Abstract
Hematopoietic stem cells (HSCs) ensure a balanced production of all blood cells throughout life. As they age, HSCs gradually lose their self-renewal and regenerative potential, whereas the occurrence of cellular derailment strongly increases. Here we review our current understanding of the molecular mechanisms that contribute to HSC aging. We argue that most of the causes that underlie HSC aging result from cell-intrinsic pathways, and reflect on which aspects of the aging process may be reversible. Because many hematological pathologies are strongly age-associated, strategies to intervene in aspects of the stem cell aging process may have significant clinical relevance.
Introduction
The average human life expectancy has increased consistently during the past 150 years. The absolute number of elderly people has increased substantially in many societies, and this is not likely to come to an end soon. Although it is evident that many elderly people reach advanced ages in healthy conditions, the prevalence of age-dependent disease and the average age of patients who enter the clinic are increasing. This is also true for patients that suffer from hematological diseases because many hematological conditions are strongly age-dependent. From a clinical perspective, this raises 2 related, yet distinct, considerations. First, how does the hematopoietic system change with age, how does this lead to a spectrum of hematological conditions, and would it be possible to pharmacologically intervene in this process? Second, if hematological disease presents in an elderly patient, should this be treated differently than if it had occurred in a young patient? Although the latter is also of significant clinical interest,1 this manuscript will focus on our understanding of the molecular mechanisms that make hematopoietic stem cells (HSCs) age. Only if we understand how HSC age, we can begin exploring opportunities to prevent, delay, or even reverse aspects of the aging process.
HSC self-renewal and aging
Although research on aging has for a long time been relatively descriptive, much progress has been made in the past decade to uncover the molecular drivers of biological aging. An influential review paper that describes the hallmarks of aging provides a comprehensive overview of the various pathways that are believed to result in age-dependent cellular and organismal functional decline.2 One of these so-called hallmarks relates to stem cell exhaustion. Stem cell exhaustion refers to the gradual functional decline of adult, tissue-specific stem cells to maintain homeostasis of the tissue in which they reside. Stem cells are critically defined by their ability to self-renew, a concept that seems difficult to reconcile with the notion of aging; either stem cells are able to self-renew and therefore not age, or alternatively, stem cells age and therefore do not truly self-renew.
In the hematopoietic system, there are multiple indications that stem cells do not formally self-renew, or at least have a restricted self-renewal potential and are therefore fundamentally different from pluripotent embryonic stem or pluripotent stem cells. What are these indications? Historically, experimental hematologists have carried out serial stem cell transplantations in mice to assess the potential of these cells to multiply in vivo in myeloablatively conditioned recipients.3 Although serial transplantations are obviously quite artificial (yet, they have also been performed in rare patients),4 collectively these studies document that after transplant the HSC compartment never returns to normal values.5-7 If stem cells are submitted to increased proliferative stress (by transplanting a low number, or even a single stem cell8 or by initiating serial transplantations in short intervals),9 self-renewal is impeded more severely. Most notably, if stem cells that were first harvested from an aged donor mouse are serially transplanted in young recipients, the self-renewal capacity is much lower compared with stem cells isolated from young donor mice.10 If stem cells from a short-lived mouse strain are compared with stem cells from a long-lived mouse strain, the latter stem cells outcompete their shorter lived counterparts.11 In general, in all studies in which young HSC were competed in transplantation studies against aged stem cells, without exception the young stem cells are functionally superior.12-16 These young stem cells produce, at the single-cell level, more mature peripheral blood cells10,14 and are better able to produce both myeloid and lymphoid cells in a balanced manner.10,17
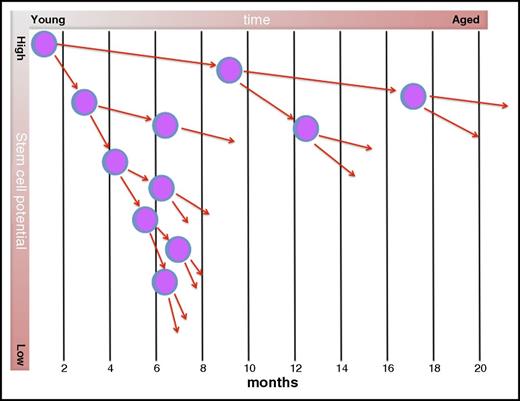
There is ample evidence that during steady-state conditions the most primitive HSC are quiescent and only divide rarely. The concept of HSC quiescence emerges from studies that have shown that stem cells are refractory to cell cycle–specific cytotoxic drugs, such as 5-fluorouracil,18 that stem cells are not easily labeled with 5-bromo-2′-deoxyuridine,19,20 and that it can take many months before they contribute to hematopoiesis after transplant.21,22 Very recently, an elegant study showed that the most primitive of all HSC may undergo only 4 to 5 divisions in the lifetime of a mouse.23 This study suggested that HSC possess some sort of cellular memory and that the typical age-dependent phenotype of HSC only emerges after HSCs have divided 5 times. A very similar concept had been hypothesized to exist by 1 of us more than 2 decades ago.24 Collectively, this indicates that most of the proliferative burden resides with committed progenitors, and that during steady-state hematopoiesis the most primitive stem cells are largely inactive. It seems plausible that with each cell division, the potential of a HSC to contribute to blood cell production declines and that, simultaneously, the pool of stem cells with reduced potential increases to compensate for loss of function of individual stem cells (Figure 1).
Hypothetical tracing of the offspring of a single HSC during aging. In mice, the most primitive HSC are believed to cycle only once every ∼4 months.23 With each cell division, daughter cells lose developmental (long-term repopulating) potential, such that each daughter is less potent than its ancestor. Cell-cycle times decrease with developmental stage. In young mice, the pool of stem cells is small, but the potency of each stem cell is high. In aged mice, the pool of stem cells has expanded, but their functionality is restricted. Adapted from Van Zant et al24 and Jung et al.25
Hypothetical tracing of the offspring of a single HSC during aging. In mice, the most primitive HSC are believed to cycle only once every ∼4 months.23 With each cell division, daughter cells lose developmental (long-term repopulating) potential, such that each daughter is less potent than its ancestor. Cell-cycle times decrease with developmental stage. In young mice, the pool of stem cells is small, but the potency of each stem cell is high. In aged mice, the pool of stem cells has expanded, but their functionality is restricted. Adapted from Van Zant et al24 and Jung et al.25
Most of what we know about HSC aging is based on studies that have used the mouse as an experimental model. As a consequence, it is not fully clear to what extent these molecular mechanisms also play a role in human HSC aging. However, decades of research have shown that, conceptually, the formation of blood cells in mouse and human is identical, and it is plausible that mechanisms that contribute to stem cell aging in mice also do so in human. Indeed, it has been demonstrated that telomeres progressively shorten in human HSCs isolated from fetal liver, cord blood, or adult bone marrow, which is associated with a strongly reduced proliferative potential.26 In addition, an age-dependent increase in the frequency of putative stem cells, coinciding with impaired functionality and reduced lymphoid potential, has also been in the human system.27-29 However, it should be recognized that essentially all experimental studies have used C57BL/6 mice and that aging (also of the hematopoietic system) is at least qualitatively different in distinct strains of mice.30,31 These genetic disparities are also likely to be prominent in human.
Heterogeneity of HSC aging
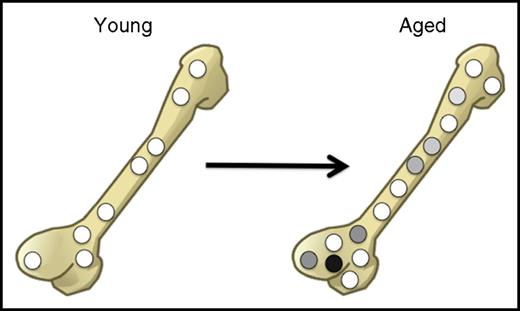
Although there is consensus on the functional decline of aged HSC in the mouse, the molecular mechanisms that contribute to such stem cell aging are less clear and are at times disputed. Our limited insight into the molecular mechanisms that cause HSC to age may result from the fact that most studies in this field have been performed using populations of inevitably heterogeneous HSCs. Although flow cytometry has allowed the prospective isolation of single cells, the purity of HSCs, at least when assayed in transplantation experiments, is never more than 50%. Therefore, even after the most stringent enrichment protocols, many non-HSCs (mostly progenitors) remain. This is particularly problematic in the aging field because there is solid evidence that the functional heterogeneity of the HSC population increases with age. Although many HSCs in young mice behave qualitatively similarly, in aged mice that display an overall increase in HSC pool size, individual HSCs behave very differently.10,13,17,32,33 Therefore, it remains possible that even in aged mice, there are still substantial numbers of very potent (“young-like”) HSCs, which are diluted by an expanded pool of less potent aged cells (Figure 2).
In aged mice, the absolute number of cells with regenerative potential increases, but the extent to which individual aged cells contribute to blood cell production becomes highly variable. The relative frequency of stem cells with high regenerative potential (white) compared with cells with low regenerative potential (gray and black) thus decreases upon aging.
In aged mice, the absolute number of cells with regenerative potential increases, but the extent to which individual aged cells contribute to blood cell production becomes highly variable. The relative frequency of stem cells with high regenerative potential (white) compared with cells with low regenerative potential (gray and black) thus decreases upon aging.
To provide further insight into the age-dependent increase in heterogeneity of the HSC pool, the identification of cell surface markers that allow prospectively isolating of these different populations will be required. Multiple single-cell RNA sequencing studies have been performed to elucidate at the molecular level how heterogeneity may be explained.34-36 Combinatorial single-cell techniques such as single-cell transplants, flow cytometry, and single-cell RNA have already begun to and will continue to identify HSC subsets that contribute to aging phenotypes. This strategy will boost research into molecular mechanisms of aging through the ability of identifying candidate marker genes (eg, receptors) for the isolation and functional characterization of “aged” HSCs.
Cell-intrinsic vs cell-extrinsic mechanisms
A topic of much dispute is whether HSC aging is caused by cell-intrinsic or cell-extrinsic parameters. This is not only of academic importance, but also has major implications for potential future interventions. If stem cell aging were largely intrinsically controlled, this would render putative interventions more cumbersome than if aging were the result of an extrinsic perturbation. Experiments with parabiotic mice have attracted attention (understandably, also from the lay press) because the suggestion has been raised that unidentified humoral factors that circulate in the blood of young mice could possibly restore cellular functioning in aged mice. Several studies have reported beneficial, arguably antiaging, effects of young blood on aged vasculature, brain, and muscle,37-41 but no beneficial effects on aged HSC have been reported. It is important here to remember that essentially all we know on the phenotype of aged HSC is based on studies in which old stem cells were transplanted into lethally irradiated young recipients.5,10-12,15 That aged HSC are functionally impaired compared with their young counterparts upon transplantation in young mice strongly suggests that HSC aging manifests largely as a consequence of cell-intrinsic molecular changes. Thus, although cell-intrinsic changes may be partially dependent on and initiated by changes that occur in the bone marrow microenvironment, transplanting aged HSCs in a young environment apparently cannot reverse these changes. It is evident that during aging many cellular and structural changes occur in the bone marrow microenvironment,42 and it is possible that these as-yet poorly understood cell extrinsic changes affect stem cell intrinsically. Indeed, it has been demonstrated that young bone marrow cells, when transplanted into aged recipients, engraft worse than when transplanted into young recipients.12,43 Also, hematological malignancies can result from a defective bone marrow environment.44
Cell-intrinsic mechanisms that cause HSC aging
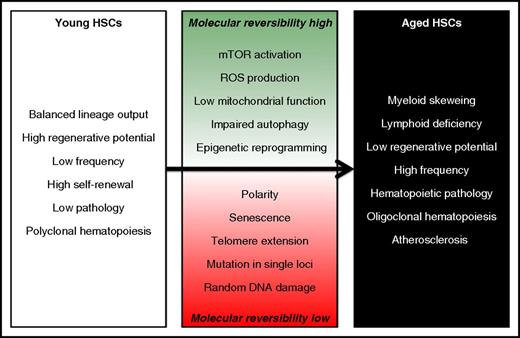
Multiple molecular and cell-intrinsic mechanisms have been reported to contribute to the age-associated decline of HSC functioning (partially reviewed in Geiger et al45 ). Although mechanistically it may be feasible, and even useful, to separately discuss these multiple aging pathways, in effect they are likely to be highly interdependent and interconnected. Although some of these cell-intrinsic causes of aging are unlikely to be reversible, several others might allow interventions and thus could potentially be explored for pharmacological targeting (Figure 3).
Cell-intrinsic mechanisms that contribute to HSC aging. Although some of these molecular events are difficult to revert, other may be amenable to pharmacological interventions and could be exploited to rejuvenate HSCs. ROS, reactive oxygen species.
Cell-intrinsic mechanisms that contribute to HSC aging. Although some of these molecular events are difficult to revert, other may be amenable to pharmacological interventions and could be exploited to rejuvenate HSCs. ROS, reactive oxygen species.
DNA damage
The most irreversible cause of HSC aging relates to the accumulation of random DNA damage. Mice, or patients for that matter, suffering from mutations in genes encoding for proteins involved in DNA repair display many aspects of premature stem cell aging.46-48 In addition, aged HSC accumulate signatures of widespread DNA damage, including γ-H2AX foci.49 To what extent normal HSC aging is caused by genetic damage remains unclear. Conceptually, if indeed HSC are largely quiescent and divide only rarely in the lifetime of a mouse, it is difficult to understand how accumulation of DNA damage could causally contribute to stem cell dysfunctioning. However, it is possible that cells immediately downstream of quiescent HSCs, which have been shown to comprise mostly myeloid-biased HSCs,50 are target cells to accumulate DNA damage.51
Beyond random DNA damage, it has recently been shown that in healthy elderly individuals, DNA mutations in specific loci are associated with the establishment of clonal hematopoiesis52 (discussed later). A specific kind of DNA damage is caused by erosion of telomeres. Although the involvement of telomere shortening in the functional decline of HSC is particularly evident in human,53 in mice with long telomeres, the inability to maintain telomere length is associated with severe HSC malfunctioning.54 Although the length of telomeres in HSC can be increased by enforced overexpression of telomerase, in mice this does not rescue functional impairment.55
Senescence
In many tissues, irreversibly cell cycle–arrested senescent cells accumulate during normal aging.56 Conceptually, senescence of stem cells is a “contradictio in terminis” (because an irreversibly arrested stem cell ceases to be a stem cell). Senescence is believed to be predominantly induced by activation of p16, and indeed expression of p16 is considered to be a marker for the presence of senescent cells. The genetic or pharmacological depletion of senescent cells in mice has been shown to enhance regenerative potential and extend life span.57 Although expression of p16 in aged primitive HSC has been contested,58 pharmacological targeting of senescent bone marrow cells has been shown to have beneficial effects.59 This suggests that putative senescent cells in the bone marrow may secrete factors that negatively affect HSC potential.
Polarity
It has been reported that the asymmetric distribution of specific proteins, collectively referred to as “increased polarity,” is a prominent feature of aged HSCs, whereas in young HSCs this is much less obvious.33,60 Unequal distribution of these proteins is believed to be caused by elevated activity of Cdc42. Interestingly, inhibitors of Cdc42 restore polarity in aged HSCs and improve HSC functioning after transplant. Although it is not straightforward to understand how an acute reversal of the asymmetric distribution of proteins can have long-term effects on HSC functioning, this study demonstrates that at least some aspects of the aging process appear to be reversible.
Impaired autophagy and mitochondrial activity
A large fraction of aged HSCs show impaired levels of autophagy.33 Autophagy is generally associated with recycling of organelles, and impaired autophagy in aged HSCs appears to specifically result in the accumulation of mitochondria, which in turn induces metabolic stress. It has been demonstrated that high levels of reactive oxygen species, generated by mitochondria, accumulate in aged HSCs and compromise their functioning.61,62 In addition, reducing mitochondrial stress in aged HSCs can reverse loss of stem functioning.62 Mitochondrial dysfunctioning caused by accumulating mitochondrial DNA mutations has been shown to cause multiple hematopoietic defects that are typically seen in the elderly, but HSCs themselves appear to relatively resistant.63 Taken together, cellular metabolism, controlled by mitochondrial status and reactive oxygen species and mTOR signaling, play an important role in maintaining HSC function throughout life, but the molecular cause of age-dependent metabolic derailment remains unclear. Importantly, however, pharmacological interventions in these signaling pathways are feasible and may be exploited to restore function in aged HSCs.
Epigenetic reprogramming
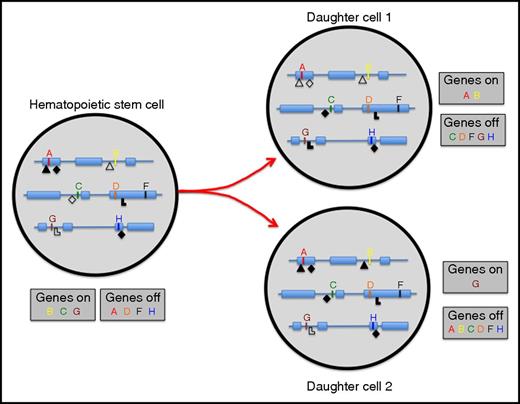
Whereas it is not immediately evident how large-scale random DNA damage would accumulate in aged HSCs, which do barely proliferate, it is not difficult to see how an accumulation of aberrant epigenetic marks could readily but gradually lead to loss of stem cell potential. For a true self-renewal division to occur, an HSC must, in the timeframe of a single division, not only copy and distribute its entire genome across 2 daughter cells, but also the plethora of epigenetic marks that cover each and genomic locus must be faithfully reproduced in at least 1 of the 2 daughter cells. It seems highly likely that not all epigenetic moieties that are required to specify stem cell functioning are properly maintained after a stem cell has divided (Figure 4).
Repressive (closed symbols) and activating (open symbols) epigenetic marks cover all genes and affect transcriptional status. If an HSC divides, all genomic and epigenomic information must be properly propagated to daughter cells. If epigenetic marks are lost or gained, genes that should be expressed in HSC (B, C, and G) may become repressed; conversely, genes that should be repressed (A, D, F, and H) may become activated. As a consequence, functional stem cell activity of daughter cell 1 and 2 may be reduced compared with the HSC from which they derive.
Repressive (closed symbols) and activating (open symbols) epigenetic marks cover all genes and affect transcriptional status. If an HSC divides, all genomic and epigenomic information must be properly propagated to daughter cells. If epigenetic marks are lost or gained, genes that should be expressed in HSC (B, C, and G) may become repressed; conversely, genes that should be repressed (A, D, F, and H) may become activated. As a consequence, functional stem cell activity of daughter cell 1 and 2 may be reduced compared with the HSC from which they derive.
Whereas loss or gain of specific epigenetic marks at defined loci may remain inconsequential, collectively chromatin modifications are important to maintain transcriptional fidelity. The relevance of these epigenetic marks is best exemplified by the fact that perturbation of a large number of epigenetic writers or erasers severely affects HSC function.64-68 The gradual and random erosion of epigenetic marks as stem cells divide and age, provides a conceptual framework which can explain why, at least in the mouse, stem cells can make only a very limited number of true self-renewal divisions. Because the erosion of epigenetic marks is likely to be different for each stem cell, with age increased functional cell-to-cell variability is expected to develop. Indeed, this is exactly what has been observed in experimental studies.10,32 Such increased functional heterogeneity is likely the result of increased transcriptional “noise,” caused by aberrant epigenetic fidelity. This scenario would also suggest that there are no specific “aging” genes, but rather that aging is caused by the cumulative and combinatorial effect of large collections of stochastically differentially expressed genes. In agreement, several distinct gene expression profile studies have not been able to identify many commonly differentially expressed transcripts, and conversely, transcripts that have shown to be affected during aging in 1 study have often not been confirmed in others.12,69-73
The involvement of epigenetic regulation in maintaining proper stem cell transcriptional activity and functioning during aging is also suggested by an increasing number of clinical studies in which mutations in genes encoding for epigenetic enzymes have been found in either elderly people whose hematopoietic system has become oligoclonal, or in patients with myelodysplastic syndromes or acute myeloid leukemia.52 Epigenetic genes that are relatively frequently affected in these conditions include DNMT3A, EZH2, TET2, and SETDB1.74-77 Although the enzymatic activity of these proteins is rather well understood (DNA methylation, H3K27 tri-methylation, DNA demethylation, and H3K9 tri-methylation, respectively), their effect on stem cell functioning is far from clear. Without going into depth here on the specific molecular pathways in which these epigenetic writers are involved, it appears very likely that mutations in these genes subtly alter the epigenetic memory of stem cells that, because of large-scale (but again, potentially subtle) transcriptional consequences, increases self-renewal potential of mutant cells. Thus, with time, in the bone marrow cells in which these self-renewal–enhancing mutations have occurred are expected to expand clonally. Indeed, studies in mice have revealed that (enforced) altered expression of wild-type or mutant epigenetic writers affects self-renewal of HSCs.64,68,78
Clonal hematopoiesis in human
As referred to previously, clonal hematopoiesis is a frequent event in elderly people. The first studies that reported on this phenomenon assessed whether X-chromosome inactivation patterns were random or skewed in peripheral blood cells of elderly females.79,80 Indeed, these early studies suggested that during aging, blood cells are derived from fewer and fewer stem cells. Much more recently, these early findings have been confirmed and significantly extended by multiple independent large-scale sequencing studies.74-77 In addition, these latter studies have shown that clonal hematopoiesis in otherwise healthy individuals increases the risk to develop leukemia, and, interestingly, cardiovascular disease, and thus is associated with increased mortality. This raises the question of whether clonal hematopoiesis is detrimental, and if so, why? It is important to realize that in many perfectly healthy very aged individuals, prominent clonal hematopoiesis is present without any signs of disease.74,81 Yet, that age-dependent clonal hematopoiesis is associated with disease suggests that, at least in these individuals, there may be a causal relationship. Whether mutations in epigenetic genes believed to cause clonal hematopoiesis are truly oncogenic remains unclear. It is possible that these mutations merely increase self-renewal of benign HSC and thus lead to clonal expansion of healthy stem cells. Mutations that are truly leukemogenic are then more likely to occur in such a pool of actively self-renewing stem cells; thus, when patients present with full-blown disease, leukemic cells frequently display mutations in epigenetic genes. In such a scenario, it would probably not be appropriate to refer to these genes as leukemia-initiating events.
That subjects with clonal hematopoiesis are more susceptible to develop nonhematological, most notably cardiovascular, disease, suggests that these mutations also affect the functioning of fully differentiated cells, such as monocytes and macrophages. These end-stage cells have been long known to play a role in vascular remodeling, and an age-dependent impaired functioning could explain their association with cardiovascular problems. In fact, whereas ample attention has focused on the aging of HSC themselves (including the present manuscript), we know much less about the functioning of fully mature peripheral blood cells that are derived from these aged stem cells. It is well established that aged red cells and aged platelets display impaired functioning,82 but is it also true that red cells and platelets derived from aged stem cells show loss of functioning? It is intriguing that clonal hematopoiesis has been associated with increased incidence of atherosclerotic cardiovascular disease, suggesting that the function of monocytic cells derived from these aged stem cell clones is impaired.83,84
Although it has now been well established that clonal hematopoiesis is relatively commonly seen in elderly individuals, it is important to realize that our general understanding of clonal stem cell contribution in hematopoiesis is very limited. We do not know at present how many stem cells actively contribute to blood cell formation during life. Various models have been proposed,85 ranging from clonal succession (at any given time, a few clones are present that become exhausted and are replaced by new clones),86 clonal stability (many clones stably contribute and do so throughout life),87 dynamic repetition (many clones contribute but do so at highly distinct efficiencies),88 or stochastic behavior (clones contribute randomly, their contribution may vary dramatically with time, they may become extinct or resurface without any apparent pattern).89 The uncertainty as to how many stem cells contribute to steady-state hematopoiesis during aging results from the fact that historically clonal descent of blood cells has been difficult to trace. For clonal analyses unique heritable markers must be present that discriminate cells from 1 clone from the other.
Whereas large-scale sequencing efforts that detect spontaneous mutations and their allele frequencies in humans provided insight into clonal makeup in humans, much more precise measurements have been made in experimental conditions using a variety of transgenic approaches. Initial approaches aimed to specifically provide insight into clonal stem cell contribution involved ex vivo barcoding of purified HSC with retro- or lentiviral vectors that contain unique DNA tags, which were subsequently transplanted into recipient mice90-93 (reviewed in Bystrykh et al85 ). Later studies embarked on in vivo DNA barcoding of stem cells, thereby avoiding the transplantation process.94,95 Most recently, in vivo clonal marking in transgenic mice and fish has been carried out using fluorescent dyes as tags.96,97 Potentially as a result of the variety of clonal marking and analyses approaches used, the consistency among these studies is limited. Whereas some studies indicate that only a limited number of stem cells robustly contributes to blood cell formation,14,96 others suggest that hematopoiesis is highly polyclonal.90,94 Irrespective of how these differences will ultimately be reconciled, it is evident from many experimental studies that mice in which blood cell production is derived from even a single stem cell, are not necessarily prone to develop leukemia.10,13,32 Thus, oligo- or even monoclonality is in and of itself not a (preclinical) sign of pathology.
Is stem cell aging similar in different tissues?
In the field of stem cell biology, comparisons between different stem cell–containing tissues are frequently made, with the assumption that at least some general characteristics of stem cells may be conserved across tissues. Although by definition all tissue-specific stem cells are characterized by their self-renewal potential, it is not at all clear whether stem cells age similarly in distinct tissues. In fact, in comparing intestinal stem cells with those of the hematopoietic system, it appears as if aging is very different in these 2 tissues. Whereas in the hematopoietic system, stem cell turnover is very low, proliferation rates in the intestine are very high.98 Intestinal stem cells do, as expected, accumulate random DNA damage,99 but they do not show functional decline during normal aging.100 It will be interesting to assess why rapidly turning over intestinal stem cells do not age and slow-turning over HSC do. In this respect, it is interesting that another important difference between blood and gut is the extent to which plasticity occurs in progenitor cells in these respective tissues. In the intestine, progenitors have been shown to be able to revert to a stem cell fate,101 but in the hematopoietic system such progenitor-to-stem cell conversions have never been described, at least not during steady-state in vivo hematopoiesis. However, enforced expression of transcription factors or microRNAs has been shown to be able to generate transplantable stem cells from committed cell types.102-104
Future perspectives
Assuming that in human HSC aging is not fundamentally different than in mice, it is interesting to speculate that the age-dependent increase of the stem cell pool size is an important factor in the increase of prevalence of myeloproliferative diseases and leukemia in the elderly. An expansion of the pool of primitive cells would increase the number of target cells for malignant derailment. Alternatively, aged stem cells would intrinsically be more susceptible to malignant transformation, possibly as a result of an altered epigenetic landscape that may have accumulated as a result of repeated proliferation. Also, there is some evidence (but not a lot) that the same oncogenic mutation results in a more aggressive disease in aged stem cells compared with young cells.105 If indeed, as in mice, aged human stem cells undergo very few divisions, it seems implausible that these primitive cells themselves are prone to accumulate multiple mutations.
Would monitoring of HSC functionality during human aging be possible or indeed useful? At this point, the answer to both of these questions is negative; there is no reliable assay that can quantify human stem cell aging, and if there were such an assay, there are no obvious intervention strategies available. However, our understanding of HSC aging is increasing very rapidly, and we may in the near future well be able to identify individuals who display enhanced stem cell aging. This may offer opportunities to intervene in the kinetics with which HSCs age. Antiaging interventions may be aimed to prevent, delay, or, most speculatively, reverse aspects of the stem cell aging process. Studies in other tissues have demonstrated that at least delaying components of the aging process can be a viable strategy,106 and there is no fundamental reason to believe that the hematopoietic system would be exempt for such approaches. In fact, reversal of some aging phenotypes has been achieved by reprogramming old HSCs to pluripotency and subsequently generating definitive hematopoiesis from these induced pluripotent stem cells.107 Future interventions may be dietary, pharmacological, or eventually cell therapeutically.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Studies in the laboratory of G.d.H. are supported by grants from the European Union FP7 MARie CuRIe AGEing Network (contract 316964), the Dutch Cancer Society (RUG 2014-7178), The Netherlands Organization for Scientific Research/ZonMW, and the Mouse Clinic for Cancer and Ageing, funded by a grant from the Netherlands Organisation of Scientific Research.
Authorship
Contribution: G.d.H. and S.S.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerald de Haan, European Research Institute for the Biology of Ageing, University Medical Center Groningen, University of Groningen, A. Deusinglaan 1, 9713 AV Groningen, The Netherlands; e-mail: g.de.haan@umcg.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal