Key Points
Lactadherin promotes the clearance of circulating microvesicles through phagocytosis.
Promoting microvesicle clearance prevents coagulopathy, reduces cerebral edema, and improves neurological function in severe TBI mice.
Abstract
Coagulopathy is common in patients with traumatic brain injury (TBI) and predicts poor clinical outcomes. We have shown that brain-derived extracellular microvesicles, including extracellular mitochondria, play a key role in the development of TBI-induced coagulopathy. Here, we further show in mouse models that the apoptotic cell-scavenging factor lactadherin, given at a single dose of 400 μg/kg 30 minutes before (preconditioning) or 30 minutes after cerebral fluid percussion injury, prevented coagulopathy as defined by clotting time, fibrinolysis, intravascular fibrin deposition, and microvascular bleeding of the lungs. Lactadherin also reduced cerebral edema, improved neurological function, and increased survival. It achieved these protective effects by enhancing the clearance of circulating microvesicles through phosphatidylserine-mediated phagocytosis. Together, these results identify the scavenging system for apoptotic cells as a potential therapeutic target to prevent TBI-induced coagulopathy and improve the outcome of TBI.
Introduction
In response to injury, cells shed membrane fragments and release intracellular granules through active microvesiculation1,2 or apoptosis.3,4 The former is initiated by intracellular signals that activate the cysteine protease calpain, which then disrupts the membrane cytoskeleton by cleaving cytoskeletal proteins.5,6 Both microvesiculation and apoptosis produce extracellular vesicles called microvesicles (MVs), which have increasingly been recognized as a new class of mediators or delivery vehicles for diverse biological processes and disease development.
We recently identified a causal role of brain-derived MVs (BDMVs) in the pathogenesis of TBI-induced coagulopathy.7 Trauma-induced uncontrolled hemorrhage is a leading cause of preventable deaths, accounting for 30% to 40% of all trauma fatalities.8,9 It is caused by direct injury to the vasculature and secondary coagulopathy. Retrospective and observational studies have consistently shown that coagulopathy is common in patients with TBI,10-12 even though patients with isolated TBI lack the key causal factors for coagulopathy found in patients with injuries to the body and limbs; that is, substantial blood loss and hemodilution resulting from fluid resuscitation.13,14 The underlying cause of TBI-induced coagulopathy remains poorly understood. We have recently demonstrated that TBI-induced coagulopathy develops from a hypercoagulable state induced by BDMVs that are suddenly and substantially released from an injured brain into the circulation.7,15 Among these BDMVs, 55.2% are cell-free or membrane-embedded mitochondria (mtMVs).15 Both BDMVs and mtMVs express abundant anionic phospholipids on their surfaces: phosphatidylserine (PS) on membrane vesicles and cardiolipin (CL) on mtMVs. These anionic phospholipid-bearing MVs induce a hypercoagulable state that rapidly turns into consumptive coagulopathy. These observations led us to hypothesize that enhancing the clearance of these procoagulant MVs would prevent the development or reduce the severity of TBI-induced coagulopathy.
Several molecules have been extensively demonstrated to participate in the clearance of apoptotic cells. Among them is lactadherin (milk fat globule-epidermal growth factor 8).16 This 41- to 46-kDa glycoprotein has an N-terminal epidermal growth factor-like domain or domains that contain an integrin-binding tripeptide Arg-Gly-Asp (RGD) sequence and 2 C-terminal discoidin domains (C1 and C2), which bind PS with a high affinity.16-20 This structure allows lactadherin to couple apoptotic cells with monocytes/macrophages to facilitate phagocytosis.21-23 Because BDMVs and mtMVs share a key structural element with apoptotic cells, that is, the surface exposure of the anionic PS and CL, which are recognized by annexin V in a similar fashion,7,15 we hypothesize that lactadherin enhances the clearance of BDMVs and mtMVs to prevent coagulopathy and improve outcomes of TBI. Here we report findings from a study designed to test this hypothesis.
Materials and methods
This study was designed primarily to determine whether a high basal rate of microvesicle clearance reduces or prevents TBI-induced coagulopathy. Most of the experiments were therefore performed in mice preconditioned with lactadherin before being exposed to TBI. However, lactadherin was also given to a subset of mice after TBI to test its therapeutic potential.
Mouse model of TBI
Adult male C57BL/6J mice (12-16 weeks and 22-25 g; Jackson Laboratory, Bar Harbor, ME) were subjected to fluid percussion injury (FPI).7,15 Briefly, saline from a Plexiglas cylindrical reservoir was rapidly injected at a controlled pressure of 1.9 ± 0.2 atm by a FPI device (Custom Design & Fabrication, Richmond, VA) into an unstrained mouse through a 3-mm-diameter cranial cavity with the dura matter intact (2.0 mm posterior from the bregma and 2.0 mm lateral to the sagittal suture). Thirty minutes before FPI, the mice received a single tail vein infusion of either 400 μg/kg (∼10 μg/mouse) purified lactadherin (Biomatik, Wilmington, DE) or an equal volume of the vehicle phosphate-buffered saline (PBS). A sham mouse received PBS and underwent the craniotomy but was not exposed to FPI. Blood samples were collected 1 hour before and 3 and 6 hours after FPI to monitor acute changes in microvesicle release and coagulation. In addition, the mice were recorded for 7-day survival, and the surviving mice were also evaluated for neurological function, using a modified Neurological Severity Score system24 on post-TBI days 1, 3, and 7. In addition to the full-length lactadherin, a truncated variant (Q188-C312) containing the PS-binding C1C2 domains, but not the integrin-binding epidermal growth factor domain,25 was similarly tested to distinguish between MV scavenging and potential anticoagulant activities of lactadherin. For data validation, lactadherin null mice and their wild-type littermates were identically examined for FPI-induced release of BDMVs and coagulopathy. Lactadherin-null mice (B6;129-Mfge8 <tm1Osa>/OsaRbrc mice, no. RBRC01726; supplemental Methods, available on the Blood Web site) were obtained from RIKEN BioSource Center (Ibaraki 305-0074, Japan) with the consent of Dr. Shigekazu Nagata from the Immunology Frontier Research Center, Osaka University.21 The use of lactadherin−/− mice is approved by the Institutional Animal Care and Use Committee of the Bloodworks Research Institute.
In reciprocal experiments, noninjured mice were infused with 1.5 × 107 purified BDMVs/mouse, followed immediately by 400 μg/kg lactadherin or an equal volume of PBS. They were evaluated similarly as those subjected to FPI. BDMVs were made by freeze-thawing normal mouse brains. They have been found to be indistinguishable from those purified from the plasma of TBI mice in morphology and procoagulant activity.7 The number of BDMVs we infused was approximately 50% of what we had previously detected in plasma samples from TBI mice.7 This number was used because more than 85% of mice died within 30 minutes of an infusion of 3.0 × 107 MVs, as is found in TBI mice, making the collection and analysis of blood and tissue samples very difficult. This high mortality was determined to be caused by a rapid accumulation of MVs in the circulation through direct vascular infusion of BDMVs compared with the relatively slow release of BDMVs from a traumatically injured brain.
Evans blue dye extravasation
To measure FPI-induced vascular leakage and cerebral edema, mice subjected to FPI received 100 μL of 2% Evans blue (Sigma Aldrich, St. Louis, MO) through the tail vein.26 The mice were killed 2 hours after injection and perfused with PBS. The brains were dissected, embedded on Tissue-Tek OCT medium (Sakura Fineteck, Torrance, CA), sectioned (10 μm thickness), and fixed in ice-cold acetone for 10 minutes in the dark. The brain sections were stained with 4′,6-diamidino-2-phenylindole for 30 minutes at room temperature and reviewed under a fluorescence microscope (Olympus IX81, Waltham, MA). The brains were also snap-frozen in liquid nitrogen, homogenized in formamide (1:20 wt/vol), and incubated at 60°C overnight. The brain homogenates were centrifuged at 16 000g for 30 minutes. Evans blue in the supernatant was measured at OD620 nm in a SpectraMax M5 plate-reader (Molecular Devices, Sunnyvale CA).
Flow cytometry
Neuronal MVs in the peripheral blood samples of TBI and control mice were identified using flow cytometry, first by the particle size (<1 μm determined using 0.5-, 0.9-, and 3-μm standard microbeads; Biocytex, Marseille, France) and then by their expression of neuron-specific enolase (NSE) detected by a rabbit anti-mouse NSE antibody (Abcam, Cambridge, MA), followed by a phycoerythrin-conjugated anti-rabbit immunoglobulin G (eBioscience, San Diego, CA) on an LSR II flow cytometer (Beckon Dickinson, San Jose, CA).7 An isotype-specific immunoglobulin G was used as the control. mtMVs were detected by the mitochondrial dye MitoTracker Green (Thermo Fisher Scientific).15 Sphero Accucount beads were used to quantify MVs. Flow cytometry was also used to measure the binding of fluorescein isothiocyanate-conjugated lactadherin (Haematologic Technologies, Inc., Essex Junction, VT) to purified BDMVs after 30 minutes of incubation at room temperature. All buffers used for MV detection were filtered with a 0.1-μm filter (EDM Millipore, Billerica, MA) to reduce noncellular MV contamination. Annexin V, which is widely used to detect PS exposure on microvesicles, was not used in most experiments, except when lactadherin−/− mice were studied, because annexin V binding to PS was competitively inhibited by lactadherin.
Coagulation and fibrinolysis assays
To quantify a lactadherin-mediated reduction of MVs on plasma clotting time, MVs from fixed volumes of plasma from TBI mice receiving lactadherin or PBS were mixed with phospholipid-free normal plasma and the coagulation factor Xa (0.02 U/mL) to induce clot formation, as previously described.7,15 The clot formation was monitored at 37°C on a CoaScreener coagulation analyzer (American Labor Corp., Durham, NC). Plasma levels of the fibrinolytic product d-dimer were measured with a commercial kit according to the manufacturer’s instructions (Cloud-Clone Corp., Houston, TX).
Microvesicle clearance and phagocytosis
For the MV clearance experiments, purified BDMVs were biotinylated using an EZ-Link Sulfo-NHS-Biotin kit (Thermo Fisher Scientific, Waltham, MA). The biotinylation of the BDMVs was validated by their binding to fluorescein isothiocyanate-conjugated streptavidin, using flow cytometry (supplemental Figure 1). After baseline blood sampling, 1.5 × 107 biotinylated BDMVs were infused into a mouse through the tail vein, and blood samples were collected 3 and 6 hours postinfusion. The livers were dissected, fixed in 4% paraformaldehyde, and processed for histology to detect BDMV accumulation in the liver by horseradish peroxidase–conjugated streptavidin (ZSGB-Bio SP-9000). For in vitro phagocytosis testing, we used mtMVs as the surrogate to reduce the heterogeneity of BDMVs in their compositions (membrane vesicles and intracellular granules) and sizes (0.1-1.0 μm). Using mtMV as the surrogate for BDMV is valid because mtMVs account for 55.2% of all annexin V+ MVs detected in FPI mice.15 mtMVs labeled with MitoTracker Green were incubated with purified monocytes for 30 minutes at 37°C. After washing with PBS, the cells were incubated with a V450-conjugated CD45 antibody for 30 minutes at 37°C. After washing, at least 20 000 cells were analyzed, using the Amnis ImageStreamX Mk II Imaging Cytometer (Amnis, Seattle, WA) with a 60× objective lens at a focal distance of 2.5 μm. Purified PS, CL, and phosphatidylcholine (200 μM each; Avanti Polar Lipids, Inc., Alabaster, AL) were tested for blocking mtMV phagocytosis.
Tissue histology
Mice were euthanized 24 hours after FPI to collect the lungs, which were fixed with 4% formaldehyde overnight at room temperature, embedded, and sectioned. The sections were stained with hematoxylin & eosin to detect TBI-induced extravascular erythrocyte accumulation, and with phosphotungstic acid hematoxylin (PTAH) to detect fibrin deposition to the vessel wall.7,15 The livers from mice injected with biotinylated BDMVs were similarly processed and stained with horseradish peroxidase–conjugated streptavidin to measure microvesicle clearance.
Statistical analysis
Categorical variables were expressed as percentages and continuous variables as the mean ± SEM. The survival data were analyzed by Kaplan-Meier plot. All data were analyzed using Sigma plot (V. 11.2) for paired t test, 1-way or repeated measures ANOVA, as specified in each data set. A P value of <.05 was considered to be statistically significant.
Results
Lactadherin prevented FPI-induced coagulopathy, cerebral edema, and death
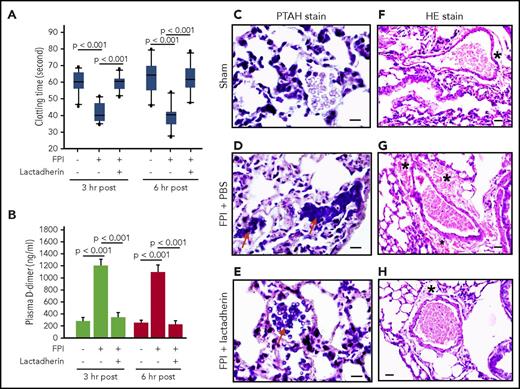
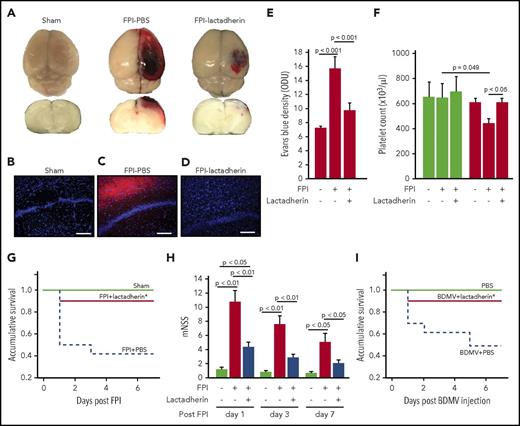
Lactadherin preconditioning prevented an FPI-induced hypercoagulable state and consumptive coagulopathy by reversing shortened clotting time (Figure 1A), reducing the level of plasma d-dimer (Figure 1B), and preventing intravascular fibrin deposition in the lungs (Figure 1C-E). The lactadherin-preconditioned mice also had reduced vascular leakage, as defined by the extravascular accumulation of erythrocytes (Figure 1F-H) and an enlarged perivascular space of the lungs (supplemental Figure 2), resulting in significantly reduced cerebral edema (Figure 2A-E). The FPI-induced thrombocytopenia was also corrected by lactadherin preconditioning (Figure 2F) without significant changes to the total leukocyte and erythrocyte counts (supplemental Figure 3). Lactadherin neither induced platelet aggregation nor altered the platelet aggregation induced by collagen (supplemental Figure 4), suggesting it did not affect platelet reactivity. By preventing coagulopathy and cerebral edema, 7-day mortality was reduced from 46.7% in FPI mice receiving PBS to 8.3% in those preconditioned with lactadherin (Figure 2G). The surviving mice receiving lactadherin developed less severe neurological dysfunction, measured by modified Neurological Severity Score during the 7-day monitoring period (Figure 2H).
Lactadherin reduced TBI-induced coagulopathy and vasculopathy. Clotting time (A) and plasma d-dimer (B) of sham mice and FPI mice preconditioned with either PBS or lactadherin (n = 16, 1-way ANOVA). Representative images of PTAH-stained lungs from a sham mouse (C) and FPI mice receiving PBS (D, red arrow indicates extensive blue PTAH stain for intravascular fibrin deposition) or lactadherin (E, arrow indicates significantly reduced intravascular PTAH stain, bar in C-E = 10 μm). Representative images of H&E-stained lungs from a sham mouse (F) and FPI mice receiving PBS (G) or lactadherin (H, bar in F-H = 20 μm). Perivascular space (denoted with an asterisk) is enlarged with extravascular accumulation of erythrocytes (hemorrhage) in the lungs of TBI mice receiving PBS. The C-H images are representatives of the 26 mice examined.
Lactadherin reduced TBI-induced coagulopathy and vasculopathy. Clotting time (A) and plasma d-dimer (B) of sham mice and FPI mice preconditioned with either PBS or lactadherin (n = 16, 1-way ANOVA). Representative images of PTAH-stained lungs from a sham mouse (C) and FPI mice receiving PBS (D, red arrow indicates extensive blue PTAH stain for intravascular fibrin deposition) or lactadherin (E, arrow indicates significantly reduced intravascular PTAH stain, bar in C-E = 10 μm). Representative images of H&E-stained lungs from a sham mouse (F) and FPI mice receiving PBS (G) or lactadherin (H, bar in F-H = 20 μm). Perivascular space (denoted with an asterisk) is enlarged with extravascular accumulation of erythrocytes (hemorrhage) in the lungs of TBI mice receiving PBS. The C-H images are representatives of the 26 mice examined.
Lactadherin reduced cerebral edema and improved outcomes of FPI. (A) Representative topical and cross-sectional views of brains from a sham mouse (left) and from FPI mice preconditioned with PBS (middle) or lactadherin (right). (B-D) Representative fluorescence images of brain cryosections from a sham mouse and mice receiving lactadherin or PBS. (E) Levels (OD unite) of Evans blue in the supernatants of tissue homogenates from FPI mice receiving different treatments (n = 9, 1-way ANOVA). (F) Platelet counts at the baseline (white bars) and 3 hours after FPI (black bars) of sham mice and TBI mice preconditioned with PBS or lactadherin (n = 16, 1-way ANOVA). A Kaplan-Meier survival analysis (G, n = 18; P < .005 vs mice received PBS) and modified Neurological Severity Score (H, n = 18, 1-way ANOVA) of sham and FPI mice receiving PBS or lactadherin. (I) A Kaplan-Meier survival analysis of BDMV-infused and control mice (P < .005 vs mice that received PBS).
Lactadherin reduced cerebral edema and improved outcomes of FPI. (A) Representative topical and cross-sectional views of brains from a sham mouse (left) and from FPI mice preconditioned with PBS (middle) or lactadherin (right). (B-D) Representative fluorescence images of brain cryosections from a sham mouse and mice receiving lactadherin or PBS. (E) Levels (OD unite) of Evans blue in the supernatants of tissue homogenates from FPI mice receiving different treatments (n = 9, 1-way ANOVA). (F) Platelet counts at the baseline (white bars) and 3 hours after FPI (black bars) of sham mice and TBI mice preconditioned with PBS or lactadherin (n = 16, 1-way ANOVA). A Kaplan-Meier survival analysis (G, n = 18; P < .005 vs mice received PBS) and modified Neurological Severity Score (H, n = 18, 1-way ANOVA) of sham and FPI mice receiving PBS or lactadherin. (I) A Kaplan-Meier survival analysis of BDMV-infused and control mice (P < .005 vs mice that received PBS).
To specifically examine the effect of lactadherin on BDMV-induced coagulopathy without the confounding influence of FPI, we infused noninjured mice with purified BDMVs (1.5 × 107/mouse), followed immediately by lactadherin (400 μg/kg). In these reciprocal experiments, lactadherin also prevented the development of a hypercoagulable state, as measured by clotting time and plasma d-dimer (supplemental Figure 5B-C). Thrombocytopenia similar to that found in FPI mice was also prevented by lactadherin (supplemental Figure 5D), again without affecting leukocyte or erythrocyte counts (supplemental Figure 6). Lactadherin reduced 7-day mortality from 58.2% in BDMV-infused mice to 10% (Figure 2I).
Lactadherin reduced circulating microvesicles
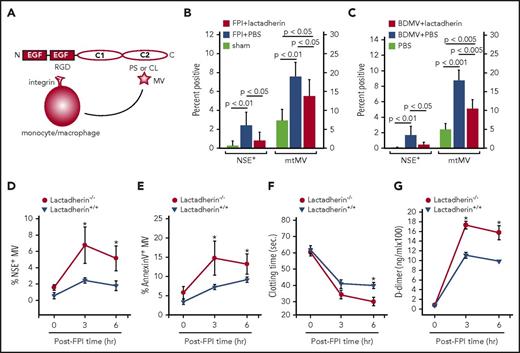
Lactadherin is known to remove apoptotic cells by coupling them with monocytes or macrophages to facilitate phagocytosis.16 Because this scavenging activity is mediated by the anionic phospholipid PS exposed on apoptotic cells, we hypothesized that lactadherin could also promote the clearance of procoagulant BDMVs and mtMVs that express PS and CL, respectively, to prevent FPI-induced coagulopathy (Figure 3A). We found that FPI mice preconditioned with lactadherin had significantly lower levels of neuronal microvesicles (NSE+) and mtMVs (MitoTracker Green+) than those receiving PBS (Figure 3B). The total number of annexin V-binding MVs was also reduced, going from 3.3 × 105 ± 5.8 × 103/μL in FPI mice to 1.2 × 105 ± 2.1 × 103/μL in those preconditioned with lactadherin (preinjury baseline, 0.9 × 104 ± 1.9 × 102/μL; supplemental Figure 5D). We focused on annexin V-binding MVs because our focus was on an anionic phospholipid-mediated procoagulant activity of MVs and lactadherin primarily removed anionic phospholipid-expressing MVs through the mechanism depicted in Figure 3A. The reduction was similarly observed in noninjured mice infused with BDMVs followed by lactadherin (Figure 3C). In contrast, higher levels of circulating NSE+ (Figure 3D) and PS-expressing (Annexin V+) MVs (Figure 3E), a shortened clotting time (Figure 3F), and a higher level of plasma d-dimer (Figure 3G) were observed in lactadherin−/− mice subjected to FPI than in their wild-type littermates.
Lactadherin reduced MPs and its deficiency increased TBI-induced BDMV release and coagulopathy. (A) A schematic illustration of lactadherin-mediated MV clearance. (B) Levels of circulating NSE+ (scale on the left) and mtMVs (MitoTracker Green+, scale on the right) measured 3 hours after injury of sham mice and FPI mice preconditioned with PBS or lactadherin (n = 15, 1-way ANOVA). (C) Levels of circulating NSE+ MVs (scale on the left) and mtMVs (scale on the right) in noninjured mice infused with 1.5 × 107/mouse of BDMVs followed by lactadherin or PBS (n = 12, 1-way ANOVA). Plasma samples from lactadherin−/− mice and their wild-type littermates were examined for dynamic changes in plasma levels of (D) NSE+ MVs, (E) annexin V-binding MVs, (F) clotting time, and (G) plasma levels of d-dimer (n = 24, repeated-measures ANOVA; *P < .001).
Lactadherin reduced MPs and its deficiency increased TBI-induced BDMV release and coagulopathy. (A) A schematic illustration of lactadherin-mediated MV clearance. (B) Levels of circulating NSE+ (scale on the left) and mtMVs (MitoTracker Green+, scale on the right) measured 3 hours after injury of sham mice and FPI mice preconditioned with PBS or lactadherin (n = 15, 1-way ANOVA). (C) Levels of circulating NSE+ MVs (scale on the left) and mtMVs (scale on the right) in noninjured mice infused with 1.5 × 107/mouse of BDMVs followed by lactadherin or PBS (n = 12, 1-way ANOVA). Plasma samples from lactadherin−/− mice and their wild-type littermates were examined for dynamic changes in plasma levels of (D) NSE+ MVs, (E) annexin V-binding MVs, (F) clotting time, and (G) plasma levels of d-dimer (n = 24, repeated-measures ANOVA; *P < .001).
Lactadherin promoted microvesicle clearance
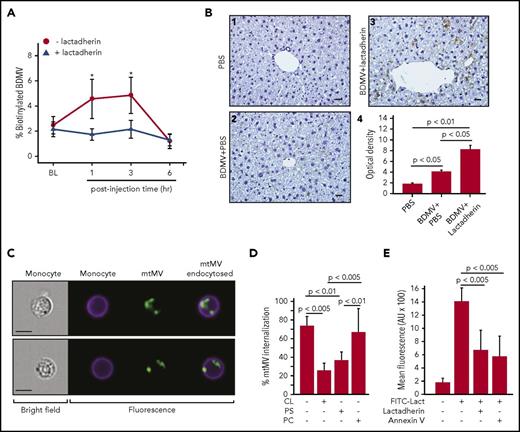
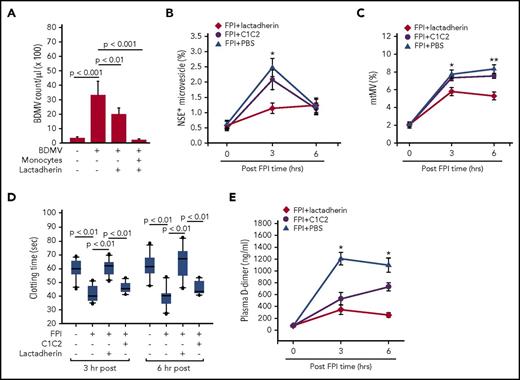
Several lines of experimental evidence support our hypothesis that lactadherin reduces circulating MVs by promoting their clearance through phagocytosis. First, the plasma level of biotinylated BDMVs injected into noninjured mice was significantly lower in the mice receiving lactadherin than in those receiving PBS (Figure 4A). Second, more biotinylated BDMVs were detected in sinusoids of the livers of mice receiving lactadherin than those receiving PBS (Figure 4B). Third, mtMV phagocytosis by monocytes was directly visualized using imaging flow cytometry (Figure 4C). The phagocytosis was blocked by the anionic phospholipids CL or PS, but not by the neutral phosphatidylcholine (Figure 4D), suggesting that anionic phospholipids were involved in the process. This notion is further supported by the ability of the PS-binding annexin V to block lactadherin binding to BDMVs (Figure 4E). Fourth, the transmigration of BDMVs through a monolayer of cultured endothelial cells measured in a transwell culture system (supplemental Methods) was prevented by lactadherin in the presence of monocytes and, to a lesser extent, in their absence (Figure 5A). Finally, truncated lactadherin lacking the integrin-binding epidermal growth factor domain (C1C2) was significantly less active in reducing plasma NSE+ microvesicles (Figure 5B) and mtMVs (Figure 5C). Mice receiving C1C2 developed an FPI-induced hypercoagulable state, as demonstrated by a shortened clotting time (Figure 5D) and an elevated level of plasma d-dimer (Figure 5E).
Lactadherin promoted BDMV clearance through phagocytosis. (A) Plasma levels of biotinylated BDMVs infused into noninjured mice that also received lactadherin or PBS (n = 12, paired t test; *P < .01). (B) Representative images of liver sections from mice infused with (1) PBS, (2) biotinylated BDMVs, and (3) biotinylated BDMVs with lactadherin were stained with horseradish peroxidase–conjugated streptavidin (bar = 20 μm, biotinylated BDMVs are stained in brown color); (4) integrated optical densities from scans from multiple mice (n = 9, 1-way ANOVA). (C) Bright field (left) and fluorescence images (right) of CD45+ monocytes and MitoTracker Green+ mtMVs (right overlay) detected by imaging flow cytometry. (D) mtMV binding to CD45+ monocytes in the absence and presence of PS, CL, and phosphatidylcholine (PC; all 200 nM, n = 12, 1-way ANOVA). (E) Blocking the binding of fluorescein isothiocyanate-lactadherin to BDMVs by 100-fold excess of either unlabeled lactadherin or annexin V (n = 24, 1-way ANOVA).
Lactadherin promoted BDMV clearance through phagocytosis. (A) Plasma levels of biotinylated BDMVs infused into noninjured mice that also received lactadherin or PBS (n = 12, paired t test; *P < .01). (B) Representative images of liver sections from mice infused with (1) PBS, (2) biotinylated BDMVs, and (3) biotinylated BDMVs with lactadherin were stained with horseradish peroxidase–conjugated streptavidin (bar = 20 μm, biotinylated BDMVs are stained in brown color); (4) integrated optical densities from scans from multiple mice (n = 9, 1-way ANOVA). (C) Bright field (left) and fluorescence images (right) of CD45+ monocytes and MitoTracker Green+ mtMVs (right overlay) detected by imaging flow cytometry. (D) mtMV binding to CD45+ monocytes in the absence and presence of PS, CL, and phosphatidylcholine (PC; all 200 nM, n = 12, 1-way ANOVA). (E) Blocking the binding of fluorescein isothiocyanate-lactadherin to BDMVs by 100-fold excess of either unlabeled lactadherin or annexin V (n = 24, 1-way ANOVA).
BDMV clearance required the integrin-binding domain of lactadherin. (A) The transmigration of PKH26-labeled BDMVs through activated endothelial cells in the presence and absence of lactadherin and monocytes (n = 15, 1-way ANOVA). C57BL/6J mice (n = 16) were preconditioned with an equal molar concentration of either lactadherin or its C1C2 domain (PBS as control) before being subjected to FPI. They were then examined for plasma levels of (B) NSE+ MVs, (C) mtMVs, (D) clotting time, and (E) plasma levels of d-dimer. Data presented in C-F were analyzed with repeated measures ANOVA, *P < .01 and **P < .001 vs PBS-injected mice.
BDMV clearance required the integrin-binding domain of lactadherin. (A) The transmigration of PKH26-labeled BDMVs through activated endothelial cells in the presence and absence of lactadherin and monocytes (n = 15, 1-way ANOVA). C57BL/6J mice (n = 16) were preconditioned with an equal molar concentration of either lactadherin or its C1C2 domain (PBS as control) before being subjected to FPI. They were then examined for plasma levels of (B) NSE+ MVs, (C) mtMVs, (D) clotting time, and (E) plasma levels of d-dimer. Data presented in C-F were analyzed with repeated measures ANOVA, *P < .01 and **P < .001 vs PBS-injected mice.
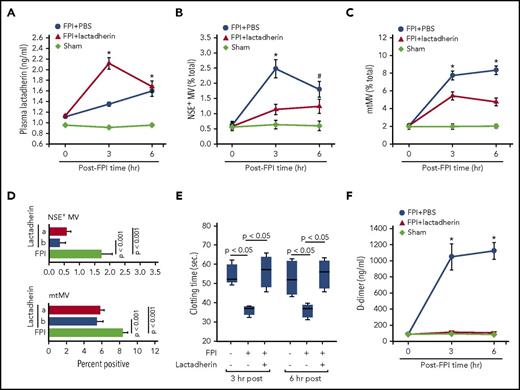
Dynamic changes of plasma lactadherin during acute TBI
We determined that lactadherin was present in the circulation of noninjured C57BL/6J mice at 1.1 ± 0.6 ng/mL. The ability of exogenous lactadherin to prevent FPI-induced coagulopathy would, therefore, suggest that either the basal level of circulating lactadherin is not sufficient to remove the excessive microvesicles acutely released from an injured brain or the lactadherin activity is suppressed by the injury. To distinguish the 2 possibilities, we measured dynamic changes of plasma lactadherin during acute FPI. We found that mouse plasma lactadherin increased steadily by 21.5% ± 8.5% and 43.2% ± 13.9% from the baseline at 3 and 6 hours after TBI, respectively (Figure 6A). Because the antigen level was closely correlated with the scavenging activity of lactadherin (n = 21; R2 = 0.8689; P < .001), the finding suggests that the scavenging activity was not suppressed during acute FPI. Furthermore, mice preconditioned with lactadherin had twice the level of circulating lactadherin at 3 hours but not at 6 hours after FPI (Figure 6A), indicating rapid lactadherin consumption.
Plasma lactadherin and its therapeutic potential for TBI-induced coagulopathy. (A) Plasma levels of lactadherin in sham mice and in FPI mice preconditioned with lactadherin or PBS (n = 15, repeated measures ANOVA; *P < .001 vs sham). Plasma levels of (B) NSE+ MVs and (C) mtMVs in C57Bl/6J mice subjected to FPI and that received 400 μg/kg lactadherin or PBS 30 minutes after injury (sham mice as control, repeated measures ANOVA, *P < .01, #P < .05). (D) A comparison in plasma NSE+ (top) and mtMVs (bottom) between mice preconditioned with lactadherin (a) and those receiving lactadherin after the injury (b; n = 32, 1-way ANOVA). (E) Clotting time and (F) plasma levels of d-dimer in mice receiving lactadherin 30 minutes after TBI (n = 21, repeated measures ANOVA, *P < .01 vs PBS-infused mice).
Plasma lactadherin and its therapeutic potential for TBI-induced coagulopathy. (A) Plasma levels of lactadherin in sham mice and in FPI mice preconditioned with lactadherin or PBS (n = 15, repeated measures ANOVA; *P < .001 vs sham). Plasma levels of (B) NSE+ MVs and (C) mtMVs in C57Bl/6J mice subjected to FPI and that received 400 μg/kg lactadherin or PBS 30 minutes after injury (sham mice as control, repeated measures ANOVA, *P < .01, #P < .05). (D) A comparison in plasma NSE+ (top) and mtMVs (bottom) between mice preconditioned with lactadherin (a) and those receiving lactadherin after the injury (b; n = 32, 1-way ANOVA). (E) Clotting time and (F) plasma levels of d-dimer in mice receiving lactadherin 30 minutes after TBI (n = 21, repeated measures ANOVA, *P < .01 vs PBS-infused mice).
Lactadherin as therapeutic
The rapid lactadherin consumption during acute FPI (Figure 6A) strongly suggests therapeutic potential of lactadherin to prevent TBI-induced coagulopathy. We tested this hypothesis by infusing lactadherin (400 μg/kg) into mice 30 minutes after FPI. This single dose reduced circulating NSE+ MVs (Figure 6B) and mtMVs (Figure 6C) to levels similar to the mice preconditioned with lactadherin before FPI (Figure 6D). It also reversed the FPI-induced hypercoagulable state (Figure 6E-F) to a level similar to that of mice preconditioned with lactadherin before FPI (supplemental Figure 7). For these experiments, lactadherin was given 30 minutes after FPI because TBI-induced coagulopathy develops within 60 minutes after injury in this mouse model.7,15
Discussion
We have recently demonstrated that brain-derived microvesicles, including extracellular mitochondria, are released from traumatically injured brains into the circulation and cause consumptive coagulopathy.7,15 Here, we further show that lactadherin preconditioning prevented mice subjected to severe TBI from developing coagulopathy and cerebral edema, resulting in significantly improved neurological function and survival (Figures 1 and 2). Consistent with the findings from wild-type mice, lactadherin-deficient mice had significantly higher levels of circulating neuronal and mitochondrial microvesicles and developed more severe coagulopathy after TBI (Figure 3D-E). These lactadherin-deficient mice have previously been shown to have significantly more platelet-derived microvesicles as a result of a lower rate of PS-mediated phagocytosis, leading to enhanced thrombin generation and thrombosis.25
Several lines of evidence support our hypothesis that lactadherin exerted its protective effects by enhancing phagocytosis-mediated microvesicle clearance. First, mice preconditioned with lactadherin had lower levels of neuronal and mitochondrial microvesicles (Figure 3B-C), faster clearance of biotinylated BDMVs from the circulation (Figure 4A), and enhanced hepatic accumulation of BDMVs (Figure 4B). The plasma levels of NSE+ microvesicles and mtMVs reduced by 72% ± 29%, and 29% ± 8.8%, respectively, in TBI mice preconditioned with lactadherin. Furthermore, although NSE+ microvesicles and mtMVs were measured in this study, those from platelets, endothelial cells, erythrocytes, and leukocytes are also likely released in response to TBI-induced secondary ischemic and inflammation injures. Consistent with the notion, the total number of annexin V-binding microvesicles that account for procoagulant microvesicles in the circulation increased from 0.9 × 104 ± 1.9 × 102/μL at the preinjury baseline to a post-TBI level of 3.3 × 105 ± 5.8 × 103/μL. These blood cell-derived microvesicles have been shown to express procoagulant anionic phospholipids6 and are likely also removed by lactadherin.22,25
Second, a truncated lactadherin lacking the integrin binding domain failed to promote microvesicle clearance and TBI-induced coagulopathy (Figure 5). The finding that the C1C2 domains of lactadherin are homologous to those of human coagulation factors V and VIII17,18 and bind anionic phospholipids27 raises the possibility that lactadherin can also reduce TBI-induced consumptive coagulopathy by acting as an anticoagulant. However, this is unlikely because the truncated lactadherin, which binds PS with an affinity similar to that of full-length lactadherin,27 did not have the same protective effect as the full-length lactadherin (Figure 5B-E).
Third, the phagocytosis of mtMVs (as the surrogate of BDMVs) by monocytes was directly visualized and found to be anionic phospholipid-dependent (Figure 4C-E), much like the lactadherin-mediated scavenging of apoptotic cells by monocytes and macrophages.28,29 The phagocytosis reduced the rate of BDMV transmigration through the endothelium (Figure 5A), but we cannot exclude other actions of lactadherin. For example, lactadherin may protect or restore the integrity of glycocalyx by interacting with negatively charged sialic acids and phospholipids that are enriched in glycocalyx.30-32 Trauma-induced disruption of glycocalyx has been reported to increase the permeability of the blood–brain barrier.33,34 In addition, the RGD sequence in lactadherin binds the integrins αvβ3 and αvβ5 to promote cell adhesion,16,19,34,35 through integrin-mediated intracellular signaling,36 to repair injured endothelial barriers. This lactadherin–integrin interaction may also promote phagocytosis of BDMVs by endothelial cells, which have been shown to have a phagocytic activity,37 as lactadherin also protects the endothelial barrier independent of monocytes (Figure 5A).
Finally, the rapid consumption of plasma lactadherin after TBI (Figure 6A) strongly suggests that the intrinsic level of circulating lactadherin, although sufficient to maintain a basal level of apoptotic cell-scavenging activity, is insufficient to remove a large number of microvesicles released suddenly into the circulation. This insufficiency develops despite the TBI-induced steady increase in circulating lactadherin (Figure 6A).
In summary, cellular microvesicles are heterogeneous in their morphologies, cargoes, and activities. This heterogeneity leads them to have different effects on various tissues, some detrimental and others beneficial. We have demonstrated in mouse models that enhancing the clearance of procoagulant microvesicles that express the anionic phospholipids PS and CL prevents TBI-induced coagulopathy and improves neurological function in mice subjected to severe TBI. Our findings strongly suggest that lactadherin, as an integral part of the intrinsic apoptotic cell-scavenging system, has significant therapeutic potential for improving outcomes of TBI and other microvesicle-driven pathologies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study is supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke grant NS087296 and National Institutes of Health, National Heart, Lung, and Blood Institute grant HL119391 (J.-f.D.); Natural Science Foundation of China State Key Program Grant 81330029 (J.Z.) and Research Grants 81271361, 81271359 (J.N.Z.), and 81672399 (M.L.); and the Biomedical Laboratory Research and Development Award BX000502 from the Department of Veterans Affairs (P.T.).
Authorship
Contribution: Y.Z. designed and performed experiments, analyzed data, and wrote the manuscript; W.C. designed and performed experiments; Z.Z. designed and performed experiments and wrote the manuscript; T.H. designed and performed experiments and wrote the manuscript; M.W. designed experiments and analyzed data; J.Y. designed and performed experiments; W.L. performed experiments and analyzed data; X.W. designed and performed experiments, analyzed data, and wrote the manuscript; F.Z. designed the study and wrote the manuscript; F.-D.S. wrote the manuscript; P.T. designed experiments, provided reagents, and wrote the manuscript; M.L. developed hypotheses, designed experiments, analyzed data, and wrote the manuscript; J.Z. developed hypotheses, designed experiments, analyzed data, and wrote the manuscript; and J.-f.D. developed hypotheses, designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jing-fei Dong, Bloodworks Research Institute, 1551 Eastlake Avenue East, Seattle, WA; e-mail: jfdong@psbc.org; Jianning Zhang, Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, China; e-mail: jianningzhang@hotmail.com; Min Li, Institute of Pathology, Lanzhou University School of Basic Medical Sciences, Lanzhou, China; e-mail: limin@lzu.edu.cn.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal