TO THE EDITOR:
Mixed phenotype acute leukemia (MPAL) represents a heterogeneous group of leukemias accounting for 2% to 5% of all newly diagnosed acute leukemias.1-5 Although most cases of acute leukemia display the differentiation pattern of a single lineage, either B-cell lymphoid, T-cell lymphoid, or myeloid, MPAL blasts express markers of >1 lineage.1-5 Expression of different lineage markers may occur in a single-blast population, a so-called biphenotypic pattern, or multiple-blast populations, a so-called bilineal pattern. According to the World Health Organization (WHO) 2016 MPAL criteria, assigning myeloid lineage to a single-blast population in MPAL requires either expression of myeloperoxidase (MPO) by flow cytometry, immunohistochemistry, or cytochemistry or evidence of monocytic differentiation by expression of at least 2 of the following: nonspecific esterase, CD11c, CD14, CD64, or lysozyme.6 Thus, a logical corollary of the WHO MPAL criteria is that patients with isolated MPO expression on a single-blast population, with an otherwise typical B-cell acute lymphoblastic leukemia (B-ALL) immunophenotype, meet criteria for the MPAL B/myeloid category. The WHO 2016 MPAL criteria acknowledge the existence of such cases, classified here as MPAL B/myeloidisoMPO, but recommends “that care be taken before making a diagnosis of B/myeloid MPAL when low-intensity myeloperoxidase is the only myeloid-associated feature,”6(p2399) because the clinical significance of this finding has not been established. Therefore, the goal of this study was to determine if there were differences in treatment approaches and survival outcomes between patients classified as having MPAL B/myeloidisoMPO and other patients with MPAL.
We performed a retrospective medical record review of all patient cases of MPAL diagnosed between 2001 and 2016 at Children’s Healthcare of Atlanta. Patient cases were identified through our institutional tumor registry and pathology department flow cytometry database. Patients were eligible if they were between the ages of 1 and 21 years and their leukemia met WHO 2016 MPAL criteria. We excluded patients with therapy-related disease, nonmyeloid disease (rare B/T subtype), and t(9;22) translocation. Data were extracted from clinical records for patient-, disease-, and treatment-related variables. All flow cytometry data were reviewed by study hematopathologists. MPO expression was reported in accordance with consensus recommendations from clinical flow cytometry experts in the field.7,8 Details regarding specific flow cytometry methods are included in the supplemental Material, available on the Blood Web site.
Patients were divided into 2 groups: MPAL B/myeloidisoMPO and other MPAL. As described before, MPAL B/myeloid patient cases were classified as MPAL B/myeloidisoMPO if there was a single B-cell lineage blast population that expressed MPO as the only WHO MPAL myeloid marker. Time to first event, death, and last follow-up were recorded. Events were defined as treatment failure requiring therapy change before the end of induction (EOI), induction failure, induction death, remission death, or relapse. Event-free survival (EFS) and overall survival (OS) were calculated using Kaplan-Meier survival analysis. Groups were compared using the log-rank test. The χ2 test and Fisher’s exact test were used for sampling distribution analyses. All statistics were calculated using SAS 9.4 and SigmaPlot 13.0 software, with a P value < .05 representing statistical significance. The study was approved by our institutional review board.
Of the 1182 patient cases of de novo acute leukemia diagnosed between 2001 and 2016 at our institution, 38 (3.2%) met WHO 2016 MPAL criteria. Of these, 3 patients were excluded from the study: 1 had the rare nonmyeloid B/T subtype, 1 had a t(9;22) translocation and was treated with imatinib, and 1 had severe underlying comorbidities resulting from 13q deletion syndrome. Of the 35 study cases, 21 (60%) met criteria for MPAL B/myeloidisoMPO, with MPO expression ranging from partial dim to moderate (supplemental Figure 1). The remaining other patient cases of MPAL consisted of 5 MPAL B/myeloid (nonisolated MPO), 7 MPAL T/myeloid, and 2 MPAL with t(v;11q23), MLL rearranged. One patient in the MPAL B/myeloidisoMPO group had an 11q23 translocation. The MPAL B/myeloidisoMPO group also had some common B-ALL cytogenetic features such as presence of ETV6-RUNX1 (2 patient cases), trisomy 4 (5 patient cases), and trisomy 10 (8 patient cases). Supplemental Tables 1 and 2 show immunophenotypic and cytogenetic details for each patient. Some patients with MPAL B/myeloidisoMPO expressed myeloid-associated markers, such as CD13 (52%), CD33 (10%), and CD15 (14%), that are not considered lineage defining by the WHO.
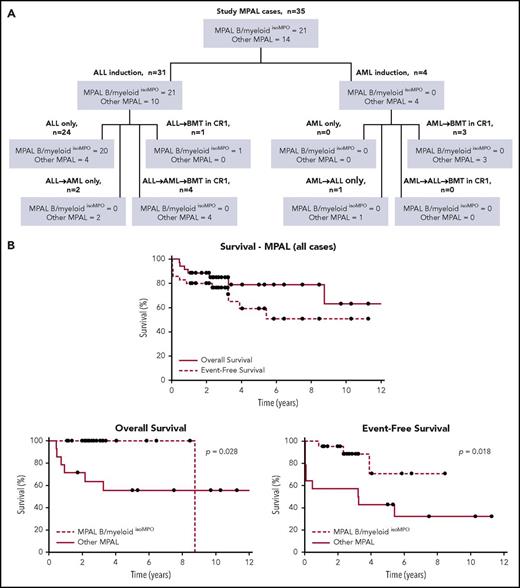
Baseline patient, disease, and treatment characteristics are summarized in Table 1. More patients with MPAL B/myeloidisoMPO were age <10 years at presentation (71% vs 43%) and had a white blood cell count <50 × 109/L (76% vs 57%), although these differences were nonsignificant. Significant differences were seen in treatment approaches. All patients with MPAL B/myeloidisoMPO received ALL induction regimens, whereas 29% (4 of 14) in the other group received AML induction. No patient with MPAL B/myeloidisoMPO was switched to AML therapy; however, 6 of 10 patients who received ALL induction in the other group were switched to AML therapy: 3 were switched because of treatment failure before EOI, 2 because of induction failure, and 1 (who was MRD negative at EOI) because of physician preference. Only 1 patient with MPAL B/myeloidisoMPO underwent BMT in CR1, and the indication for BMT was hypodiploidy. Comparatively, 50% (7 of 14) of the other patients with MPAL underwent transplantation in CR1. An overall treatment schema for the study population is depicted in Figure 1A. Individual patient treatment information including BMT details are summarized in supplemental Tables 3, 4, and 5.
Demographic and treatment characteristics
| Characteristic . | All MPAL patient cases (n = 35) . | MPAL B/myeloidisoMPO patient cases (n = 21) . | Other MPAL patient cases (n = 14) . | P . |
|---|---|---|---|---|
| No. (%) . | No. (%) . | No. (%) . | ||
| Sex | .491 | |||
| Male | 19 (54.3) | 10 (47.6) | 9 (64.3) | |
| Female | 16 (45.7) | 11 (52.4) | 5 (35.7) | |
| Age at diagnosis, y | .181 | |||
| <10 | 21 (60.0) | 15 (71.4) | 6 (42.9) | |
| ≥10 | 14 (40.0) | 6 (28.6) | 8 (57.1) | |
| Race | 1.000 | |||
| White | 25 (71.4) | 15 (71.4) | 10 (71.4) | |
| Black | 9 (25.7) | 5 (23.8) | 4 (28.6) | |
| Asian | 1 (2.9) | 1 (4.8) | 0 (0.0) | |
| Ethnicity | .262 | |||
| Hispanic | 9 (25.7) | 7 (33.3) | 2 (14.3) | |
| Not Hispanic | 26 (74.3) | 14 (66.7) | 12 (85.7) | |
| WBC count at diagnosis, × 109/L | .283 | |||
| <50 | 24 (68.6) | 16 (76.2) | 8 (57.1) | |
| ≥50 | 11 (31.4) | 5 (23.8) | 6 (42.9) | |
| CNS status at diagnosis | 1.000 | |||
| CNS1 | 27 (77.1) | 16 (76.2) | 11 (78.6) | |
| CNS2 | 7 (20.0) | 4 (19.0) | 3 (21.4) | |
| CNS3 | 1 (2.9) | 1 (4.8) | 0 (0.0) | |
| Initial treatment regimen | .019 | |||
| ALL | 31 (88.6) | 21 (100.0) | 10 (71.4) | |
| AML | 4 (11.4) | 0 (0.0) | 4 (28.6) | |
| AML therapy during treatment course | <.001 | |||
| Yes | 10 (28.6) | 0 (0.0) | 10 (71.4) | |
| No | 25 (71.4) | 21 (100.0) | 4 (28.6) | |
| BMT in CR1 | .003 | |||
| Yes | 8 (22.9) | 1 (4.8)* | 7 (50.0) | |
| No | 27 (77.1) | 20 (95.2) | 7 (50.0) | |
| Overall treatment schema | <.001 | |||
| ALL only | 24 (68.6) | 20 (95.2) | 4 (28.6) | |
| ALL → BMT in CR1 | 1 (2.9) | 1 (4.8)* | 0 (0.0) | |
| ALL → AML | 2 (5.7) | 0 (0.0) | 2 (14.3) | |
| ALL → AML → BMT in CR1 | 4 (11.4) | 0 (0.0) | 4 (28.6) | |
| AML only | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| AML → BMT in CR1 | 3 (8.6) | 0 (0.0) | 3 (21.4) | |
| AML → ALL | 1 (2.9) | 0 (0.0) | 1 (7.1) | |
| Initial event type | .004 | |||
| Treatment failure before EOI | 3 (8.6) | 0 (0.0) | 3 (21.4) | |
| Induction failure† | 2 (5.7) | 0 (0.0) | 2 (14.3) | |
| Induction death | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Remission death | 1 (2.9) | 0 (0.0) | 1 (7.1) | |
| Relapse | 6 (17.1) | 3 (14.3) | 3 (21.4) | |
| No event | 23 (65.7) | 18 (85.7) | 5 (35.7) | |
| MRD at end of induction, % | .266 | |||
| <0.01 | 28 (80.0)‡ | 18 (85.7) | 10 (71.4)‡ | |
| ≥0.01 to <5 | 5 (14.3) | 3 (14.3) | 2 (14.3) | |
| ≥5 to <25 | 2 (5.7) | 0 (0.0) | 2 (14.3) | |
| ≥25 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Characteristic . | All MPAL patient cases (n = 35) . | MPAL B/myeloidisoMPO patient cases (n = 21) . | Other MPAL patient cases (n = 14) . | P . |
|---|---|---|---|---|
| No. (%) . | No. (%) . | No. (%) . | ||
| Sex | .491 | |||
| Male | 19 (54.3) | 10 (47.6) | 9 (64.3) | |
| Female | 16 (45.7) | 11 (52.4) | 5 (35.7) | |
| Age at diagnosis, y | .181 | |||
| <10 | 21 (60.0) | 15 (71.4) | 6 (42.9) | |
| ≥10 | 14 (40.0) | 6 (28.6) | 8 (57.1) | |
| Race | 1.000 | |||
| White | 25 (71.4) | 15 (71.4) | 10 (71.4) | |
| Black | 9 (25.7) | 5 (23.8) | 4 (28.6) | |
| Asian | 1 (2.9) | 1 (4.8) | 0 (0.0) | |
| Ethnicity | .262 | |||
| Hispanic | 9 (25.7) | 7 (33.3) | 2 (14.3) | |
| Not Hispanic | 26 (74.3) | 14 (66.7) | 12 (85.7) | |
| WBC count at diagnosis, × 109/L | .283 | |||
| <50 | 24 (68.6) | 16 (76.2) | 8 (57.1) | |
| ≥50 | 11 (31.4) | 5 (23.8) | 6 (42.9) | |
| CNS status at diagnosis | 1.000 | |||
| CNS1 | 27 (77.1) | 16 (76.2) | 11 (78.6) | |
| CNS2 | 7 (20.0) | 4 (19.0) | 3 (21.4) | |
| CNS3 | 1 (2.9) | 1 (4.8) | 0 (0.0) | |
| Initial treatment regimen | .019 | |||
| ALL | 31 (88.6) | 21 (100.0) | 10 (71.4) | |
| AML | 4 (11.4) | 0 (0.0) | 4 (28.6) | |
| AML therapy during treatment course | <.001 | |||
| Yes | 10 (28.6) | 0 (0.0) | 10 (71.4) | |
| No | 25 (71.4) | 21 (100.0) | 4 (28.6) | |
| BMT in CR1 | .003 | |||
| Yes | 8 (22.9) | 1 (4.8)* | 7 (50.0) | |
| No | 27 (77.1) | 20 (95.2) | 7 (50.0) | |
| Overall treatment schema | <.001 | |||
| ALL only | 24 (68.6) | 20 (95.2) | 4 (28.6) | |
| ALL → BMT in CR1 | 1 (2.9) | 1 (4.8)* | 0 (0.0) | |
| ALL → AML | 2 (5.7) | 0 (0.0) | 2 (14.3) | |
| ALL → AML → BMT in CR1 | 4 (11.4) | 0 (0.0) | 4 (28.6) | |
| AML only | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| AML → BMT in CR1 | 3 (8.6) | 0 (0.0) | 3 (21.4) | |
| AML → ALL | 1 (2.9) | 0 (0.0) | 1 (7.1) | |
| Initial event type | .004 | |||
| Treatment failure before EOI | 3 (8.6) | 0 (0.0) | 3 (21.4) | |
| Induction failure† | 2 (5.7) | 0 (0.0) | 2 (14.3) | |
| Induction death | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Remission death | 1 (2.9) | 0 (0.0) | 1 (7.1) | |
| Relapse | 6 (17.1) | 3 (14.3) | 3 (21.4) | |
| No event | 23 (65.7) | 18 (85.7) | 5 (35.7) | |
| MRD at end of induction, % | .266 | |||
| <0.01 | 28 (80.0)‡ | 18 (85.7) | 10 (71.4)‡ | |
| ≥0.01 to <5 | 5 (14.3) | 3 (14.3) | 2 (14.3) | |
| ≥5 to <25 | 2 (5.7) | 0 (0.0) | 2 (14.3) | |
| ≥25 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Bold font indicates significance in P column.
AML, acute myeloid leukemia; BMT, bone marrow transplantation; CNS, central nervous system; CR1, first complete remission; MRD, minimal residual disease; WBC, white blood cell.
BMT performed because of hypodiploidy.
Induction failure defined as M2 or M3 bone marrow at EOI.
3 patients switched from ALL to AML therapy before EOI, thus affecting EOI minimal residual disease.
Treatment and survival data. (A) Schematic showing different treatment approaches for entire study population. (B) Kaplan-Meier curves showing OS and EFS for all study MPAL cases (top), as well as OS (bottom left) and EFS (bottom right) of MPAL B/myeloidisoMPO compared with other MPAL cases.
Treatment and survival data. (A) Schematic showing different treatment approaches for entire study population. (B) Kaplan-Meier curves showing OS and EFS for all study MPAL cases (top), as well as OS (bottom left) and EFS (bottom right) of MPAL B/myeloidisoMPO compared with other MPAL cases.
Median time to last follow-up in survivors was 3.14 years (range, 1.09 to 13.03 years). Three-year EFS was 76.4% and 3-year OS was 84.9% for all study patients (Figure 1B, top). Patients with MPAL B/myeloidisoMPO had a significantly better 3-year EFS compared with other patients with MPAL (88.4% vs 57.1%; P = .018; Figure 1B, bottom right). All patients with MPAL B/myeloidisoMPO (100%) were alive at 3 years compared with only 63.1% in the other MPAL group (P = .028; Figure 1B, bottom left). Interestingly, there was no correlation between degree of MPO expression and survival in the MPAL B/myeloidisoMPO group (supplemental Figure 2). Survival comparisons between different MPAL subgroups were difficult given the small study numbers and did not yield any significant results. Survival analysis of different treatment subgroups was not feasible given the heterogeneity in treatment approaches.
Although there are several limitations to our study given its retrospective nature and small sample size, we demonstrated significant differences between MPAL B/myeloidisoMPO and other MPAL patient cases in terms of treatment approach and survival. This raises the question of whether this patient population would be better considered within the spectrum of B-ALL rather than MPAL. Oberley et al9 reported that patients with isolated MPO expression but an otherwise typical B-ALL phenotype showed outcomes similar to those of patients with MPAL B/myeloid, and there were no OS differences in those with otherwise typical ALL.9 However, of their 21 patients with MPAL B/myeloid, at least 19 expressed MPO as their only WHO myeloid marker and would have been categorized as having MPAL B/myeloidisoMPO in our study, along with all the B-ALLisoMPO patient cases. Thus, it is difficult to compare the 2 studies given the differences in study design.
There continues to be no consensus on the treatment of MPAL. All treatment data have come from retrospective studies, and the changing definition of MPAL has contributed to the difficulty in understanding this rare and heterogeneous disease.6,9-15 Although our findings need to be further validated with larger studies, the implications of our results could significantly alter how MPAL is studied. If MPAL B/myeloidisoMPO clinically behaves like B-ALL, as our data would suggest, then collectively including these patients in MPAL studies creates a significant bias toward ALL-directed therapy. Although the current WHO criteria have simplified the definition of MPAL, they now formally incorporate the MPAL B/myeloidisoMPO subset but then simultaneously recommend “that care be taken” before labeling this population as having MPAL B/myeloid. Our findings support such caution, because they imply that the underlying biology of these leukemias may more closely resemble that of B-ALL, at least in terms of response to therapy. We suggest that the current WHO MPAL criteria may benefit from another revision, in which MPAL B/myeloidisoMPO is more clearly segregated from other MPAL subsets.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the leukemia/lymphoma team at Children’s Healthcare of Atlanta for its constructive review of the manuscript.
No funding was used to conduct this study.
Authorship
Contribution: S.S.R., S.I.P., and W.G.W. designed the study; S.S.R. collected and analyzed the data and wrote the manuscript; S.I.P. and D.L.J. reviewed the flow cytometry data; S.S.R. and T.L. performed all statistical analyses; and S.I.P., D.L.J., F.G.K., J.T.H., and W.G.W. reviewed and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sunil S. Raikar, Aflac Cancer and Blood Disorders Center, Children’s Healthcare of Atlanta, Emory University School of Medicine, 1760 Haygood Dr, Health Sciences Research Building, Room W-345, Atlanta, GA 30322; e-mail: sraikar@emory.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal