In this issue of Blood, Palladini et al address one of the most important, yet befuddling questions in immunoglobulin light chain (AL) amyloidosis treatment: what triggers should be used to reinstitute plasma cell clone directed therapy among patients with AL amyloidosis?1
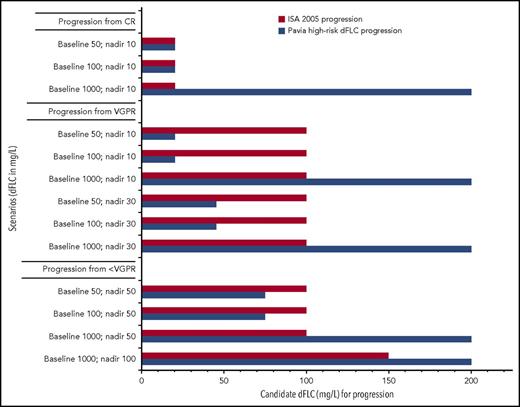
Progression scenarios comparing ISA 2005 progression criteria to “Pavia high-risk dFLC progression.” Y-axis, Different examples of baseline and nadir values; x-axis, candidate dFLC value at time of progression for each system. For this figure, the assumption is made that patients do not have measurable M-spikes in the urine or serum at the time of progression. ISA hematologic progression (1) from CR, any detectable monoclonal protein, or an abnormal FLC ratio (light chain must double) and (2) from PR or stable response, a 50% increase in serum M-protein to >5 g/L or 50% increase in urine M-protein to >200 mg per day or FLC increase of 50% to at least 10 mg/L. Pavia “high-risk dFLC progression” requires all of the following: (1) an absolute value of dFLC of >20 mg/L; (2) a dFLC level that is at least 20% of the baseline value; and (3) a dFLC that is at least 50% higher than the nadir dFLC achieved posttherapy.
Progression scenarios comparing ISA 2005 progression criteria to “Pavia high-risk dFLC progression.” Y-axis, Different examples of baseline and nadir values; x-axis, candidate dFLC value at time of progression for each system. For this figure, the assumption is made that patients do not have measurable M-spikes in the urine or serum at the time of progression. ISA hematologic progression (1) from CR, any detectable monoclonal protein, or an abnormal FLC ratio (light chain must double) and (2) from PR or stable response, a 50% increase in serum M-protein to >5 g/L or 50% increase in urine M-protein to >200 mg per day or FLC increase of 50% to at least 10 mg/L. Pavia “high-risk dFLC progression” requires all of the following: (1) an absolute value of dFLC of >20 mg/L; (2) a dFLC level that is at least 20% of the baseline value; and (3) a dFLC that is at least 50% higher than the nadir dFLC achieved posttherapy.
In general, patients whose serum difference between involved and uninvolved free light chain (dFLC) is driven to the lowest levels have the highest likelihoods of improved organ function and better overall survival in both the upfront and relapsed settings.2 Moreover, among patients with newly diagnosed AL amyloidosis, those who present with very low light chains (eg, dFLC <50 mg/L) have significantly better prognoses than those who do not.3-5 The presumption is that these low dFLC patients are diagnosed earlier than those with higher dFLC, and this lower tumor-burden state and lead-time bias lead to less organ dysfunction and better outcomes.
In the relapsed setting, however, we have not fully come to terms with the issue of how much rise from nadir is sufficient to reinstate therapy.3,6,7 This ambiguity is in part the legacy of our knowledge of how fragile patients with AL amyloidosis can be, the history of having very limited options to treat the plasma cell clone, our partial reliance on modeling response and progression on myeloma models, and limitations of the assays used to measure response and progression. Unlike in myeloma, we are largely dependent on the serum free light chain (FLC) alone to measure hematologic response.
In the setting of previously treated patients, it is logical to think of progression of FLC in the context of the initial baseline of FLC at diagnosis, the best FLC achieved (ie, the nadir), and the context of the limitations of the FLC assay. Palladini and colleagues do just that and present us with a definition of “high-risk dFLC progression,” which is satisfied if all of the following criteria are met: an absolute value of dFLC of >20 mg/L, a dFLC level that is at least 20% of the baseline value, and a dFLC that is at least 50% higher than the nadir dFLC achieved posttherapy. Examples of scenarios of baseline and nadir dFLC values and the dFLC that would satisfy progression using the 2005 International Symposium of Amyloidosis (ISA) definition of progression8 and the Pavia “high-risk dFLC progression” are shown in the figure. If we compare the performance between the “high-risk dFLC progression” and that of the 2005 ISA criteria, both systems perform similarly for patients relapsing from complete response (CR) with the exception of patients who have a very high dFLC at baseline. The disagreement between the systems is most evident for those patients whose best response is very good partial response (VGPR), and the differences are less dramatic for patients progressing from less than VGPR.
Using the “high-risk dFLC progression” definition in the subset of 92 patients these experts had decided to retreat (their definition of relapse for the purposes of their analyses), the authors found that the 60 patients who satisfied criteria for “high-risk dFLC progression” had a significantly higher risk of death and were more likely to have signs of cardiac progression. The implication of suggesting that a patient with “high-risk dFLC progression” who may have a dFLC of only 20 mg/L be started on therapy opens an additional can of worms in terms of trying to measure hematologic response, especially in the context of clinical trials targeting patients with progressive or relapsing disease. The current consensus for “measurable” clonal disease in AL amyloidosis is a dFLC of at least 50 mg/L,9 which is based in part on the knowledge that the analytic coefficient of variance for the serum FLC is about 6% and the within-person biologic variation has been calculated to have a coefficient of variance of 34%.10 Palladini and colleagues and other recent papers6,7 challenge this consensus definition of 50 mg/L being the minimal measurable disease due to concerns about the risk of waiting too long to reinstitute therapy.
Besides the fact that this study was a single-center retrospective analysis, it has other limitations that will need to be clarified and validated in other series, including: (1) no transplanted patients were included in the series; (2) only patients who achieved a VGPR or better or a partial response (PR) with organ response were included; (3) no data are provided to demonstrate how this definition would apply to the 167 patients the authors continue to observe without reinstituting therapy; (4) no information is provided to inform how this system will work in patients with renal impairment; and (5) the multivariate analysis was underpowered to clarify the interaction between “high-risk dFLC progression” and baseline characteristics like extent of response (less than VGPR) and baseline cardiac function (more than cardiac stage I).
Despite these limitations, their definition of “high-risk dFLC progression” is at a minimum a starting point to move the amyloid community to a consensus of when to reinstitute therapy. This article sets the stage for amyloid experts to work together to answer 2 important questions. First, is “high-risk dFLC progression” a reproducible concept? Second, can meaningful hematologic response end points be derived from therapeutic trials that allow patients to be enrolled with a dFLC of 20 mg/L? Time will tell, but these authors are definitely onto something of importance.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal