In this issue of Blood, Zhou and coworkers show that prophylaxis and treatment with the apoptotic cell-scavenging protein lactadherin prevent traumatic brain injury (TBI)–associated coagulopathy in mice, resulting in markedly improved outcome of this potentially lethal injury.1
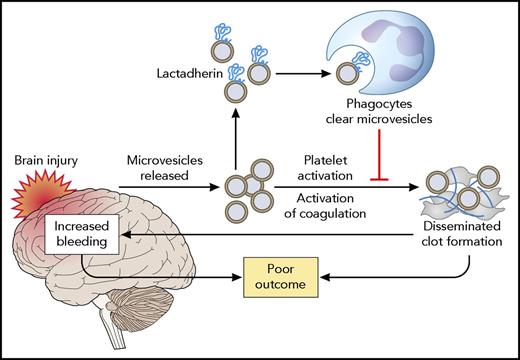
Mode of action of lactadherin in alleviating the consequences of TBI. TBI results in the release of brain-derived microvesicles, which activate coagulation and result in a consumption coagulopathy. Both systemic thrombosis and secondary brain hemorrhage as a result of exhaustion of platelets and coagulation factors result in poor outcome. Lactadherin binds to brain-derived microvesicles, which are subsequently cleared by phagocytes. Professional illustration by Patrick Lane, ScEYEnce Studios.
Mode of action of lactadherin in alleviating the consequences of TBI. TBI results in the release of brain-derived microvesicles, which activate coagulation and result in a consumption coagulopathy. Both systemic thrombosis and secondary brain hemorrhage as a result of exhaustion of platelets and coagulation factors result in poor outcome. Lactadherin binds to brain-derived microvesicles, which are subsequently cleared by phagocytes. Professional illustration by Patrick Lane, ScEYEnce Studios.
TBI is a leading cause of death from trauma. It is well established that brain injury is associated with major changes in the hemostatic system, including thrombocytopenia and platelet function defects, decreased plasma levels of coagulation factors and inhibitors, and activation of fibrinolysis.2 The causes of these hemostatic changes are not completely understood but likely include release of tissue factor from the damaged brain, platelet activation by local tissue or vessel injury, platelet activation by systemically activated endothelial cells (eg, related to shock), activation of fibrinolysis in response to activation of coagulation, and release of fibrinolytic activators from the injured brain. The consequences of hemostatic activation following TBI paradoxically include both bleeding and thrombotic complications. Initially, activation of coagulation may result in disseminated intravascular coagulation, which leads to consumption of platelets and coagulation factors. Subsequently, bleeding complications occur, which are likely related to the consumption of platelets and hemostatic factors in the initial phase. Finally, those patients that survive the initial injury are at substantial risk for development of venous thrombotic disease, which may be related to a rebound hypercoagulable state that may or may not be related to prohemostatic therapy given in the acute phase.
The hemostatic changes associated with TBI are frequently referred to as “coagulopathy,” which is a somewhat vague and misleading term, as both thrombotic and bleeding complications can occur in these patients, sometimes even simultaneously. Nevertheless, patients with severe hemostatic changes (eg, defined as a prolonged prothrombin time or activated partial thromboplastin time or significant thrombocytopenia) have a worse outcome compared with patients without coagulopathy.3 This relation is related, at least in part, to an increased risk of (intracranial) bleeding. Treatment of the coagulopathy by blood product transfusions or factor concentrates has been shown to increase survival,4 which is presumably linked to decreased bleeding complications. Although much progress in the treatment of TBI-associated coagulopathy has been made, current treatments are solely directed at reversal of the coagulopathy. The study by Zhou and coworkers suggests that it might be possible to prevent coagulopathy by specifically neutralizing procoagulant brain-borne microvesicles.
Earlier studies by this group have demonstrated that brain-derived microvesicles are released into the systemic circulation following TBI in mice.5,6 These microvesicles were shown to induce thrombocytopenia, fibrinogen consumption, and fibrin deposition in the lung, kidney, and heart. The procoagulant activity of brain-derived microvesicles, which include both vesicles derived from the plasma membrane of brain cells and brain-derived mitochondrial microvesicles, is related to their negative charge, which is provided by phosphatidylserine for plasma membrane–derived vesicles and by cardiolipin for mitochondrial vesicles. The present study builds on these findings to show that enhanced removal of these procoagulant brain-derived microvesicles by infusion of lactadherin prevents coagulopathy. Importantly, lactadherin infusion markedly improved the outcome of TBI, as assessed by the extent of injury, survival, and neurological performance in surviving mice (see figure).
Lactadherin is a protein that naturally occurs in plasma. It binds negatively charged phospholipids and has been implicated in regulation of coagulation and removal of negatively charged microvesicles from circulation.7-9 The phagocytic effect of lactadherin involves an Arg-Gly-Asp (RGD) motif that interacts with integrins such as αvβ3 on macrophages and other phagocytic cells. Zhou and coworkers demonstrate that administration of exogenous lactadherin decreases coagulopathy and improves outcome of TBI, whereas lactadherin deficient mice have exaggerated coagulopathy. In addition, they demonstrate that lactadherin administration specifically promotes clearance of brain-derived microvesicles via a mechanism involving the lactadherin RGD motif, and clearance of these microvesicles underlies the neutralizing effects of lactadherin on TBI-associated coagulopathy. Although lactadherin was administered prophylactically in the majority of experiments, it was also demonstrated that lactadherin blunted the coagulopathy when administered after brain injury.
In aggregate, these preclinical experiments suggest that therapeutic strategies aimed at removal of procoagulant brain-derived microvesicles would have merit in preventing severe coagulopathy in patients with TBI, with potentially substantial effects on outcome. Because lactadherin is an endogenous protein, infusion of lactadherin concentrates might be a feasible clinical strategy. What steps are required before such a strategy is ready to be tested clinically? First, additional studies on the therapeutic effects of lactadherin in this model will be required to assess whether administration of lactadherin after the brain injury will improve not only coagulopathy but also outcome. Second, studies on the clinical relevance of brain-derived microvesicles in humans should be performed. A recent small study found increased levels of brain-derived microvesicles in plasma from patients after TBI compared with controls.10 However, the increase in levels appears much more modest in humans than in the mouse model. Larger human studies assessing a relation between brain-derived microvesicle level and outcome, and studies on the thrombogenicity of human brain-derived microvesicles will be of interest. Third, although the mode of action of lactadherin to prevent TBI-induced coagulopathy was shown to involve microvesicle clearance, a second biological effect of lactadherin in circulation is a direct anticoagulant effect.8 This anticoagulant effect may overrule the beneficial effects of microvesicle clearance, potentially leading to aggravation of TBI-induced bleeding. Notwithstanding these hurdles, the series of studies performed by this laboratory on the causes and potential treatment options of TBI-associated coagulopathy in mice has provided exciting new insight in the pathogenesis of the poorly understood hemostatic changes in these patients. The concept of removal of pathogenic brain-derived microvesicles to prevent or halt coagulopathy, which results in improved outcome, is exciting and will hopefully receive attention in the clinical arena.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal