In this issue of Blood, Lausten-Thomsen et al challenge the notion that TEL-AML1 transcripts are prevalent in newborns, raising questions about our understanding of the natural history of childhood ALL and the potential utility of newborn screening.1
The t(12;21) translocation results in the TEL-AML1 fusion and is present in approximately 25% of childhood acute lymphoblastic leukemia (ALL).2 In approximately 60% of cases of TEL-AML1+ childhood ALL, the TEL-AML1 fusion can be traced back to genomic DNA isolated from drops of blood taken from the patient soon after birth for the purposes of newborn screening (Guthrie cards).3 In such cases, the TEL-AML1 fusion is thought to develop in a lymphoid precursor in utero, establishing a detectable preleukemic clone that, over the course of years, acquires the additional mutations necessary for full leukemic transformation.
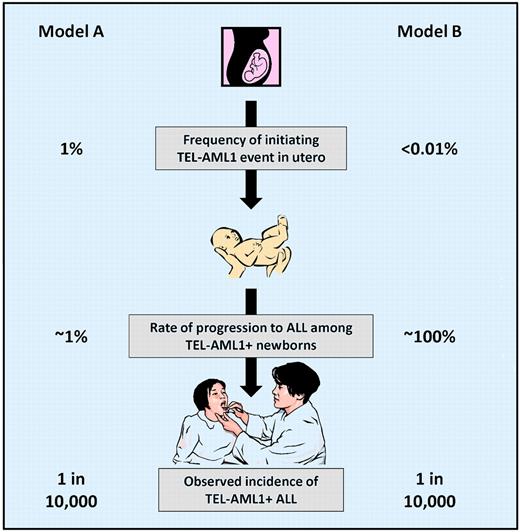
There is a commonly held belief that a relatively high proportion (∼ 1%) of newborns have detectable, functional TEL-AML1 fusion transcripts in umbilical cord blood. Because the cumulative incidence of TEL-AML1+ ALL is approximately 1 in 10 000 (0.01%), this suggests that only 1 of 100 newborns born with detectable TEL-AML1 transcripts are destined to develop ALL. This model of TEL-AML1+ leukemogenesis (see figure, Model A), in which the initiating genetic event (TEL-AML1 fusion) is 100-fold more common than the disease, limits the utility of newborn screening. Of the large number of babies expected to test positive, approximately 99% would never develop ALL, needlessly causing anxiety for families and pediatricians. Furthermore, it is unclear how a positive test would be handled in clinical practice. Preventative treatment with chemotherapy during infancy is out of the question. Frequent physical exams and blood counts could be contemplated for the babies with positive screens, but this would be invasive, expensive, and of dubious benefit, even for the 1% of babies for whom the surveillance program might aid in early diagnosis.
Until now, the only published direct evidence in support of Model A was a single study by Mori et al in which 567 umbilical cord blood samples from babies in the United Kingdom and Italy were screened and 6 were found to contain TEL-AML1 transcripts, with estimated TEL-AML1+ cell frequencies of 10-3 to 10-4.4 In addition to this direct evidence, data from identical twin studies are often cited as indirect evidence in support of Model A.5 These studies suggest that if one identical twin is diagnosed with ALL between the ages of 1 and 10 years, the risk of the second twin developing ALL is relatively low (5%-10%). The identical twins would have shared a preleukemic clone acquired in utero and the majority (∼ 60%) of TEL-AML1+ ALL can be traced to birth, suggesting that a high proportion of babies with a prenatally acquired TEL-AML1 preleukemic clone do not develop ALL. It is important to realize, however, that there are no published data regarding the identical twin concordance in cases of TEL-AML1+ ALL. It is certainly possible that the concordance rate is much higher in this subset. If so, this would challenge the notion that 99% of babies born with a TEL-AML1 transcript will not go on to develop ALL.
The provocative article by Lausten-Thomsen et al in this issue of Blood challenges Model A by providing direct evidence that the proportion of newborns with detectable TEL-AML1 transcripts may actually be much lower than 1%. The study was undertaken in an effort to confirm the findings of the previous study in a larger population. The results, however, were strikingly different. Of 1417 umbilical cord blood samples studied, none could be shown conclusively to contain TEL-AML1 transcripts. There were 14 positive cases in the initial TaqMan quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) screen, all at estimated cell levels of less than 10-5. Of these 14, 12 were positive in only 1 of 3 triplicate wells, and 2 were positive in 2 of 3. Only 9 of the 14 were verified as positive using “dot-blot” hybridization with a 32P-labeled probe. None of the 14 produced positive findings in parallel or subsequent PCRs, including assays done on CD19+ cell-enriched subpopulations.
Understandably, online publication of this manuscript has generated several responses, 2 of which are published as letters to the editor in this issue.6,7 Greaves et al, representing the laboratory from which the Mori et al report originated, provide 5 additional fluorescence in situ hybridization (FISH) images, bringing to 6 the total of published FISH images from the 2 TEL-AML1+ cord blood samples that could be confirmed by FISH testing. The focus on FISH is appropriate, because FISH is not as susceptible as PCR to false-positive results due to contamination.6 In another letter, Zuna et al describe the results of an unpublished study of 253 cord blood samples, in which 5 (2%) were found to be positive for TEL-AML1 using RT-PCR. Of these, 1 sample was found to be positive by FISH, although the TEL-AML1 transcript sequence in this case suggested breakpoints that are not typical of TEL-AML1+ ALL.7
There are many possible explanations for these divergent results, which the article and the letters begin to address. Lausten-Thomsen et al make the case that the differences in results are due primarily to differences in the applied methods of screening and confirmation, arguing that methodologic shortcomings in the Mori et al study may have resulted in false positives. The letter from Greaves et al challenges this assertion. Another possibility that is briefly considered by Lausten-Thomsen et al is that the population in their study (a predominately white Danish population) could somehow differ from the predominately British/Italian population studied in the Mori et al report or from the Czech population studied in the Zuna et al report. Is it possible, for example, that there are differences in susceptibilities (based on polymorphisms), or prenatal/postnatal exposures, that might explain these divergent results? Is it possible that the frequencies of TEL-AML1+ ALL differ between the geographic regions covered by the studies?
While the Lausten-Thomsen et al paper is not conclusive, it suggests an alternative model of TEL-AML1 leukemogenesis (see figure, Model B). In this model, the initiating event (TEL-AML1 fusion) is as rare as the disease itself, implying that a high proportion (perhaps 100%) of babies born with a detectable TEL-AML1 fusion are destined to develop TEL-AML1+ ALL. Could newborn screening for TEL-AML1 be considered in this scenario? Assuming a sensitive and specific clinical test could be developed, the problem of what to do with the small number of babies with positive screens would remain. How would one determine whether treating TEL-AML1+ newborns during the preleukemic phase could prevent the development of ALL? Or whether using intensive surveillance to detect the development of ALL very early in the disease process could translate into improved outcomes? These important questions can only be answered with more research in this fascinating aspect of leukemia biology.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal