukemia.
In 2002, Mel Greaves’ group analyzed the frequency of ETV6-RUNX1 translocations in human newborns by independent reverse transcriptase (RT)–polymerase chain reaction (PCR) and quantitative PCR (qPCR) screens confirmed by multicolor fluorescence in situ hybridization. They observed that ≈1% of cord bloods from newborns (n = 6 of 567) contained ETV6-RUNX1+ cells.4 The translocation seemed to occur frequently during normal fetal development and to be 100-fold more common than the corresponding leukemia (1 in 10 000 children). This indicated a very low oncogenic potential of the ETV6-RUNX1 transcription factor and suggested that the usefulness of preventive screenings of ETV6-RUNX1–carrying newborns is limited because 99% would never develop the disease.
Since this initial investigation, several studies have carefully analyzed the incidence of the translocation in newborns, children, and adults using fresh or frozen cord blood or peripheral blood (summarized in supplemental Table 1, available on the Blood Web site).4-14 Remarkably, the suggested incidence ranged from 0.01% (equaling the corresponding leukemia rate)7-9 to ≈8% (exceeding it by a factor of 800).5 Because the investigated cohorts and specimens were similar, the variation was assumed to have technical explanations. Also, the frequency of preleukemic cells in healthy blood or cord blood was controversially discussed, ranging from <10−5 to 10−3.
All of the previous studies used RNA and RT-PCR for prevalence determination. A drawback of RT-PCR is the possible generation of false-positive results caused by contamination. These false-positive results cannot be distinguished from real ones, as for a given translocation the identical amplification products are created in different samples.9 In addition, low levels of positivity can be due to RNA instability, different processing of RNA, detection methods, and, possibly, low expression of ETV6-RUNX1 transcripts in cord blood.
To analyze the frequency of ETV6-RUNX1+ preleukemic cells in newborns, we therefore developed a DNA-based method termed genomic inverse PCR for exploration of ligated breakpoints (GIPFEL)15 because DNA, in contrast to RNA, is very stable. By DNA-based approaches, patient-specific amplification products of the translocation site that differ from one another in size and sequence are obtained and contaminations can be easily identified (Figure 1A). The advantages of DNA-based screening methods have led to an increased use in clinical applications (eg, DNA-based monitoring of minimal residual disease in BCR-ABL+ leukemia16 ).
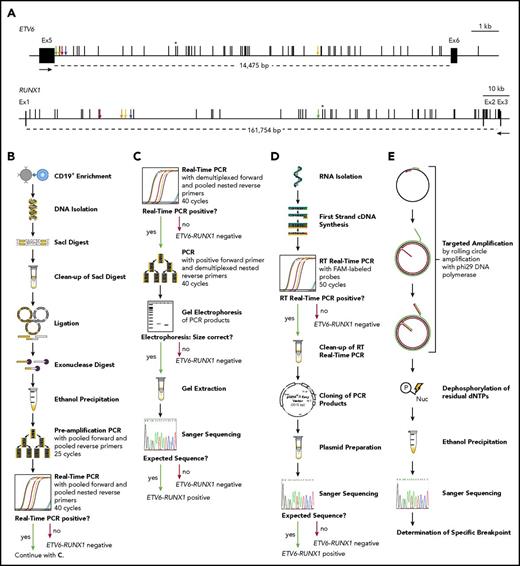
The incidence of ETV6-RUNX1 fusions in healthy newborns can be determined by DNA-based GIPFEL screening. (A) Only DNA- and not RNA-based ETV6-RUNX1 detection provides individual-specific breakpoints. The breakpoint cluster regions (BCRs; horizontal lines, exact regions are marked by dotted lines, sizes are given in base pairs) of ETV6 (top panel, intron 5) and RUNX1 (bottom panel, intron 1 and 2) are presented. Black boxes indicate exons and vertical black lines on top of the BCRs indicate reported patient-specific breakpoints.21-25 Asterisks mark the breakpoint present in the REH cell line. The breakpoints identified in the present study are indicated by orange (N424), blue (N726), red (N817), green (N823), and yellow (N890) arrows. Black arrows represent primers that are usually used for ETV6-RUNX1 screening by RT-PCR. They generate 1 of 2 PCR products for every possible breakpoint within the BCRs: exon 5 of ETV6 fused to either exon 2 or exon 3 of RUNX1. Only DNA-based techniques can differentiate between breakpoints that are specific for each individual patient and localized in intronic regions. (B-E) The workflow of cord blood screening using a modified GIPFEL technique15 is presented. (B) CD19+ B cells were enriched from newborn cord blood. DNA was isolated, fragmented using SacI, and purified. The DNA was ligated to achieve circularization and residual linear DNA was digested. DNA circles were purified and PCR (preamplification) and real-time PCR were used to detect the ligation joints produced by circularization. The protocol was carried out with each sample. (C) Optional continuation protocol that was carried out if the real-time PCR in panel B generated a putative positive result. Then a new real-time PCR was carried out with demultiplexed forward primers. If 1 of the forward primers produced a positive result, the PCR was repeated with this forward primer and demultiplexed reverse primers. The products were then analyzed on an agarose gel and Sanger sequenced. In case of negative results, no further validation steps were done. (D) Workflow of the transcriptional validation. RNA was reverse transcribed and subjected to real-time PCR. In case of a putative positive result, the PCR product was purified, cloned, and subjected to Sanger sequencing. (E) Further development of the original GIPFEL technique allowed the identification of patient-specific breakpoints with base-pair resolution. To this end, circularized DNA was amplified using specific primers that hybridized to the known ligation joint region and faced toward the unknown breakpoint. Amplification was carried out with Phi29 DNA polymerase, leading to linear products. With Phi29, amplification occurs at a constant temperature and new primers can constantly bind to the original and the amplified DNA, leading to further amplification. The amplified DNA was subsequently purified and Sanger sequenced. cDNA, complementary DNA; dNTP, deoxynucleotide triphosphate; Ex, exon; FAM, fluorescein amidite; Nuc, deoxynucleoside diphospate; P, phosphate.
The incidence of ETV6-RUNX1 fusions in healthy newborns can be determined by DNA-based GIPFEL screening. (A) Only DNA- and not RNA-based ETV6-RUNX1 detection provides individual-specific breakpoints. The breakpoint cluster regions (BCRs; horizontal lines, exact regions are marked by dotted lines, sizes are given in base pairs) of ETV6 (top panel, intron 5) and RUNX1 (bottom panel, intron 1 and 2) are presented. Black boxes indicate exons and vertical black lines on top of the BCRs indicate reported patient-specific breakpoints.21-25 Asterisks mark the breakpoint present in the REH cell line. The breakpoints identified in the present study are indicated by orange (N424), blue (N726), red (N817), green (N823), and yellow (N890) arrows. Black arrows represent primers that are usually used for ETV6-RUNX1 screening by RT-PCR. They generate 1 of 2 PCR products for every possible breakpoint within the BCRs: exon 5 of ETV6 fused to either exon 2 or exon 3 of RUNX1. Only DNA-based techniques can differentiate between breakpoints that are specific for each individual patient and localized in intronic regions. (B-E) The workflow of cord blood screening using a modified GIPFEL technique15 is presented. (B) CD19+ B cells were enriched from newborn cord blood. DNA was isolated, fragmented using SacI, and purified. The DNA was ligated to achieve circularization and residual linear DNA was digested. DNA circles were purified and PCR (preamplification) and real-time PCR were used to detect the ligation joints produced by circularization. The protocol was carried out with each sample. (C) Optional continuation protocol that was carried out if the real-time PCR in panel B generated a putative positive result. Then a new real-time PCR was carried out with demultiplexed forward primers. If 1 of the forward primers produced a positive result, the PCR was repeated with this forward primer and demultiplexed reverse primers. The products were then analyzed on an agarose gel and Sanger sequenced. In case of negative results, no further validation steps were done. (D) Workflow of the transcriptional validation. RNA was reverse transcribed and subjected to real-time PCR. In case of a putative positive result, the PCR product was purified, cloned, and subjected to Sanger sequencing. (E) Further development of the original GIPFEL technique allowed the identification of patient-specific breakpoints with base-pair resolution. To this end, circularized DNA was amplified using specific primers that hybridized to the known ligation joint region and faced toward the unknown breakpoint. Amplification was carried out with Phi29 DNA polymerase, leading to linear products. With Phi29, amplification occurs at a constant temperature and new primers can constantly bind to the original and the amplified DNA, leading to further amplification. The amplified DNA was subsequently purified and Sanger sequenced. cDNA, complementary DNA; dNTP, deoxynucleotide triphosphate; Ex, exon; FAM, fluorescein amidite; Nuc, deoxynucleoside diphospate; P, phosphate.
GIPFEL is capable of detecting gene fusions without prior knowledge of the exact breakpoint by exploiting the presence of genomic fragments that join material from known regions of 2 different chromosomes. These fragments can be digested and circularized by ligation, creating a junction across the restriction site whose sequence can be predicted from published genome data and used for the design of real-time PCRs.
For ETV6-RUNX1 fusion detection, genomic DNA is digested by SacI and circularized by intramolecular ligation (Figure 1B-E; supplemental Methods; supplemental Figure 1). A multiplexed, semi-nested real-time PCR is performed to quantify translocation-specific ligation products. Demultiplexed qPCR, gel electrophoresis, and sequencing are used for validation of the ligation product and to narrow down the breakpoint region. GIPFEL can detect 1 translocation-carrying cell within 10 000 normal cells (sensitivity ≈10−4) and is highly specific. The likelihood that GIPFEL produces false-positive signals is extremely low (<<10−10; supplemental Figure 2) and has so far never been observed (eg, >180 negative controls for ETV6-RUNX1).
Here, we applied GIPFEL to a retrospective screening of 1000 newborns. The study was approved by the Danish Data Protection Agency and the Danish Scientific Ethics Committee. Written informed consent was obtained. After B-cell enrichment, genomic DNA was prepared from cryopreserved cord bloods and GIPFEL was carried out. In 50 of 1000 samples, sequencing of the ligation joints validated a signal compatible with ETV6-RUNX1 translocation (Table 1; supplemental Figures 3 and 4). These data suggest that ≈5% of newborns harbor the ETV6-RUNX1 fusion at levels detectable by GIPFEL.
Fifty of 1000 cord bloods from healthy newborns were ETV6-RUNX1 translocation-positive in GIPFEL screening
| Healthy newborn . | Multiplexed primer group . | Forward primer . | RUNX1 intron . | Reverse primer . | ETV6 intron . | Estimated frequency . |
|---|---|---|---|---|---|---|
| N005 | 3 | RUNX1-S6f | 1 | ETV6-S1r-n | 5 | 1 × 10−4 |
| N059 | 4 | RUNX1-S13f | 1 | ETV6-S2r-n | 5 | 6 × 10−3 |
| N099 | 3 | RUNX1-S2f | 2 | ETV6-S2r-n | 5 | 2 × 10−4 |
| N260 | 2 | RUNX1-S14f | 1 | ETV6-S2r-n | 5 | 1.5 × 10−4 |
| N285 | 2 | RUNX1-S11f | 1 | ETV6-S1r-n | 5 | 8 × 10−4 |
| N286 | 3 | RUNX1-S8f | 1 | ETV6-S1r-n | 5 | 1 × 10−4 |
| N382 | 2 | RUNX1-S11f | 1 | ETV6-S3r-n | 5/6 | 1 × 10−3 |
| N424* | 1 | RUNX1-S23f | 1 | ETV6-S1r-n | 5 | 5 × 10−4 |
| N439 | 1 | RUNX1-S23f | 1 | ETV6-S1r-n | 5 | 4 × 10−4 |
| N440 | 3 | RUNX1-S18f | 1 | ETV6-S2r-n | 5 | 1 × 10−3 |
| N441 | 1 | RUNX1-S22f | 1 | ETV6-S2r-n | 5 | 1 × 10−4 |
| N447 | 1 | RUNX1-S28f | 1 | ETV6-S3r-n | 5/6 | 2 × 10−3 |
| N463 | 2 | RUNX1-S10f | 1 | ETV6-S2r-n | 5 | 3 × 10−4 |
| N472 | 4 | RUNX1-S21f | 1 | ETV6-S3r-n | 5/6 | 1 × 10−4 |
| N479 | 4 | RUNX1-S13f | 1 | ETV6-S2r-n | 5 | 1 × 10−3 |
| N493 | 3 | RUNX1-S18f | 1 | ETV6-S2r-n | 5 | 1 × 10−2 |
| N496 | 4 | RUNX1-S5f | 1 | ETV6-S1r-n | 5 | 1.5 × 10−3 |
| N505 | 2 | RUNX1-S11f | 1 | ETV6-S3r-n | 5/6 | 4 × 10−3 |
| N505 | 4 | RUNX1-S13f | 1 | ETV6-S1r-n | 5 | 7 × 10−4 |
| N506 | 1 | RUNX1-S28f | 1 | ETV6-S1r-n | 5 | 5 × 10−3 |
| N521 | 2 | RUNX1-S4f | 1 | ETV6-S2r-n | 5 | 4 × 10−2 |
| N522 | 2 | RUNX1-S10f | 1 | ETV6-S2r-n | 5 | 1 × 10−2 |
| N527 | 4 | RUNX1-S21f | 1 | ETV6-S1r-n | 5 | 4 × 10−3 |
| N531 | 3 | RUNX1-S29f | 1 | ETV6-S2r-n | 5 | 5 × 10−3 |
| N531 | 4 | RUNX1-S13f | 1 | ETV6-S2r-n | 5 | 1.5 × 10−3 |
| N548 | 4 | RUNX1-S21f | 1 | ETV6-S2r-n | 5 | 9 × 10−5 |
| N563 | 2 | RUNX1-S11f | 1 | ETV6-S2r-n | 5 | 8 × 10−4 |
| N578 | 2 | RUNX1-S24f | 1 | ETV6-S3r-n | 5/6 | 8 × 10−5 |
| N590 | 1 | RUNX1-S26f | 1 | ETV6-S1r-n | 5 | 4 × 10−4 |
| N599 | 2 | RUNX1-S10f | 1 | ETV6-S3r-n | 5/6 | 5 × 10−3 |
| N619 | 4 | RUNX1-S5f | 1 | ETV6-S3r-n | 5/6 | 6 × 10−3 |
| N622 | 1 | RUNX1-S26f | 1 | ETV6-S2r-n | 5 | 3 × 10−4 |
| N630 | 1 | RUNX1-S12f | 1 | ETV6-S2r-n | 5 | 1 × 10−2 |
| N651 | 2 | RUNX1-S4f | 1 | ETV6-S2r-n | 5 | 1 × 10−4 |
| N670 | 1 | RUNX1-S12f | 1 | ETV6-S3r-n | 5/6 | 1 × 10−2 |
| N673 | 3 | RUNX1-S8f | 1 | ETV6-S2r-n | 5 | 1 × 10−2 |
| N674 | 1 | RUNX1-S28f | 1 | ETV6-S3r-n | 5/6 | 9 × 10−3 |
| N726* | 1 | RUNX1-S22f | 1 | ETV6-S1r-n | 5 | 4 × 10−5 |
| N729 | 1 | RUNX1-S23f | 1 | ETV6-S3r-n | 5/6 | 5 × 10−5 |
| N731 | 2 | RUNX1-S4f | 1 | ETV6-S3r-n | 5/6 | 1.5 × 10−5 |
| N732 | 2 | RUNX1-S10f | 1 | ETV6-S2r-n | 5 | 1 × 10−4 |
| N770 | 4 | RUNX1-S13f | 1 | ETV6-S3r-n | 5/6 | 4 × 10−4 |
| N775 | 2 | RUNX1-S14f | 1 | ETV6-S2r-n | 5 | 1 × 10−3 |
| N784 | 1 | RUNX1-S28f | 1 | ETV6-S2r-n | 5 | 3 × 10−4 |
| N791 | 3 | RUNX1-S6f | 1 | ETV6-S2r-n | 5 | 1 × 10−4 |
| N795 | 2 | RUNX1-S24f | 1 | ETV6-S3r-n | 5/6 | 1 × 10−4 |
| N817* | 4 | RUNX1-S25f | 1 | ETV6-S1r-n | 5 | 2 × 10−3 |
| N823* | 4 | RUNX1-S13f | 1 | ETV6-S1r-n | 5 | 1 × 10−3 |
| N890* | 1 | RUNX1-S23f | 1 | ETV6-S3r-n | 5/6 | 1 × 10−3 |
| N908 | 1 | RUNX1-S15f | 1 | ETV6-S3r-n | 5/6 | 6 × 10−4 |
| N912 | 2 | RUNX1-S24f | 1 | ETV6-S2r-n | 5 | 3 × 10−3 |
| N926 | 4 | RUNX1-S5f | 1 | ETV6-S3r-n | 5/6 | 3 × 10−4 |
| Healthy newborn . | Multiplexed primer group . | Forward primer . | RUNX1 intron . | Reverse primer . | ETV6 intron . | Estimated frequency . |
|---|---|---|---|---|---|---|
| N005 | 3 | RUNX1-S6f | 1 | ETV6-S1r-n | 5 | 1 × 10−4 |
| N059 | 4 | RUNX1-S13f | 1 | ETV6-S2r-n | 5 | 6 × 10−3 |
| N099 | 3 | RUNX1-S2f | 2 | ETV6-S2r-n | 5 | 2 × 10−4 |
| N260 | 2 | RUNX1-S14f | 1 | ETV6-S2r-n | 5 | 1.5 × 10−4 |
| N285 | 2 | RUNX1-S11f | 1 | ETV6-S1r-n | 5 | 8 × 10−4 |
| N286 | 3 | RUNX1-S8f | 1 | ETV6-S1r-n | 5 | 1 × 10−4 |
| N382 | 2 | RUNX1-S11f | 1 | ETV6-S3r-n | 5/6 | 1 × 10−3 |
| N424* | 1 | RUNX1-S23f | 1 | ETV6-S1r-n | 5 | 5 × 10−4 |
| N439 | 1 | RUNX1-S23f | 1 | ETV6-S1r-n | 5 | 4 × 10−4 |
| N440 | 3 | RUNX1-S18f | 1 | ETV6-S2r-n | 5 | 1 × 10−3 |
| N441 | 1 | RUNX1-S22f | 1 | ETV6-S2r-n | 5 | 1 × 10−4 |
| N447 | 1 | RUNX1-S28f | 1 | ETV6-S3r-n | 5/6 | 2 × 10−3 |
| N463 | 2 | RUNX1-S10f | 1 | ETV6-S2r-n | 5 | 3 × 10−4 |
| N472 | 4 | RUNX1-S21f | 1 | ETV6-S3r-n | 5/6 | 1 × 10−4 |
| N479 | 4 | RUNX1-S13f | 1 | ETV6-S2r-n | 5 | 1 × 10−3 |
| N493 | 3 | RUNX1-S18f | 1 | ETV6-S2r-n | 5 | 1 × 10−2 |
| N496 | 4 | RUNX1-S5f | 1 | ETV6-S1r-n | 5 | 1.5 × 10−3 |
| N505 | 2 | RUNX1-S11f | 1 | ETV6-S3r-n | 5/6 | 4 × 10−3 |
| N505 | 4 | RUNX1-S13f | 1 | ETV6-S1r-n | 5 | 7 × 10−4 |
| N506 | 1 | RUNX1-S28f | 1 | ETV6-S1r-n | 5 | 5 × 10−3 |
| N521 | 2 | RUNX1-S4f | 1 | ETV6-S2r-n | 5 | 4 × 10−2 |
| N522 | 2 | RUNX1-S10f | 1 | ETV6-S2r-n | 5 | 1 × 10−2 |
| N527 | 4 | RUNX1-S21f | 1 | ETV6-S1r-n | 5 | 4 × 10−3 |
| N531 | 3 | RUNX1-S29f | 1 | ETV6-S2r-n | 5 | 5 × 10−3 |
| N531 | 4 | RUNX1-S13f | 1 | ETV6-S2r-n | 5 | 1.5 × 10−3 |
| N548 | 4 | RUNX1-S21f | 1 | ETV6-S2r-n | 5 | 9 × 10−5 |
| N563 | 2 | RUNX1-S11f | 1 | ETV6-S2r-n | 5 | 8 × 10−4 |
| N578 | 2 | RUNX1-S24f | 1 | ETV6-S3r-n | 5/6 | 8 × 10−5 |
| N590 | 1 | RUNX1-S26f | 1 | ETV6-S1r-n | 5 | 4 × 10−4 |
| N599 | 2 | RUNX1-S10f | 1 | ETV6-S3r-n | 5/6 | 5 × 10−3 |
| N619 | 4 | RUNX1-S5f | 1 | ETV6-S3r-n | 5/6 | 6 × 10−3 |
| N622 | 1 | RUNX1-S26f | 1 | ETV6-S2r-n | 5 | 3 × 10−4 |
| N630 | 1 | RUNX1-S12f | 1 | ETV6-S2r-n | 5 | 1 × 10−2 |
| N651 | 2 | RUNX1-S4f | 1 | ETV6-S2r-n | 5 | 1 × 10−4 |
| N670 | 1 | RUNX1-S12f | 1 | ETV6-S3r-n | 5/6 | 1 × 10−2 |
| N673 | 3 | RUNX1-S8f | 1 | ETV6-S2r-n | 5 | 1 × 10−2 |
| N674 | 1 | RUNX1-S28f | 1 | ETV6-S3r-n | 5/6 | 9 × 10−3 |
| N726* | 1 | RUNX1-S22f | 1 | ETV6-S1r-n | 5 | 4 × 10−5 |
| N729 | 1 | RUNX1-S23f | 1 | ETV6-S3r-n | 5/6 | 5 × 10−5 |
| N731 | 2 | RUNX1-S4f | 1 | ETV6-S3r-n | 5/6 | 1.5 × 10−5 |
| N732 | 2 | RUNX1-S10f | 1 | ETV6-S2r-n | 5 | 1 × 10−4 |
| N770 | 4 | RUNX1-S13f | 1 | ETV6-S3r-n | 5/6 | 4 × 10−4 |
| N775 | 2 | RUNX1-S14f | 1 | ETV6-S2r-n | 5 | 1 × 10−3 |
| N784 | 1 | RUNX1-S28f | 1 | ETV6-S2r-n | 5 | 3 × 10−4 |
| N791 | 3 | RUNX1-S6f | 1 | ETV6-S2r-n | 5 | 1 × 10−4 |
| N795 | 2 | RUNX1-S24f | 1 | ETV6-S3r-n | 5/6 | 1 × 10−4 |
| N817* | 4 | RUNX1-S25f | 1 | ETV6-S1r-n | 5 | 2 × 10−3 |
| N823* | 4 | RUNX1-S13f | 1 | ETV6-S1r-n | 5 | 1 × 10−3 |
| N890* | 1 | RUNX1-S23f | 1 | ETV6-S3r-n | 5/6 | 1 × 10−3 |
| N908 | 1 | RUNX1-S15f | 1 | ETV6-S3r-n | 5/6 | 6 × 10−4 |
| N912 | 2 | RUNX1-S24f | 1 | ETV6-S2r-n | 5 | 3 × 10−3 |
| N926 | 4 | RUNX1-S5f | 1 | ETV6-S3r-n | 5/6 | 3 × 10−4 |
Frequencies refer to B-cell population. Results for 2 newborns with concurrent fusions appear in bold.
In these cases, the translocation was confirmed by Sanger sequencing of the breakpoint.
GIPFEL screening also revealed 2 newborns (N505, N531) whose cord blood harbored 2 different ETV6-RUNX1 fusions each (Table 1; supplemental Figure 5). The estimated frequencies of the 2 fusions differed from one another (N505: 4 × 10−3 and 7 × 10−4, N531: 5 × 10−3 and 1.5 × 10−3), indicating 2 coexisting or overlapping clones.
To validate our screening results, we tested fusion transcript expression in GIPFEL+ and GIPFEL− cord bloods by qPCR (Figure 1D; supplemental Figure 6; supplemental Table 2). Due to limited sample availability, we could only investigate 2 positive (N005 and N260) and 50 negative cord bloods. In the REH cell line, ETV6-RUNX1 expression was roughly in the same range as the ABL1 control gene. In GIPFEL+ cord blood samples, expression of ETV6-RUNX1 was detected at a lower level (10−4) in accord with the lower number of translocation-carrying cells (10−4) compared with the cell line. We confirmed the fusions on the RNA level by Sanger sequencing. None of the GIPFEL− cord bloods showed transcription of ETV6-RUNX1. In addition, 9 translocation-negative cell lines were both GIPFEL− and qPCR− (supplemental Table 3).
To finally confirm positive GIPFEL results, we sequenced individual-specific breakpoints with base-pair resolution on the DNA level using targeted amplification of translocation-carrying circles and Sanger sequencing (Figure 1E). Due to limited sample availability, ETV6-RUNX1 breakpoints of 5 cord bloods (N424, N726, N817, N832, N890) have been identified so far (Figure 1A; supplemental Figure 7). The breakpoints were specific for each proband and never reported before.
Because of anonymized sample processing, no tracking of leukemia cases within the analyzed cohort could be carried out. However, the determined frequencies of ETV6-RUNX1 translocations (≈5% GIPFEL screen or ≈0.5% if taking into account only GIPFEL screen results validated by breakpoint sequencing) are both far above the leukemia frequency of ≈0.01%. The incidence of ETV6-RUNX1 fusions may even exceed ≈5% because complex rearrangements, rearrangements outside of the known breakpoint regions, and not-yet-expanded cell clones (<10−4) evade detection by GIPFEL.15 Thus, translocation-carrying clones (and potentially >1) are likely present in a high number of healthy individuals who will never develop leukemia. Similarly, other leukemia- or lymphoma-associated translocations have been detected in peripheral blood of healthy individuals (reviewed in Janz et al17 ). Illegitimate genetic recombination seems to occur frequently in hematopoietic precursors. For preleukemic clones to arise, the fusion has to occur in an early precursor with self-renewal capacity and must produce a functional oncoprotein that promotes clonal expansion.
In the case of ETV6-RUNX1, the leukemia-inducing potential of the fusion seems to be very low. This is in accordance with (1) the low concordance rate of ≈10% in identical twins,18 (2) the long postnatal latency phase (≈2-14 years),2 (3) the presence of recurrent secondary leukemia inducing genetic lesions (ETV6 deletions in 70%, extra copies of RUNX1 in 23%, or extra der(21)t(12;21) in 10% of cases),19 and (4) evidence from transgenic animal studies.20
These results strengthen the importance of environmentally or spontaneously caused secondary hits in ETV6-RUNX1+ ALL. Future studies correlating GIPFEL screens with genetic and epidemiological data will provide closer insight into the pathogenesis of ETV6-RUNX1+ leukemia.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Sabine Hornhardt (Federal Office for Radiation Protection, Oberschleissheim, Germany) for advice and support. The authors thank Mel Greaves and Tony Ford (The Institute of Cancer Research, London, United Kingdom) and Jan Trka (Childhood Leukaemia Investigation Prague, Prague, Czech Republic) for valuable comments. The authors thank their colleague Cyrill Schipp for valuable support and Daniel Scholtyssik and Bianca Killing for excellent technical assistance.
This work was supported by the German Federal Office for Radiation Protection (grant 36 14 S 30034), by an intramural grant (2016-70) of the Research Commission of the Medical Faculty of Heinrich Heine University Düsseldorf, by the Katharina-Hardt-Stiftung, by the Danish Cancer Society, and by the Danish Childhood Cancer Foundation.
Authorship
Contribution: D.S. performed laboratory work, designed research, analyzed data, and wrote the paper; D.L. designed research and critically reviewed the paper; M.S. provided clinical samples and analyzed clinical data; R.S. designed research; M.O. recruited the probands, analyzed clinical data, and designed and performed laboratory work; K.S. designed and supervised research; A.B. designed research and wrote the paper; U.F. designed research, analyzed data, and wrote the paper; and U.F. and A.B. were the principal investigators and take primary responsibility for the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ute Fischer, Department of Pediatric Oncology, Hematology, and Clinical Immunology, Center for Child and Adolescent Health, Heinrich Heine University, Moorenstr 5, 40225 Düsseldorf, Germany; e-mail: ute.fischer@med.uni-duesseldorf.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal