Abstract
The majority of blood cancers occur in the elderly. This fact conspires with an aging population in many countries to make rigorous assessment for frailty increasingly important for hematologic oncologists. In this review, we first define frailty and its relevance for patients with hematologic malignancy. Next, we review current data regarding the effect of domains of frailty on outcomes for blood cancers including myelodysplastic syndromes, acute leukemia, non-Hodgkin lymphomas such as chronic lymphocytic leukemia, and multiple myeloma. Finally, after presenting assessment and treatment options for the practicing hematologist, we propose elements of a new research agenda for geriatric hematology: the exchange of age limits for rigorous frailty screening, development of disease-specific measures, and inclusion of functional and patient-reported outcomes alongside survival.
Introduction to frailty
Although recent years have seen an explosion in new treatments for hematologic malignancies such as chronic lymphocytic leukemia (CLL)1 and multiple myeloma,2 including the expansion of age limits for hematpoietic cell transplantation (HCT) for acute leukemia and myelodysplastic syndromes (MDS),3 there is overall little specific evidence to guide treatment decisions for older adults. This is largely because they are underrepresented in cancer-related clinical trials4 ; indeed, about one-third of hematology trials listed in the National Institutes of Health registry include age-based exclusions.5 Moreover, even when there are no age restrictions, older patients who are eligible are most often not representative of the older patients commonly seen in practice. Hematologists are thus left with great clinical uncertainty, and must either assume that new treatments will be effective despite data that hail primarily from younger patients, or avoid such treatments altogether for their older patients.
Like all patients, older patients with blood cancers want to know, “What is the optimal treatment for me?” To answer them, we must consider the features of the malignancy, potential efficacy and toxicity of treatment, and each patient’s individual characteristics such as life expectancy, personal values, and presence or absence of frailty. This is a tall order, and the goal of the burgeoning field of geriatric hematology is to fill this knowledge gap. Integrating frailty assessment (“staging the aging”6 ) into routine clinical care, so that current evidence can be better applied to older patients regardless of chronological age, is part of this agenda. We must also advocate for the inclusion of older patients and rigorous frailty assessment into clinical trials of new agents, so that there are directly applicable data.
Frailty is a vulnerable state that arises from “decreased reserves in multiple organ systems, which are initiated by disease, lack of activity, inadequate nutritional intake, stress, and/or the physiologic changes of aging.”7,8 Although chronological age, comorbidity, and performance status are relatively easy to assess, they have only limited utility in capturing the heterogeneity of older patients with blood cancers9 ; indeed, determining “physiological age” requires measures of function. Although frailty is associated with comorbid burden,10,11 comorbidity is a distinct concept. Some patients have many comorbidities that are optimally managed such that they are not frail, whereas others have limited comorbidity that is so poorly managed as to make them frail. Scales such as the well-known Charlson Comorbidity Index (CCI)12 and the Sorror index for HCT (HCT-CI)13 and its derivatives14 are clinically useful and predict survival for many blood cancers,15,16 but in this review, we will focus on work that specifically relates to the broader concept of frailty.
The implications of frailty vary in different clinical scenarios. For example, a phenotypically frail older adult may do well with low-intensity therapy for indolent disease but poorly with intensive therapy for aggressive disease. The goal of frailty screening, or enhanced functional assessment, is to estimate a patient’s physiological age when considering treatment options and goals of care. Such assessment takes several domains into account, including comorbidity, psychological health, current quality of life, medication burden, physical health, cognitive function, and social support. Moreover, many geriatricians do not consider frailty a binomial construct; although some patients are clearly robust and others clearly frail, there is a third category of patients considered “vulnerable,” “unfit,” or “prefrail.”
Many frailty assessments are based on Fried’s phenotype model,17 which focuses on items such as weight loss, poor grip strength, slow gait speed, low physical activity, and self-reported exhaustion. The Rockwood model18 alternatively assesses frailty as the cumulative effect of 30 or more “deficits” and considers additional factors such as comorbidity and cognition. There are also hybrid models19 and simple questionnaires,20-22 but screening is best captured through in-person functional examination, known as geriatric assessment. This approach has been repeatedly shown to detect unrecognized vulnerabilities in large cohorts of patients with cancer and predict treatment tolerance, survival, and quality of survivorship.23-29
Frailty has also been associated with poor therapeutic response, increased toxicity, and worse survival for patients with blood cancers.16,30-33 Because these patients are often at an immunologic and hematopoietic disadvantage as a result of the malignancy itself, chemotherapy can work hand-in-hand with the blood cancer to intensify frailty. Moreover, a recent review of 19 publications from 15 studies of elderly patients with hematologic cancers9 found that 75% revealed an association between objective measures of physical capacity and survival, 67% an association between nutritional status and survival, and 50% an association between comorbidity and survival. Indeed, frail older patients with MDS,16,34 acute myeloid leukemia (AML),32,35 lymphoma,36,37 and myeloma38,39 have all been shown to have worse disease-related outcomes when compared with robust counterparts of similar age. We consider each of these diseases, reviewing evidence for the utility of frailty screening and/or geriatric assessment in decision-making, and building the rationale for routine enhanced functional assessment for patients with blood cancers. Importantly, the following is not intended to be a meta-analysis or even a systematic review, which the nascent nature of this field currently precludes.
Evidence for the effect of frailty among specific blood cancers
AML and MDS
In patients with AML, geriatric assessment has been demonstrated to add information to the standard oncology assessment based on Eastern Cooperative Oncology Group (ECOG) performance status.40 In a prospective study,41 50 patients aged 60 years or older underwent geriatric assessment by a nurse within 5 days of initial hospitalization; 63% were found to be impaired in more than one functional domain (depression, distress, impairment of activities of daily living, physical function, cognitive impairment, or comorbidity). Moreover, participants with good ECOG performance status were often impaired; for example, more than 50% of patients with cognitive impairment had ECOG performance status of 0 or 1. Another study of patients with blood cancers (including AML) had subjects assess their own ECOG performance status and compared these appraisals with physician assessment. Agreement was only fair/moderate (weighted κ = 0.41 [95% confidence interval, 0.37, 0.44]),42 arguing that enhanced functional assessment is worthwhile.
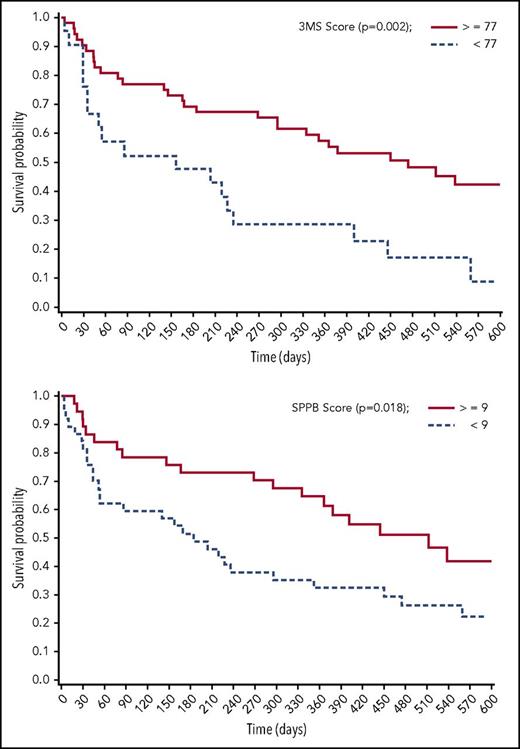
Frail patients with AML have been demonstrated to have worse survival. In a retrospective study of 101 patients aged 65 years or older,35 in addition to increased comorbidity, difficulty with strenuous activity (hazard ratio [HR] = 2.2 [1.2, 4.0]) and reports of pain (HR = 2.2 [1.2, 4.0]) were independent prognostic factors in a multivariable model including cytogenetic risk group. These remained independent predictors even for patients with ECOG performance status of 0 or 1. In another prospective study of patients with AML (n = 74; median age, 70 years), pretreatment geriatric assessment included evaluation of cognition, depression, distress, physical function, and comorbidity.32 Adjusting for age and disease risk, impaired cognition and physical function were independently associated with worse survival (Figure 1). Baseline clinical characteristics (age, risk group, performance status, hemoglobin, and creatinine) explained 20% of variation in survival, and comorbidity another 1%. Impaired physical and cognitive functioning explained an additional 5% and 6%, respectively.
Impaired cognitive function and physical performance are associated with worse survival for patients with AML (n = 73). 3MS, 100-point Modified Mini-Mental State Exam; SPPB, Short Physical Performance Battery. For both, a higher score reflects better function. Reprinted with permission.32
Impaired cognitive function and physical performance are associated with worse survival for patients with AML (n = 73). 3MS, 100-point Modified Mini-Mental State Exam; SPPB, Short Physical Performance Battery. For both, a higher score reflects better function. Reprinted with permission.32
Frailty has also been associated with poor survival in MDS. For example, a retrospective study (n = 114) demonstrated that low serum albumin (a marker of poor nutrition) and poor physical function both added prognostic information to the International Prognostic Scoring System (IPSS).34 Moreover, a large prospective study (n = 445) characterized the effect of frailty among older patients with MDS (median age, 73 years).8 Using the Clinical Frailty Scale,43 in a multivariate analysis that included age-adjusted revised IPSS (IPSS-R)44 and comorbidity score, frailty independently predicted survival (HR = 2.7 [1.7, 4.2]) and was more predictive than comorbidity, as measured by the CCI (HR = 1.8 [1.1, 2.8]). Integration of frailty significantly improved prognostication of the IPSS-R in all but the highest-risk group.
A multisite European study of 195 older patients (median age, 71 years) with MDS (n = 63) and AML (n = 132)45 similarly measured frailty domains. These included impairments in ability to perform routine daily activities and poor quality of life/fatigue. In a multivariable analysis of patients treated nonintensively, both pretreatment impairment in routine daily activities (HR = 2.60 [1.37, 5.46]) and poor quality of life/fatigue (HR = 1.82 [1.02, 3.23]) were associated with worse survival.
Finally, it is critically important to assess the robustness of older patients with MDS or AML being considered for HCT. In a small prospective study, investigators screened 50 older patients (mean age, 65 years) eligible for allogenic HCT (70% had either MDS or AML).46 The assessment included the following domains: comorbidity, polypharmacy, nutritional status, physical performance, functional status, social support, psychological status, and cognition. In the total cohort, 66% of patients had an abnormal assessment, and about 1 in 5 (22%) were ultimately considered frail. The authors concluded that older HCT candidates should undergo formal frailty screening.
Lymphoma and CLL
Several small studies, some prospective and some retrospective, have demonstrated the effect of frailty on outcomes for non-Hodgkin lymphoma, including ability to complete chemotherapy and poor overall survival.47-50 For example, an analysis from the Netherlands47 recruited 44 patients aged 70 years or older, most with diffuse large B-cell lymphoma (91%) for geriatric assessment. The assessment included the Groningen Frailty Indicator,22 a 15-item questionnaire that assesses physical, cognitive, social, and psychological domains of frailty. In multivariable analyses, an abnormal Groningen Frailty Indicator was associated with early termination of chemotherapy (odds ratio [OR] = 9.2 [1.5, 55.8]), as well as worse overall survival (HR = 2.6 [1.1, 6.1]). In another analysis,50 143 German patients with lymphoproliferative malignancies were recruited for geriatric assessment (median age, 63 years). Advanced age, poor performance status, dependence in activities of daily living (ADL; patients needed help with simple daily activities such as dressing), dependence in instrumental activities of daily living (IADL; patients needed help with more complex skills correlating with ability to live alone), and presence of severe comorbidity were all significantly associated with shorter survival. In multivariable analysis, impaired IADL and comorbidity were both independently associated with worse survival.
In contrast to AML/MDS, several studies have also assessed specific chemotherapy agents for frail older patients with non-Hodgkin lymphoma. For example, there are data for vinorelbine and prednisone for frail patients with intermediate- to high-grade non-Hodgkin lymphoma,51 and another study demonstrated that R-CVP (rituximab, cyclophosphamide, vincristine, prednisone) is active in frail patients aged 80 years or older with diffuse large B-cell lymphoma (although not without substantial toxicity).52 Another large study aimed to tailor initial treatment of older patients with diffuse large B-cell lymphoma through geriatric assessment.53 After 334 assessments from 2003 to 2006, 99 patients were identified as frail (median age, 78 years). Robust patients were enrolled in a randomized trial comparing R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) with R-mini CEOP (rituximab, cyclophosphamide, epirubicin, vinblastine, prednisone); frail patients were treated at physician discretion. Compared with the robust, frail patients had worse overall survival (HR = 3.1 [2.2, 4.3]), but did a bit better when treated with rituximab-containing chemotherapy (HR = 2.4 [1.5, 3.9]).
As older patients with aggressive lymphoma may present with potentially curable disease, it is important to determine whether they should be treated with curative intent. An Italian multicenter study54 aimed to assess whether geriatric assessment could identify elderly patients who were neither robust nor frail but were “unfit,” such that they might still benefit from curative treatment. In total, 173 patients aged 70 years or older were treated by clinical judgement (curative intent was rigorously defined by regimen), and later grouped according to geriatric assessment into fit (46%), unfit (16%), and frail (38%) categories. Treatment with curative intent improved overall survival in unfit (75% vs 45%) but not in frail (44% vs 39%) patients, arguing that enhanced functional assessment can identify patients who are not robust but may still benefit from a curative approach.
Hodgkin lymphoma less often affects the elderly, but can be difficult to treat when it does, especially for the frail. A phase 2 study of first-line brentuximab for patients aged 60 years or older (n = 27)55 found that 81% were impaired in at least one geriatric domain. Treatment yielded a 92% response rate, with 73% achieving complete remission. The authors concluded that brentuximab is reasonable for frail older patients.
Finally, given its predilection for older patients, understanding the contribution of frailty to outcomes for CLL seems especially important. Frailty results from the CLL9 trial of the German CLL study group, in which 75 of 97 patients underwent geriatric assessment (median age, 75 years) before treatment with low-dose fludarabine ± an erythropoiesis-stimulating agent, were recently published.36 The study evaluated a geriatric assessment that included the Timed Up and Go (TUG) test56 for physical function and the Dementia Detection Test (DemTect)57 for cognition, finding 61% were impaired in either physical or cognitive functioning. Poor performance on either was associated with worse survival (P = .005 and .007, respectively), prompting the authors to conclude that these tools should be integrated into both future trials and routine management of CLL.58
Multiple myeloma
Perhaps because of its relatively long natural history, treatment trajectory, and associated comorbid diagnoses such as renal failure and bone disease, efforts to assess fitness for older patients with myeloma have been the most concerted. The effort began with the initial and revised myeloma-specific comorbidity index (R-MCI)59-61 but has more recently focused on the creation and validation of the International Myeloma Working Group (IMWG) frailty score. After a pooled analysis of 869 newly diagnosed elderly patients from 3 multicenter randomized trials, the IMWG developed a frailty score and assessed its effect on clinical outcome and toxicity.39 Importantly, the associated trials were for patients who were deemed ineligible for autologous HCT.
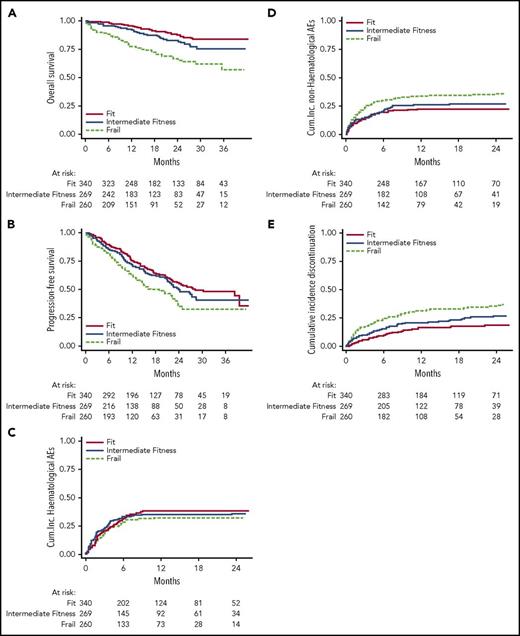
The European Myeloma Network and Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) conducted baseline geriatric assessments in these trials, using the Katz ADL,62 Lawton IADL,63 and CCI12 instruments. The median age was 74 years, and median follow-up was 18 months. The most frequent comorbidities were diabetes without organ damage (13%), cardiopulmonary disease (10%), and mild renal failure (7%). The most prevalent abnormal ADL were independence in bathing, transferring, and dressing. Among IADL, transportation, housekeeping, shopping, and laundry were most often abnormal. Using weights derived from survival analysis that adjusted for disease factors to create a scoring system, including age category as well as performance on ADL, IADL and CCI, 3 risk groups were ultimately identified: “fit” (39% of the cohort), “intermediate fit” (31%), and “frail” (30%).
The resulting IMWG frailty score predicted both survival and risk for toxicity (Figure 2). For example, the 3-year overall survival was 84% among the fit, 76% among the intermediate-fit, and 57% among the frail. Moreover, the cumulative incidence of grade ≥3 nonhematologic adverse events at 1 year in these groups was 22%, 26%, and 34%, respectively. The IMWG score has since been prospectively assessed in an external cohort of 125 newly diagnosed patients who were not part of a clinical trial.38 In that cohort, multivariate analysis confirmed that cytogenetics, ADL, IADL, and CCI each added to risk for poor outcomes. Three-year overall survival was 91% for the fit, 77% for the intermediate-fit, and 47% for the frail, suggesting the IMWG score is useful in the “real-world” of clinical practice.
Performance on geriatric assessment predicts survival, nonhematologic adverse events, and treatment discontinuation in multiple myeloma (n = 869). Reprinted with permission.39
Performance on geriatric assessment predicts survival, nonhematologic adverse events, and treatment discontinuation in multiple myeloma (n = 869). Reprinted with permission.39
Frailty assessment and management in practice: opportunities and challenges
Feasibility of screening
Routine geriatric assessment of older adults with blood cancers is feasible. Most assessments include a self-administered questionnaire with an administered component (cognitive, physical performance) typically performed by a nurse or another trained professional. In the study of elderly inpatients with AML mentioned earlier, of 54 patients approached for bedside geriatric assessment, 50 (93%) completed it.41 Another group demonstrated that during a 1-year period, 85% of new patients with blood cancer aged 75 years or older agreed to a brief geriatric screening and that scores correlated with intensity of subsequent treatments received.64 In another study, community and tertiary patients with cancer aged 65 years or older from North Carolina (n = 1088) underwent frailty assessment including tests for cognitive function, Timed Up and Go,56 and a questionnaire.65 The median time to complete the assessment was 23 minutes in the tertiary center and 30 minutes in the community (31% of the sample). The authors concluded that routine geriatric assessment could be relatively brief and effective, even in community settings.
Multidisciplinary care models
Several models have been created to enhance assessment and management of frailty in older adults with cancer. In a recent review,66 Dale and colleagues discuss four that have emerged in the United States. The first, the primary provider model, uses formally trained geriatric oncologists who perform an initial comprehensive assessment and manage all geriatric and oncology needs. Unfortunately, this model is limited because of the small number of geriatric oncologists available, even at tertiary centers. The second, a multidisciplinary consultative model, includes an initial assessment with a team that includes oncologists, geriatricians, physical and occupational therapists, pharmacists, social workers, and nutritionists who work together to develop an initial treatment plan. Although this model is more comprehensive, it only offers limited ongoing input from geriatricians and other specialists, and is dependent on resources that may not be available at many centers.
The third model is the geriatrics-driven and embedded consultative model, which is similar to the multidisciplinary consultative model except that patients are seen in parallel by their oncology team and a physically distinct geriatric service. It offers benefits of comanagement but is also limited by availability of geriatric specialists to function in this capacity. Finally, the screen and refer model employs a screening tool that is the responsibility of the primary oncology team, which then seeks geriatrics care when needed. This last model may make the most sense for hematologic oncologists in community practice; however, it can be limited by the availability of geriatrics appointments and by communication delays. Indeed, all optimal models of care depend on available resources, which may differ at the local and national level, and partially depend on a country’s overall system of healthcare delivery.
What can the individual hematologist do?
Many tools can efficiently screen for frailty in hematology practice, with the results used to inform treatment decisions and tailor treatment approaches. For some patient populations, such as those with myeloma, disease-specific screening measures have been developed; however, for the majority, these do not yet exist. No instrument alone has been shown to be a substitute for comprehensive geriatric assessment by a geriatric physician, but several tools can be integrated into hematology practice (Table 1).
Tools for frailty screening in hematologic oncology
| Tool . | Description . |
|---|---|
| Vulnerable Elders Survey (VES-13)21 | 13-item survey that does not require observation or laboratory data. Age included. Can be administered by nonclinicians. Available at https://www.rand.org/health/projects/acove/survey.html |
| Geriatric 8 (G8)20 | 8-item tool includes questions about food intake, weight loss, mobility, neuropsychological function, body mass index, number of prescriptions, self-reported heath status, and age. Most often administered by clinicians. Available at http://www.siog.org/content/comprehensive-geriatric-assessment-cga-older-patient-cancer |
| Geriatric Assessment in Hematology (GAH) Scale19 | 30-item measure covers 8 domains including medications, gait speed, mood, ADLs, subjective health status, nutrition, mental status, and comorbidity. Can be administered by a nonclinician. Scoring still under development. Available at http://www.geriatriconcology.net/article/S1879-4068(15)00053-3/fulltext (supplement) |
| Clinical Frailty Scale (CFS)43 | 9-point scale fits patients into categories based on written descriptions and simple line art images. Most often administered by clinicians. Available at http://geriatricresearch.medicine.dal.ca/clinical_frailty_scale.htm |
| Timed Up and Go (TUG)56 | Clinician asks patient to get up from a chair, walk a short distance (3 m), turn around, and come back and sit down; patient is graded depending on the time this takes. Available at http://www.siog.org/content/comprehensive-geriatric-assessment-cga-older-patient-cancer |
| International Myeloma Working Group (IMWG) Frailty Score39 | 30-item composite scale including age, Katz Activities of Daily Living, Lawton Instrumental Activities of Daily Living, and Charlson Comorbidity Index. Myeloma-specific. Can be administered by nonclinician. Score and calculator available at http://195.88.6.191/Frailtyscore/ |
| Tool . | Description . |
|---|---|
| Vulnerable Elders Survey (VES-13)21 | 13-item survey that does not require observation or laboratory data. Age included. Can be administered by nonclinicians. Available at https://www.rand.org/health/projects/acove/survey.html |
| Geriatric 8 (G8)20 | 8-item tool includes questions about food intake, weight loss, mobility, neuropsychological function, body mass index, number of prescriptions, self-reported heath status, and age. Most often administered by clinicians. Available at http://www.siog.org/content/comprehensive-geriatric-assessment-cga-older-patient-cancer |
| Geriatric Assessment in Hematology (GAH) Scale19 | 30-item measure covers 8 domains including medications, gait speed, mood, ADLs, subjective health status, nutrition, mental status, and comorbidity. Can be administered by a nonclinician. Scoring still under development. Available at http://www.geriatriconcology.net/article/S1879-4068(15)00053-3/fulltext (supplement) |
| Clinical Frailty Scale (CFS)43 | 9-point scale fits patients into categories based on written descriptions and simple line art images. Most often administered by clinicians. Available at http://geriatricresearch.medicine.dal.ca/clinical_frailty_scale.htm |
| Timed Up and Go (TUG)56 | Clinician asks patient to get up from a chair, walk a short distance (3 m), turn around, and come back and sit down; patient is graded depending on the time this takes. Available at http://www.siog.org/content/comprehensive-geriatric-assessment-cga-older-patient-cancer |
| International Myeloma Working Group (IMWG) Frailty Score39 | 30-item composite scale including age, Katz Activities of Daily Living, Lawton Instrumental Activities of Daily Living, and Charlson Comorbidity Index. Myeloma-specific. Can be administered by nonclinician. Score and calculator available at http://195.88.6.191/Frailtyscore/ |
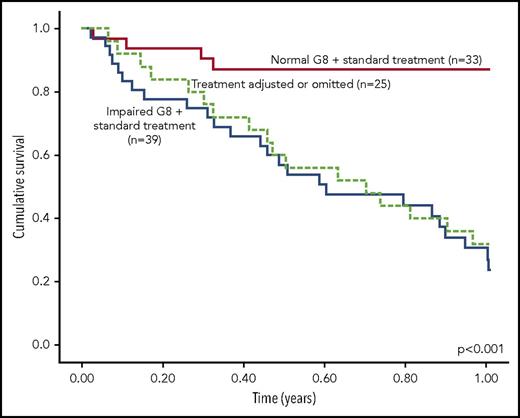
The Vulnerable Elders Survey (VES-13)21 is a questionnaire that has been successfully used in cancer-related studies67 and can provide basic screening for frailty, including for patients with blood cancers in need of HCT.46 Another screening tool is the Geriatric 8 (G8),20 which includes seven questions in addition to patient age, and was first developed to identify older patients who would benefit from subsequent comprehensive geriatric assessment. It includes questions about food intake, weight loss, mobility, neuropsychological function, body mass index, number of prescription medications, self-reported heath status, and age. Its performance was recently compared with comprehensive geriatric assessment among 97 patients with hematologic malignancy from Austria with an indication for treatment at the time of enrollment.30 As expected, the G8 lacked discriminative ability compared with full geriatric assessment, but was still an independent predictor of mortality within the first year after assessment (HR = 3.9 [1.7, 9.2]; Figure 3).
Survival by performance on the G8 frailty screening tool among patients with blood cancer (n = 97). Reprinted with permission.30
Survival by performance on the G8 frailty screening tool among patients with blood cancer (n = 97). Reprinted with permission.30
Given the aspects of hematologic oncology that are unique (eg, continued potential curability despite the presence of advanced disease), Spanish researchers developed the Geriatric Assessment in Hematology (GAH) scale, a 30-item measure that covers 8 geriatric domains.19 Taking about 12 minutes to complete, the scale was piloted with 363 patients aged 65 years or older with MDS, AML, multiple myeloma, and CLL. Almost 90% of patients completed all items. In psychometric analyses, no floor or ceiling effects were identified, comparison with other scales (known groups validity) was satisfactory, internal consistency reliability was acceptable, and test–retest reliability was excellent. A follow-up study to determine whether the GAH scale can predict chemotherapy tolerance in elderly patients with blood cancers (NCT02842229) has just been completed.
Although the gold standard for enhanced functional assessment is comprehensive assessment with a trained geriatrician,6 such an approach is not practical at most facilities. The 9-item Clinical Frailty Scale used in the abovementioned study of MDS16 uses written descriptions and line drawings to depict different states of fitness/frailty and is highly correlated with the more comprehensive Canadian Study of Health and Aging Frailty Index (which assesses 30-100 deficits).43 After assessing a patient’s history and discussing the different descriptions with the patient and family,68 clinicians can fit patients into one of three categories: 1 to 3 (robust to prefrail), 4 (vulnerable), and 5 to 8 (frail). An additional category (9) is for those who are not frail but approaching the end of life. Those who score as frail or vulnerable can be offered comprehensive geriatric assessment and management by a geriatrician if available.
Even if geriatrician comanagement is not possible, providers can focus on several strategies to help frail elderly patients.69 Although specific guidelines are beyond the scope of this review, Table 2 provides recommendations regarding optimizing frailty domains.69 In the absence of specific data for treatments tailored to frail older patients with blood cancer (apart from the exceptions detailed earlier), dose reduction and palliation are possible approaches, but not necessarily the optimal plan for all frail older adults, depending on the malignancy and the treatment. As an example, a frail older adult with chronic myeloid leukemia may still tolerate a tyrosine kinase inhibitor, whereas a frail older adult with AML will not likely tolerate standard induction therapy.
Goals for frailty assessment and treatment among patients with blood cancer
| Goals and approach . |
|---|
| 1. Screen for frailty |
| a. Refer to geriatrician, if appropriate |
| b. Even if robust, repeat screen(s) after treatment of disease progression |
| 2. Tailor treatment goals |
| a. Assess and frequently reassess goals |
| b. Integrate frailty-specific treatment data when available |
| c. Balance potential for longer life and/or cure with treatment toxicity, visit burden, and potential for hospitalization |
| 3. Improve strength and address exhaustion |
| a. PT and/or OT |
| b. Consider medication side effects |
| c. Judicious use of transfusions and/or ESAs (MDS) |
| d. Evaluate for mood disorders |
| 4. Address weight loss |
| a. Nutritionist evaluation |
| b. Socialized meals |
| c. Food access (eg, Meals on Wheels) |
| d. Replace/fix dentures |
| e. Liberalize diet (beware of transplant or neutropenic diet) |
| 5. Reduce polypharmacy |
| a. Frequent medication reconciliation |
| b. Review evolving medication plans of other providers |
| c. “Start low and go slow” with new medications |
| d. Frequently assess for side effects |
| 6. Screen for and address social support |
| a. Obtain contact information for all caregivers |
| b. Establish surrogate or health care power of attorney |
| c. Assess for loneliness |
| d. Assess for caregiver burnout |
| e. Evaluate for financial strain and refer to social work, if appropriate |
| 7. Screen for and address cognitive impairment |
| a. Administer validated assessment tool |
| b. Anticipate potential cognitive decompensation when hospitalized |
| c. Consider role of pharmacotherapy |
| Goals and approach . |
|---|
| 1. Screen for frailty |
| a. Refer to geriatrician, if appropriate |
| b. Even if robust, repeat screen(s) after treatment of disease progression |
| 2. Tailor treatment goals |
| a. Assess and frequently reassess goals |
| b. Integrate frailty-specific treatment data when available |
| c. Balance potential for longer life and/or cure with treatment toxicity, visit burden, and potential for hospitalization |
| 3. Improve strength and address exhaustion |
| a. PT and/or OT |
| b. Consider medication side effects |
| c. Judicious use of transfusions and/or ESAs (MDS) |
| d. Evaluate for mood disorders |
| 4. Address weight loss |
| a. Nutritionist evaluation |
| b. Socialized meals |
| c. Food access (eg, Meals on Wheels) |
| d. Replace/fix dentures |
| e. Liberalize diet (beware of transplant or neutropenic diet) |
| 5. Reduce polypharmacy |
| a. Frequent medication reconciliation |
| b. Review evolving medication plans of other providers |
| c. “Start low and go slow” with new medications |
| d. Frequently assess for side effects |
| 6. Screen for and address social support |
| a. Obtain contact information for all caregivers |
| b. Establish surrogate or health care power of attorney |
| c. Assess for loneliness |
| d. Assess for caregiver burnout |
| e. Evaluate for financial strain and refer to social work, if appropriate |
| 7. Screen for and address cognitive impairment |
| a. Administer validated assessment tool |
| b. Anticipate potential cognitive decompensation when hospitalized |
| c. Consider role of pharmacotherapy |
ESA, erythropoiesis-stimulating agents; OT, occupational therapy; PT, physical therapy. Adapted with permission from Huisingh-Scheetz and Walston.69
Future research directions
Ideally, the individual hematologist should be able to access data that matches a patient’s frailty status and develop an appropriately individualized treatment plan, inclusive of therapeutic regimen and tailored supportive care. In the context of sparse data, we propose a research agenda to develop evidence for integration of frailty into the management of patients with blood cancers. Key goals are to investigate which patient characteristics (ie, physical function, cognitive function, comorbidity) predict treatment tolerance for a given malignancy and therapy, and to refine our understanding of the contributions of preexisting frailty vs frailty attributed to the blood cancer itself. Trials must also include all the outcomes important to older patients with blood cancers, including the effect of treatments on physical, cognitive, and emotional functioning. In addition, as described by Wildiers and colleagues,70 frailty should be considered not just for treatment decisions but also as an outcome, with progression in and out of frail states and treatment tolerance included alongside survival in composite endpoints. Finally, trials of nontreatment interventions (ie, exercise, meditation, cancer care delivery strategies) are also needed to optimize tolerance of disease and available treatments.
In a review of the National Institutes of Health’s clinical trial registry for phase 1, 2, or 3 trials for hematological malignancies during July 2013,71 1207 were found; however, patient-centered outcome measures such as quality of life, healthcare use, and functional capacity were incorporated in only a very small number (8%, 4%, and 0.7% of trials, respectively). The review found that even in blood cancer trials specifically developed for older patients, the primary focus was most often on standard end points such as overall survival, whereas functional and/or patient-reported measures were included in less than one-fifth. Given growing data suggesting that performance on these measures may be associated with survival (a prime example is MDS72,73 ), it also stands to reason that they be included as outcomes themselves.
Prior experience with frailty assessment suggests many issues for optimal clinical trial design for older patients with blood cancer. First and foremost is the need to remove, or at least expand, age limits. Next, core measures for both eligibility and outcomes should include physical function (self-report and objective), cognitive function, and comorbidity. Moreover, clinical trials for patients with blood cancer should include optimal models to predict treatment tolerance and complications, subset selection, treatment modifications, adaptive design for vulnerability, and effect of treatment on patient-centered outcomes including quality of life, cognitive function, and physical function.
Conclusions
In summary, varied approaches to frailty assessment have been studied in hematologic malignancies and consistently demonstrate added value in outcome prediction among patients with both indolent and aggressive blood cancers. Routine measurement of frailty in hematology practice is feasible, and several measures such as the G8 and Clinical Frailty Scale are available. Integration of the results can focus on underlying causes of frailty, frailty dynamics, and tailored treatment of the malignancy itself. Importantly, although data specific to the treatment of frail older adults with blood cancers are sparse, we now have proven tools of enhanced functional assessment to help guide trial design and to assess meaningful functional and/or patient-reported measures in addition to survival.
Acknowledgments
G.A.A. gratefully acknowledges the Leukemia and Lymphoma Society (Scholar in Clinical Research) and the American Cancer Society (Research Scholar Award) for their support.
Authorship
Contribution: G.A.A. reviewed the literature and wrote the manuscript; and H.D.K. reviewed the literature and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gregory A. Abel, Dana-Farber Cancer Institute, 450 Brookline Ave, Dana 1106, Boston, MA 02215; e-mail: gregory_abel@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal