Key Points
Selinexor is a novel XPO1 inhibitor.
Selinexor was safe and efficacious in relapsed or refractory AML.
Abstract
Selinexor is a novel, first-in-class, selective inhibitor of nuclear export compound, which blocks exportin 1 (XPO1) function, leads to nuclear accumulation of tumor suppressor proteins, and induces cancer cell death. A phase 1 dose-escalation study was initiated to examine the safety and efficacy of selinexor in patients with advanced hematological malignancies. Ninety-five patients with relapsed or refractory acute myeloid leukemia (AML) were enrolled between January 2013 and June 2014 to receive 4, 8, or 10 doses of selinexor in a 21- or 28-day cycle. The most frequently reported adverse events (AEs) in patients with AML were grade 1 or 2 constitutional and gastrointestinal toxicities, which were generally manageable with supportive care. The only nonhematological grade 3/4 AE, occurring in >5% of the patient population, was fatigue (14%). There were no reported dose-limiting toxicities or evidence of cumulative toxicity. The recommended phase 2 dose was established at 60 mg (∼35 mg/m2) given twice weekly in a 4-week cycle based on the totality of safety and efficacy data. Overall, 14% of the 81 evaluable patients achieved an objective response (OR) and 31% percent showed ≥50% decrease in bone marrow blasts from baseline. Patients achieving an OR had a significant improvement in median progression-free survival (PFS) (5.1 vs 1.3 months; P = .008; hazard ratio [HR], 3.1) and overall survival (9.7 vs 2.7 months; P = .01; HR, 3.1) compared with nonresponders. These findings suggest that selinexor is safe as a monotherapy in patients with relapsed or refractory AML and have informed subsequent phase 2 clinical development. This trial was registered at www.clinicaltrials.gov as #NCT01607892.
Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy characterized by aberrant growth of immature myeloid progenitors in bone marrow (BM) and peripheral blood (PB). Current treatment strategies for AML are dependent on several factors, including age of the patient, performance status, disease history, and cytogenetic and molecular risk.1-4 For patients with relapsed or refractory AML, meaningful treatment options are limited and treatment-related morbidity and mortality are high with intensive chemotherapy.5,6 Though some therapies targeting specific AML aberrations have demonstrated clinical response, there remains a high unmet need for novel, well-tolerated, and efficacious therapies for AML.7-10
Exportin 1 (XPO1/CRM1) is the primary karyopherin protein responsible for the facilitated nuclear export of over 200 nuclear export signal–containing cargo proteins, including nearly all known tumor suppressor proteins (TSPs) and growth regulators.11 In AML and other cancers, XPO1 overexpression leads to enhanced nuclear export-mediated inactivation of TSPs responsible for detection of DNA damage, allowing malignant cells to evade apoptosis and growth arrest.12-14 In addition, XPO1 regulates the cytoplasmic localization and, in turn, translation of key oncogenic messenger RNAs (mRNAs) that complex with the XPO1-cargo protein, eukaryotic initiation factor 4E.15,16 Higher XPO1 levels correlate with poor outcomes in patients with AML.14
Recently, a new class of orally bioavailable, small-molecule therapeutics termed selective inhibitor of nuclear export (SINE) compounds were developed. SINE compounds act through covalent modification of cysteine-528 in the cargo-binding pocket of XPO1, preventing loading and nuclear export of cargo proteins.17 The SINE-induced nuclear retention of multiple TSP cargoes restores DNA damage surveillance and promotes tumor cell apoptosis.13,18-21 In AML cell lines, animal models, and patient samples, treatment with selinexor and other SINE compounds potently induced apoptosis, cell cycle arrest, and differentiation of primary myeloid blasts at nanomolar concentrations, independent of cytogenetic risk factors.13,22 Furthermore, the cytotoxic effects of selinexor have been shown to act not only on rapidly proliferating leukemia cells, but also on leukemia-initiating cells, which are inherently drug-resistant and have unlimited self-renewal.23 SINE compounds promote nuclear accumulation of p53, p21, p27, FOXO3, Rb, BRCA1/2, and survivin, and the degradation of XPO1, c-Kit, and FLT3 protein levels in wild-type and FLT3–internal tandem duplication (FLT3-ITD)-positive cell lines.13 Furthermore, the SINE compound induced nuclear retention of the XPO1 cargo nucleophosmin 1 (NPM1), a multifunctional nucleolar protein involved in ribosomal assembly and trafficking, centrosome duplication, DNA repair, and Arf gene stability.24 NPM1 is frequently mutated in AML and known mutations create a novel binding site for XPO1, leading to enhanced nuclear export and functional deactivation of the mutant NPM1.3,25
A multicenter, international phase 1 study (NCT01607892) was initiated in June 2012 with the primary aims of assessing the safety of selinexor in patients with advanced hematological malignancies and identifying the recommended phase 2 dose (RP2D), and the secondary aims of assessing the pharmacokinetics (PK), pharmacodynamics (PDn), disease response, and overall survival (OS). The trial enrolled 286 patients with a spectrum of hematological malignancies. Based upon the promising preclinical evidence of selinexor efficacy in AML model systems, 95 patients with AML were enrolled and treated with various doses and schedules to provide a more in-depth characterization of selinexor in this population.
Methods
Eligibility
The following patients were eligible for enrollment:
patients >18 years of age with histologically confirmed diagnoses of relapsed/refractory AML (excluding acute promyelocytic leukemia),26
previously untreated older adults (>60 years) with antecedent hematological disease (eg, myelodysplastic syndrome [MDS]) or unfavorable cytogenetics,26 or
elderly patients (>70 years) unfit for chemotherapy, Eastern Cooperative Oncology Group (ECOG) status ≤1, and with no standard treatment options.
Exclusion criteria included active central nervous system (CNS) malignancy, serious systemic infection (eg, HIV, hepatitis A/B/C), treatment with another investigational anticancer drug within 3 weeks prior to first selinexor dose, or concurrent anticancer therapy other than steroids or allogeneic stem cell transplant within 2 months. Descriptions of the World Health Organization (WHO) classification, ECOG criteria, and inclusion/exclusion criteria for this study are provided in supplemental Table 1 (available on the Blood Web site).
Study design
The protocol was approved by the institutional review board or independent ethics committee at each participating center and is in accordance with the Declaration of Helsinki, the International Conference on Harmonization (ICH)–Good Clinical Practice, and local laws. The primary objectives were to determine the safety, tolerability, and RP2D and the secondary objectives were to assess tumor response, OS, PK, and PDn of selinexor. The first 2 arms included dose-escalation cohorts using a modified 3+3 design with each dose administered until disease progression, death, adverse events (AEs) that could not be managed by standard care, or consent withdrawal. Arm 1 enrolled patients with lymphoid malignancies (reported elsewhere), and arm 2 enrolled patients with AML. The initial dose of 3 mg/m2 was selected using ICH guidelines to extrapolate results from animal toxicological studies and apply appropriate species-specific safety factors. The initial dosing schedule involved a 28-day cycle of selinexor administration 3 times weekly every other day (Monday/Wednesday/Friday) for weeks 1 and 3, and 2 times weekly every other day (Monday/Wednesday) for weeks 2 and 4. The first 3 escalation doses of 3 mg/m2, 6 mg/m2, and 12 mg/m2 were completed in arm 1 and then patients with AML were enrolled in arm 2 starting at 16.8 mg/m2. Prior to initiation of arm 2, the protocol was amended to include additional dose schedules designed to reduce gastrointestinal (GI) and constitutional toxicities. In the AML-focused arm 2, the dose was escalated through to 23 and 30 mg/m2 using schedule A (lead-in week of 3 doses at 12 mg/m2 doses every other day, followed by Monday/Wednesday/Friday dosing on weeks 1 and 3, and Monday/Wednesday on weeks 2 and 4), but this schedule was ultimately abandoned for futility. Following schedule A, 30 mg/m2 was studied using schedule B (28-day cycle of twice weekly Monday/Wednesday), followed by escalation through 40, 55, and 70 mg/m2, and an expansion cohort at 40 mg/m2. In addition, 55 mg/m2 was studied using schedule C consisting of twice weekly every other day (Monday/Thursday) in a 28-day cycle; 70 mg/m2 was studied using schedule D of once-weekly dosing. Finally, schedule E of twice weekly (Monday/Wednesday) with a week 1, 2, and 3 on and week 4 off drug and schedule F of twice weekly (Monday/Wednesday) with weeks 1 and 2 on, and 3 and 4 off drug was tested. A summary of the doses and treatment schedules can be found in Table 1.
Dosing schedules
| Schedule . | Dosing interval/week . | Selinexor dose, mg/m2 . | Total patients . | Total evaluable patients . |
|---|---|---|---|---|
| A | Lead-in week of 3 doses of 12 mg/m2 M/W/F weeks 1 and 3; M/W weeks 2 and 4 4-week cycle | 16.8 | 10 | 7 |
| 23 | 6 | 6 | ||
| 30 | 8 | 5 | ||
| B | M/W 4-week cycle | 30 | 6 | 4 |
| 40 | 28 | 27 | ||
| 55 | 4 | 3 | ||
| 70 | 3 | 3 | ||
| C | M/Th 4-week cycle | 55 | 4 | 2 |
| D | Once weekly 4-week cycle | 70 | 4 | 3 |
| E | M/W weeks 1 and 2; 3-week cycle | 55 | 11 | 11 |
| F | M/W weeks 1 and 2; 4-week cycle | 55 | 11 | 10 |
| Total | — | — | 95 | 81 |
| Schedule . | Dosing interval/week . | Selinexor dose, mg/m2 . | Total patients . | Total evaluable patients . |
|---|---|---|---|---|
| A | Lead-in week of 3 doses of 12 mg/m2 M/W/F weeks 1 and 3; M/W weeks 2 and 4 4-week cycle | 16.8 | 10 | 7 |
| 23 | 6 | 6 | ||
| 30 | 8 | 5 | ||
| B | M/W 4-week cycle | 30 | 6 | 4 |
| 40 | 28 | 27 | ||
| 55 | 4 | 3 | ||
| 70 | 3 | 3 | ||
| C | M/Th 4-week cycle | 55 | 4 | 2 |
| D | Once weekly 4-week cycle | 70 | 4 | 3 |
| E | M/W weeks 1 and 2; 3-week cycle | 55 | 11 | 11 |
| F | M/W weeks 1 and 2; 4-week cycle | 55 | 11 | 10 |
| Total | — | — | 95 | 81 |
Dose and dose schedule for AML patients treated with selinexor. Schedule A incorporated a lead-in week of 3 doses at 12 mg/m2 as an effort to reduce potential AE.
—, Not applicable; F, Friday; M, Monday; W, Wednesday.
Safety
Safety and tolerability were evaluated by summary drug-related dose-limiting toxicity (DLT) (defined in supplemental Table 2), AE reports, physical examinations, and laboratory safety evaluations. National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v4.03 was used for grading of AEs, and investigators assessed causality as either unrelated, or possibly, probably, or definitely related.
PK
Patients were given an oral dose of selinexor (16.8, 23, 30, 40, 55, or 70 mg/m2) in tablet form within 30 minutes of a meal. Blood was collected in K2EDTA plasma separator tubes at 0.5, 1, 2, 4, 8, 24, and 48 hours posttreatment on day 1 and day 15 or 17 of cycle 1. Samples were centrifuged within 30 minutes of collection at 2000 × g for 10 minutes at 4°C, and plasma was collected and frozen at −80°C. Plasma concentrations of selinexor were determined by liquid chromatography tandem mass spectrometry (Sciex API 4000) using a method validated and performed at Tandem Labs (Durham, NC) under good laboratory practice compliance.
PDn
PDn studies were performed on peripheral blood mononuclear cells (PBMCs) at 0, 0.5, 1, 2, 4, 8, 24, and 48 hours posttreatment and in BM aspirate samples collected at screening and at cycle 2 day 1 of selinexor treatment. The cells were layered over Ficoll medium, centrifuged, counted, resuspended in cell-freezing medium, and stored in liquid nitrogen. DNA and total RNA was isolated using TRIzol reagent (Invitrogen, Carslbad, CA). Gene expression levels of XPO1, Kit, and FLT3 were detected using TaqMan Gene expression assays (Applied Biosystems) and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or 18S RNA. Comparative real-time quantitative polymerase chain reaction was performed in triplicate, and relative expression was calculated using the comparative cycle threshold method as previously described.13 XPO1, Kit, FLT3, and p53 protein expression was measured by western blotting using the following antibodies: XPO1 (sc-5595; Santa Cruz Biotechnology), Kit (sc-168; Santa Cruz Biotechnology), FLT3 (sc-480; Santa Cruz Biotechnology), p53 (sc-126; Santa Cruz Biotechnology), and β-actin (Cell Signaling Technology). Cytogenetic analyses were performed at the participating institutions using unstimulated short-term (24-, 48-, and 72-hour) cultures with or without a direct method and G-banding. The criteria used to describe a cytogenetic clone and description of karyotype followed the recommendations of the International System for Human Cytogenetic Nomenclature.27 The diagnosis of normal karyotype was based on ≥20 metaphases analyzed in BM specimens subjected to short-term (24- or 48-hour) unstimulated cultures. Targeted amplicon sequencing using the Miseq platform (Illumina) was used to analyze DNA samples for presence of recurrent gene mutations in AML. FLT3-ITDs were evaluated using Sanger sequencing and fragment analysis, as described previously.2
Response criteria
Objective response (OR) was assessed according to the revised recommendations of the International Working Group (IWG) for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in AML.28 Stable disease was defined as not meeting criteria for other response categories for at least 1 cycle. Patients who completed cycle 1 were evaluated for response, however, patients who did not complete cycle 1, but were considered to have PD per the investigator’s discretion, were also considered evaluable. Patients who died for any reason other than progressive AML or withdrew consent prior to first BM assessment were considered nonevaluable (NE).
Statistical analysis
All 95 patients with AML described here were included in the summaries of baseline characteristics and AEs. One-way analysis of variance (ANOVA) was used to compare PK parameters at different doses in a subset of 42 dose-escalation patients who underwent PK evaluation. The Student t test was used to analyze time dependence of XPO1 expression in leukocytes and dose dependence of changes in BM blast percentage. Kaplan-Meier analysis was used to determine the median progression-free survival (PFS) and OS with statistical significance calculated by the Mantel-Byar method.29
Results
Patient characteristics
Between January 2013 and February 2015, 95 patients with relapsed or refractory AML, newly diagnosed older adults (>60 years old) with MDS or unfavorable cytogenetics, or elderly patients (>70 years old) who were not candidates for chemotherapy and had no other standard therapeutic options were enrolled in this multi-institutional, international phase 1 trial. The date of data cutoff was September 7, 2016. The median age of the patient population was 70 years, 59% were male, and over half of the patients received 3 or more prior therapies. A full summary of patient demographics and characteristics can be found in Table 2.
Baseline characteristics
| Characteristics . | Value: n, median (range), or n (%) . |
|---|---|
| Total | n = 95 |
| Age | 70 (24-89) |
| ≥65 y | 61 (64) |
| ≥75 y | 31 (33) |
| Sex | |
| Female | 40 (42) |
| Male | 55 (58) |
| Ethnic origin | |
| Asian | 1 (1) |
| Black or African American | 5 (5) |
| Caucasian | 88 (92) |
| Not reported | 1 (1) |
| Prior AML therapies | 3 (0-8) |
| 1 | 17 (18) |
| 2 | 19 (20) |
| 3 | 22 (23) |
| ≥4 | 30 (32) |
| 0* | 7 (7) |
| ECOG performance status | |
| 0 | 26 (27) |
| 1 | 69 (73) |
| ELN classification | |
| Favorable | 5 (5) |
| Intermediate-I | 27 (28) |
| Intermediate-II | 12 (13) |
| Adverse | 27 (28) |
| Not known | 24 (25) |
| Type of cytogenetic abnormality | |
| t(8;21) | 3 (3) |
| CN-AML | 30 (32) |
| FLT3-ITD | 6 (20) |
| FLT3-TKD | 3 (10) |
| NPM1 | 5 (17) |
| Unknown | 7 (23) |
| CK-AML | 15 (16) |
| del(5q) | 4 (4) |
| +8 | 4 (4) |
| −7 | 3 (3) |
| Other abnormalities | 11 (12) |
| Karyotype failure | 2 (2) |
| Unknown | 12 (13) |
| Characteristics . | Value: n, median (range), or n (%) . |
|---|---|
| Total | n = 95 |
| Age | 70 (24-89) |
| ≥65 y | 61 (64) |
| ≥75 y | 31 (33) |
| Sex | |
| Female | 40 (42) |
| Male | 55 (58) |
| Ethnic origin | |
| Asian | 1 (1) |
| Black or African American | 5 (5) |
| Caucasian | 88 (92) |
| Not reported | 1 (1) |
| Prior AML therapies | 3 (0-8) |
| 1 | 17 (18) |
| 2 | 19 (20) |
| 3 | 22 (23) |
| ≥4 | 30 (32) |
| 0* | 7 (7) |
| ECOG performance status | |
| 0 | 26 (27) |
| 1 | 69 (73) |
| ELN classification | |
| Favorable | 5 (5) |
| Intermediate-I | 27 (28) |
| Intermediate-II | 12 (13) |
| Adverse | 27 (28) |
| Not known | 24 (25) |
| Type of cytogenetic abnormality | |
| t(8;21) | 3 (3) |
| CN-AML | 30 (32) |
| FLT3-ITD | 6 (20) |
| FLT3-TKD | 3 (10) |
| NPM1 | 5 (17) |
| Unknown | 7 (23) |
| CK-AML | 15 (16) |
| del(5q) | 4 (4) |
| +8 | 4 (4) |
| −7 | 3 (3) |
| Other abnormalities | 11 (12) |
| Karyotype failure | 2 (2) |
| Unknown | 12 (13) |
CK-AML, complex karyotype AML; CN-AML, cytogenetically normal AML; ELN, European LeukemiaNet.
Poor risk, untreated elderly patients with AML or MDS.
Safety
In the AML patient population of this study, selinexor was investigated at 6 dose levels (16.8-70 mg/m2) during dose escalation and expansion. As incremental knowledge of the toxicity profile of selinexor emerged through clinical experience, the protocol was amended to include dosing schedules designed to improve tolerability. Initially, a lead-in week of 12 mg/m2 dosed every other day was tested in an effort to acclimate the patients to treatment prior to escalation to the target dose. The lead-in week was ultimately abandoned and once- or twice-weekly dosing schedules were tested. A listing of doses and treatment schedules can be found in Table 1.
Selinexor-related AEs observed in at least 5% of patients with AML are detailed in Table 3. The most commonly reported AEs (all grades) were fatigue (60%), anorexia (53%), nausea (53%), diarrhea (39%), vomiting (38%), and weight loss (25%), which were primarily grade 1 and 2. These constitutional AEs improved with supportive care agents including D2 antagonists, 5-HT3 antagonists, dexamethasone, megestrol, and/or olanzapine. The most frequently reported grade 3 and 4 AEs were thrombocytopenia (19%), anemia (15%), fatigue (14%), and neutropenia (13%). No DLTs were reported in the AML patient population during dose escalation and there was no evidence of cumulative toxicity, consistent with lack of drug accumulation (see “PK and PDn”). Unusual toxicities such as hyponatremia (22%) and blurred vision (16%) were observed in this patient population, but were generally lower grade and reversible with medical intervention. Dose interruptions and dose reductions occurred most commonly as a result of GI AEs. There were 6 patients who withdrew consent and 14 who were temporarily withheld from therapy by the treating physician due to toxicity.
AEs related to selinexor administration
| AE . | No. of patients (%) . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16.8-23 mg/m2 . | 30-40 mg/m2 . | 55-75 mg/m2 . | Total . | |||||||||
| Grades 1-2 . | Grades 3-4 . | Total, n = 16 . | Grades 1-2 . | Grades 3-4 . | Total, n = 42 . | Grades 1-2 . | Grades 3-4 . | Total, n = 37 . | Grades 1-2 . | Grades 3-4 . | Total, n = 95 . | |
| Blood and lymphatic system disorders | ||||||||||||
| Anemia | 1 (6) | 1 (6) | 2 (13) | 2 (5) | 7 (17) | 9 (21) | 1 (3) | 8 (22) | 9 (24) | 4 (4) | 16 (17) | 20 (21) |
| Febrile neutropenia | — | — | — | — | 2 (5) | 2 (5) | — | 3 (8) | 3 (8) | — | 5 (5) | 5 (5) |
| Eye disorders | ||||||||||||
| Blurred vision | 2 (13) | — | 2 (13) | 6 (14) | — | 6 (14) | 7 (19) | — | 7 (19) | 15 (16) | — | 15 (16) |
| GI disorders | ||||||||||||
| Nausea | 11 (69) | 1 (6) | 12 (75) | 20 (48) | 1 (2) | 21 (50) | 16 (43) | 3 (8) | 19 (51) | 47 (49) | 5 (5) | 52 (55) |
| Diarrhea | 5 (31) | — | 5 (31) | 18 (43) | — | 18 (43) | 14 (38) | 1 (3) | 15 (41) | 37 (39) | 1 (1) | 38 (40) |
| Vomiting | 6 (38) | — | 6 (38) | 12 (29) | 2 (5) | 14 (33) | 13 (35) | 3 (8) | 16 (43) | 31 (33) | 5 (5) | 36 (38) |
| Mucositis oral | 1 (6) | — | 1 (6) | 4 (10) | — | 4 (10) | 3 (8) | 1 (3) | 4 (11) | 8 (8) | 1 (1) | 9 (9) |
| Constipation | 1 (6) | — | 1 (6) | 3 (7) | — | 3 (7) | 3 (8) | 1 (3) | 4 (11) | 7 (7) | 1 (1) | 8 (8) |
| General disorders and administration site conditions | ||||||||||||
| Fatigue | 9 (56) | 2 (13) | 11 (69) | 18 (43) | 7 (17) | 25 (60) | 16 (43) | 4 (11) | 20 (54) | 43 (45) | 13 (14) | 56 (59) |
| Edema limbs | 3 (19) | — | 3 (19) | 2 (5) | — | 2 (5) | 1 (3) | — | 1 (3) | 6 (6) | — | 8 (8) |
| Investigations | ||||||||||||
| Weight loss | 8 (50) | — | 8 (50) | 8 (19) | 1 (2) | 9 (21) | 10 (27) | — | 10 (27) | 26 (27) | 1 (1) | 27 (28) |
| Platelet count decreased | — | 3 (19) | 3 (19) | — | 6 (14) | 6 (14) | — | 10 (27) | 10 (27) | — | 19 (20) | 19 (20) |
| Neutrophil count decreased | — | 3 (19) | 3 (19) | 1 (2) | 6 (14) | 7 (17) | — | 3 (8) | 3 (8) | 1 (1) | 12 (13) | 13 (14) |
| Creatinine increased | 1 (6) | — | 1 (6) | 4 (10) | — | 4 (10) | 3 (8) | — | 3 (8) | 8 (8) | — | 8 (8) |
| Alanine aminotransferase increased | 2 (13) | — | 2 (13) | 1 (2) | 1 (2) | 2 (5) | 2 (5) | 1 (3) | 3 (8) | 5 (5) | 2 (2) | 7 (7) |
| Aspartate aminotransferase increased | 2 (13) | 1 (6) | 3 (19) | 2 (5) | — | 2 (5) | 2 (5) | — | 2 (5) | 6 (6) | 1 (1) | 7 (7) |
| WBC decreased | 1 (6) | 1 (6) | 2 (13) | — | 3 (7) | 3 (7) | — | 2 (5) | 2 (5) | 1 (1) | 6 (6) | 7 (7) |
| Lipase increased | — | — | — | 2 (5) | — | 2 (5) | — | 3 (8) | 3 (8) | 2 (2) | 3 (3) | 5 (5) |
| Serum amylase increased | — | — | — | 2 (5) | — | 2 (5) | 2 (5) | 1 (3) | 3 (8) | 4 (4) | 1 (1) | 5 (5) |
| Metabolism and nutrition disorders | ||||||||||||
| Anorexia | 6 (38) | 2 (13) | 8 (50) | 20 (48) | 1 (2) | 21 (50) | 18 (49) | 5 (14) | 23 (62) | 44 (46) | 8 (8) | 52 (55) |
| Hyponatremia | 3 (19) | 1 (6) | 4 (25) | 7 (17) | 2 (5) | 9 (21) | 6 (16) | 2 (5) | 8 (22) | 16 (17) | 5 (5) | 21 (22) |
| Dehydration | 1 (6) | — | 1 (6) | 3 (7) | 4 (10) | 7 (17) | 5 (14) | 1 (3) | 6 (16) | 9 (9) | 5 (5) | 14 (15) |
| Hypokalemia | — | 2 (13) | 2 (13) | 2 (5) | — | 2 (5) | 6 (16) | 2 (5) | 8 (22) | 8 (8) | 4 (4) | 12 (13) |
| Hypomagnesemia | 1 (6) | — | 1 (6) | 4 (10) | — | 4 (10) | 6 (16) | — | 6 (16) | 11 (12) | — | 11 (12) |
| Hyperglycemia | 1 (6) | — | 1 (6) | 4 (10) | 1 (2) | 5 (12) | 1 (3) | 1 (3) | 6 (16) | 6 (6) | 2 (2) | 8 (8) |
| Hypophosphatemia | — | — | — | 1 (2) | 1 (2) | 2 (5) | 2 (5) | 2 (5) | 4 (11) | 3 (3) | 3 (3) | 6 (6) |
| Hypoalbuminemia | 1 (6) | — | 1 (6) | 2 (5) | — | 2 (5) | 2 (5) | — | 2 (5) | 5 (5) | — | 5 (5) |
| Hypocalcemia | 1 (6) | — | 1 (6) | 1 (2) | — | 1 (2) | 2 (5) | 1 (3) | 3 (8) | 4 (4) | 1 (1) | 5 (5) |
| Musculoskeletal and connective tissue disorders | ||||||||||||
| Generalized muscle weakness | 2 (13) | 1 (6) | 3 (19) | 2 (5) | 1 (2) | 3 (7) | 3 (8) | 2 (5) | 5 (14) | 7 (7) | 4 (4) | 11 (12) |
| Nervous system disorders | ||||||||||||
| Dysgeusia | 2 (13) | — | 2 (13) | 12 (29) | — | 12 (29) | 5 (14) | — | 5 (14) | 19 (20) | — | 19 (20) |
| Dizziness | 3 (19) | — | 3 (19) | 1 (2) | — | 1 (2) | 7 (19) | — | 7 (19) | 11 (12) | — | 11 (12) |
| Headache | — | 1 (6) | 1 (6) | 2 (5) | — | 2 (5) | 3 (8) | 1 (3) | 4 (11) | 5 (5) | 2 (2) | 7 (7) |
| Respiratory, thoracic, and mediastinal disorders | ||||||||||||
| Epistaxis | 1 (6) | — | 1 (6) | — | 1 (2) | 1 (2) | 3 (8) | 1 (3) | 4 (11) | 4 (4) | 2 (2) | 6 (6) |
| Dyspnea | 3 (19) | — | 3 (19) | 1 (2) | 1 (2) | 2 (5) | — | — | — | 4 (4) | 1 (1) | 5 (5) |
| Vascular disorders | ||||||||||||
| Hypotension | 3 (19) | — | 3 (19) | — | — | — | 4 (11) | — | 4 (11) | 7 (7) | — | 7 (7) |
| AE . | No. of patients (%) . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16.8-23 mg/m2 . | 30-40 mg/m2 . | 55-75 mg/m2 . | Total . | |||||||||
| Grades 1-2 . | Grades 3-4 . | Total, n = 16 . | Grades 1-2 . | Grades 3-4 . | Total, n = 42 . | Grades 1-2 . | Grades 3-4 . | Total, n = 37 . | Grades 1-2 . | Grades 3-4 . | Total, n = 95 . | |
| Blood and lymphatic system disorders | ||||||||||||
| Anemia | 1 (6) | 1 (6) | 2 (13) | 2 (5) | 7 (17) | 9 (21) | 1 (3) | 8 (22) | 9 (24) | 4 (4) | 16 (17) | 20 (21) |
| Febrile neutropenia | — | — | — | — | 2 (5) | 2 (5) | — | 3 (8) | 3 (8) | — | 5 (5) | 5 (5) |
| Eye disorders | ||||||||||||
| Blurred vision | 2 (13) | — | 2 (13) | 6 (14) | — | 6 (14) | 7 (19) | — | 7 (19) | 15 (16) | — | 15 (16) |
| GI disorders | ||||||||||||
| Nausea | 11 (69) | 1 (6) | 12 (75) | 20 (48) | 1 (2) | 21 (50) | 16 (43) | 3 (8) | 19 (51) | 47 (49) | 5 (5) | 52 (55) |
| Diarrhea | 5 (31) | — | 5 (31) | 18 (43) | — | 18 (43) | 14 (38) | 1 (3) | 15 (41) | 37 (39) | 1 (1) | 38 (40) |
| Vomiting | 6 (38) | — | 6 (38) | 12 (29) | 2 (5) | 14 (33) | 13 (35) | 3 (8) | 16 (43) | 31 (33) | 5 (5) | 36 (38) |
| Mucositis oral | 1 (6) | — | 1 (6) | 4 (10) | — | 4 (10) | 3 (8) | 1 (3) | 4 (11) | 8 (8) | 1 (1) | 9 (9) |
| Constipation | 1 (6) | — | 1 (6) | 3 (7) | — | 3 (7) | 3 (8) | 1 (3) | 4 (11) | 7 (7) | 1 (1) | 8 (8) |
| General disorders and administration site conditions | ||||||||||||
| Fatigue | 9 (56) | 2 (13) | 11 (69) | 18 (43) | 7 (17) | 25 (60) | 16 (43) | 4 (11) | 20 (54) | 43 (45) | 13 (14) | 56 (59) |
| Edema limbs | 3 (19) | — | 3 (19) | 2 (5) | — | 2 (5) | 1 (3) | — | 1 (3) | 6 (6) | — | 8 (8) |
| Investigations | ||||||||||||
| Weight loss | 8 (50) | — | 8 (50) | 8 (19) | 1 (2) | 9 (21) | 10 (27) | — | 10 (27) | 26 (27) | 1 (1) | 27 (28) |
| Platelet count decreased | — | 3 (19) | 3 (19) | — | 6 (14) | 6 (14) | — | 10 (27) | 10 (27) | — | 19 (20) | 19 (20) |
| Neutrophil count decreased | — | 3 (19) | 3 (19) | 1 (2) | 6 (14) | 7 (17) | — | 3 (8) | 3 (8) | 1 (1) | 12 (13) | 13 (14) |
| Creatinine increased | 1 (6) | — | 1 (6) | 4 (10) | — | 4 (10) | 3 (8) | — | 3 (8) | 8 (8) | — | 8 (8) |
| Alanine aminotransferase increased | 2 (13) | — | 2 (13) | 1 (2) | 1 (2) | 2 (5) | 2 (5) | 1 (3) | 3 (8) | 5 (5) | 2 (2) | 7 (7) |
| Aspartate aminotransferase increased | 2 (13) | 1 (6) | 3 (19) | 2 (5) | — | 2 (5) | 2 (5) | — | 2 (5) | 6 (6) | 1 (1) | 7 (7) |
| WBC decreased | 1 (6) | 1 (6) | 2 (13) | — | 3 (7) | 3 (7) | — | 2 (5) | 2 (5) | 1 (1) | 6 (6) | 7 (7) |
| Lipase increased | — | — | — | 2 (5) | — | 2 (5) | — | 3 (8) | 3 (8) | 2 (2) | 3 (3) | 5 (5) |
| Serum amylase increased | — | — | — | 2 (5) | — | 2 (5) | 2 (5) | 1 (3) | 3 (8) | 4 (4) | 1 (1) | 5 (5) |
| Metabolism and nutrition disorders | ||||||||||||
| Anorexia | 6 (38) | 2 (13) | 8 (50) | 20 (48) | 1 (2) | 21 (50) | 18 (49) | 5 (14) | 23 (62) | 44 (46) | 8 (8) | 52 (55) |
| Hyponatremia | 3 (19) | 1 (6) | 4 (25) | 7 (17) | 2 (5) | 9 (21) | 6 (16) | 2 (5) | 8 (22) | 16 (17) | 5 (5) | 21 (22) |
| Dehydration | 1 (6) | — | 1 (6) | 3 (7) | 4 (10) | 7 (17) | 5 (14) | 1 (3) | 6 (16) | 9 (9) | 5 (5) | 14 (15) |
| Hypokalemia | — | 2 (13) | 2 (13) | 2 (5) | — | 2 (5) | 6 (16) | 2 (5) | 8 (22) | 8 (8) | 4 (4) | 12 (13) |
| Hypomagnesemia | 1 (6) | — | 1 (6) | 4 (10) | — | 4 (10) | 6 (16) | — | 6 (16) | 11 (12) | — | 11 (12) |
| Hyperglycemia | 1 (6) | — | 1 (6) | 4 (10) | 1 (2) | 5 (12) | 1 (3) | 1 (3) | 6 (16) | 6 (6) | 2 (2) | 8 (8) |
| Hypophosphatemia | — | — | — | 1 (2) | 1 (2) | 2 (5) | 2 (5) | 2 (5) | 4 (11) | 3 (3) | 3 (3) | 6 (6) |
| Hypoalbuminemia | 1 (6) | — | 1 (6) | 2 (5) | — | 2 (5) | 2 (5) | — | 2 (5) | 5 (5) | — | 5 (5) |
| Hypocalcemia | 1 (6) | — | 1 (6) | 1 (2) | — | 1 (2) | 2 (5) | 1 (3) | 3 (8) | 4 (4) | 1 (1) | 5 (5) |
| Musculoskeletal and connective tissue disorders | ||||||||||||
| Generalized muscle weakness | 2 (13) | 1 (6) | 3 (19) | 2 (5) | 1 (2) | 3 (7) | 3 (8) | 2 (5) | 5 (14) | 7 (7) | 4 (4) | 11 (12) |
| Nervous system disorders | ||||||||||||
| Dysgeusia | 2 (13) | — | 2 (13) | 12 (29) | — | 12 (29) | 5 (14) | — | 5 (14) | 19 (20) | — | 19 (20) |
| Dizziness | 3 (19) | — | 3 (19) | 1 (2) | — | 1 (2) | 7 (19) | — | 7 (19) | 11 (12) | — | 11 (12) |
| Headache | — | 1 (6) | 1 (6) | 2 (5) | — | 2 (5) | 3 (8) | 1 (3) | 4 (11) | 5 (5) | 2 (2) | 7 (7) |
| Respiratory, thoracic, and mediastinal disorders | ||||||||||||
| Epistaxis | 1 (6) | — | 1 (6) | — | 1 (2) | 1 (2) | 3 (8) | 1 (3) | 4 (11) | 4 (4) | 2 (2) | 6 (6) |
| Dyspnea | 3 (19) | — | 3 (19) | 1 (2) | 1 (2) | 2 (5) | — | — | — | 4 (4) | 1 (1) | 5 (5) |
| Vascular disorders | ||||||||||||
| Hypotension | 3 (19) | — | 3 (19) | — | — | — | 4 (11) | — | 4 (11) | 7 (7) | — | 7 (7) |
AEs observed in at least 5% of all enrolled patients at any point while on study are listed as number of patients (%). Unlisted grade 4 AEs occurring in <5% of the patient population include lymphocyte count decreased (1), pneumonitis (1), sepsis (1), and blood in stool (1).
—, No reported incidents.
There were a total of 89 serious AEs (SAEs) of any causality reported in the AML patient population of this study. Fifteen of the SAEs were deemed at least possibly related to selinexor treatment and 12 of the 95 patients experienced at least 1 SAE. Two of the SAEs were fatal and included ejection fraction decrease/respiratory failure and infection. The remaining SAEs were grade 2 or 3 and included: oral mucositis (1), dehydration (2), dyspnea (1), infection (3), febrile neutropenia (1), vomiting (1), cataract (1), CNS hemorrhage (1), fatigue w/ dehydration (1), and Guillain-Barre syndrome (1). All grade 2 and 3 SAEs resolved with supportive care, dose interruption, or surgery (in the case of the cataract) with the exception of the oral mucositis, which was ongoing at the time of the patient’s death from progressive disease (PD). Twelve of the 15 SAEs occurred at doses ≥40 mg/m2. A full summary of drug-related SAEs can be found in supplemental Table 3.
Twenty-six patients died during the first cycle or prior to first disease assessment. The deaths were reported as PD (12 patients), infection (9 patients), cardiopulmonary event (1 patient), and unknown (4 patients). None were attributed to selinexor treatment.
PK and PDn
Selinexor PK was evaluated in 42 patients on days 1 and 15 or 17 (Figure 1A). On day 1 or day 15/17, maximum concentration (Cmax) and area under the curve (AUC) (0-8 hours or 0-48 hours) were dose proportional from 16.8 to 70 mg/m2 (P ≤ .001), whereas time to peak concentration in serum (Tmax) (3.3 ± 2.0 hours), biological half-life (t1/2) (6.0 ± 1.9 hours), and volume of distribution (Vd) (2.0 ± 0.6 L/kg) were dose independent (P ≥ .5). Cmax and AUC were not significantly different at any dose on day 1 compared with day 15/17, demonstrating that drug accumulation was not occurring after administration of 5 to 6 doses. Over the doses studied in AML patients, Cmax values were 1.9- to 9.5-fold above the median in vitro potency of 195 nM (range, 5 nM-1.06 μM) (supplemental Table 4), and selinexor plasma concentrations were sustained above the median potency for 8 to 19 hours with 23 to 70 mg/m2 selinexor (Figure 1B).
Selinexor PK. (A) Selinexor plasma concentration in patients with AML as a function of time over 48 hours for doses as listed administered on day 1. Points are means and standard error for the number of patients at each dose listed in parentheses on the graph. Median in vitro IC50 is 86.4 ng/mL (or 195 nM). (B) Cmax, AUC0-8 h, and AUC0-48 h were different across doses with high statistical significance (P < .0001 by ANOVA).
Selinexor PK. (A) Selinexor plasma concentration in patients with AML as a function of time over 48 hours for doses as listed administered on day 1. Points are means and standard error for the number of patients at each dose listed in parentheses on the graph. Median in vitro IC50 is 86.4 ng/mL (or 195 nM). (B) Cmax, AUC0-8 h, and AUC0-48 h were different across doses with high statistical significance (P < .0001 by ANOVA).
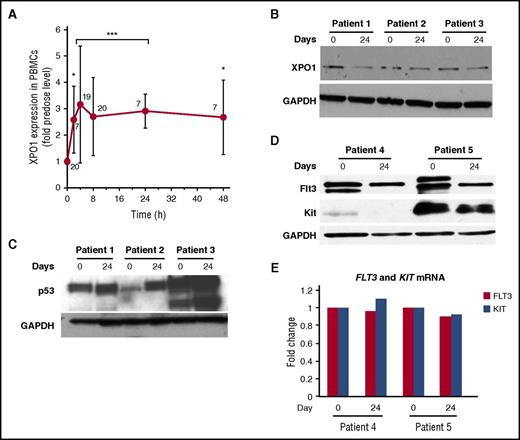
Assessment of target engagement was determined by measuring XPO1 mRNA expression in PBMCs isolated from patient blood at times up to 48 hours following the first selinexor dose. Two hours after drug administration, XPO1 expression had significantly increased and reached a maximum of about threefold induction at 4 hours, which was sustained through 48 hours (Figure 2A). Despite a half-life of about 6 hours, selinexor induced XPO1 expression through a positive feedback loop to restore XPO1 activity, and elevated XPO1 levels were sustained for at least 48 hours, providing PDn evidence of XPO1 inhibition beyond the presence of selinexor in plasma (t1/2 of ∼6 hours). This is likely due in part to the slowly reversible inhibitory mechanism of SINE compounds, which dissociate from XPO1 after several hours, but biologic effects of XPO1 inhibition may persist.30
PDn. (A) XPO1 mRNA levels in PBMCs isolated from patients before and after the first dose of selinexor. Each point represents data from patients across all doses normalized to the predose level, with the number of patients per time point indicated on the graph. The Student t test was used to determine statistical significance (*P < .05; ***P < .0001 vs predose). (B-D) Assessment of protein and mRNA levels in patient-derived BM blasts. XPO1, p53, FLT3, and Kit protein levels were analyzed by western blot at baseline and near the end of cycle 1 (day 24). GAPDH was used as a loading control. (E) FLT3 and Kit mRNA levels from corresponding BM blast samples assayed in panel D.
PDn. (A) XPO1 mRNA levels in PBMCs isolated from patients before and after the first dose of selinexor. Each point represents data from patients across all doses normalized to the predose level, with the number of patients per time point indicated on the graph. The Student t test was used to determine statistical significance (*P < .05; ***P < .0001 vs predose). (B-D) Assessment of protein and mRNA levels in patient-derived BM blasts. XPO1, p53, FLT3, and Kit protein levels were analyzed by western blot at baseline and near the end of cycle 1 (day 24). GAPDH was used as a loading control. (E) FLT3 and Kit mRNA levels from corresponding BM blast samples assayed in panel D.
XPO1 protein levels were decreased in BM blasts of patients after 1 cycle of selinexor treatment, compared with baseline (Figure 2B). As expected, p53 upregulation and FLT3/Kit protein downregulation was observed in BM blasts after treatment with selinexor (Figure 2C-D). However, there were no changes in mRNA expression or Kit mRNA levels in the same samples, indicating a posttranslational effect (Figure 2E).
A targeted recurrent AML mutation panel was performed for a subset of patients (n = 14) for whom DNA was available from BM before treatment. Supplemental Table 5 shows the frequency of recurrent mutations observed in this cohort. Given the limited number of samples, no correlation could be made between mutational status and best response.
Efficacy
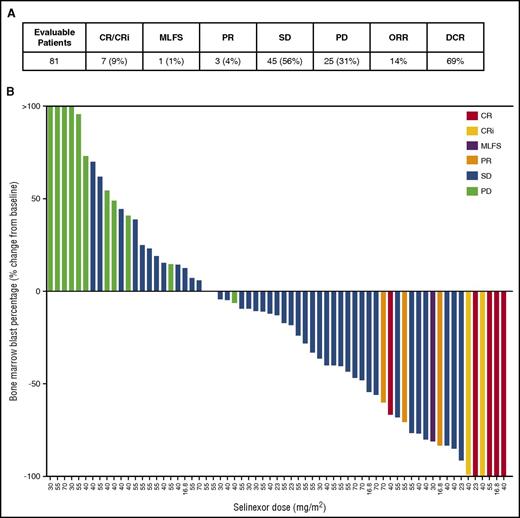
Eleven of the 81 evaluable patients (14%) achieved OR, which included 5 complete response (CR), 2 complete response with incomplete blood count recovery (CRi), 1 morphologic leukemia-free state (MLFS), and 3 partial response (PR) (Figure 3A). Forty-five patients achieved stable disease (SD), for a disease control rate (DCR) of 69%, with 8 patients exhibiting SD for over 3 months and 3 patients for over 6 months. SD was determined when there was ≤50% increase in BM blasts from baseline, whereas PD required >50% increase in BM blasts as per the IWG criteria for response in AML.28 Fourteen patients (15%) were NE due to death other than progressive AML (n = 12) or consent withdrawal (n = 2) prior to first BM assessment (supplemental Table 6). Four patients were assigned a best response call without BM blast assessments, which included 2 SD (based on lack of or inconclusive BM blast evaluation after cycle 1) and 2 PD (based on percentage blast count measured in peripheral blood and investigator’s discretion).
Efficacy. (A) CR, CRi, MLFS, PR, SD, PD, ORR = (CR+CRi+MLFS+PR)/number of evaluable patients; disease control rate (DCR) = (CR+CRi+MLFS+PR+SD)/number of evaluable patients. (B) The best response based upon change in BM blast percentage is depicted for the 65 evaluable patients with available blast data postselinexor administration. Best response category is color-coded as indicated in the legend. The remaining 30 patients lacking post–drug blast data were assessed as SD (2), PD (14), or were NE (14).
Efficacy. (A) CR, CRi, MLFS, PR, SD, PD, ORR = (CR+CRi+MLFS+PR)/number of evaluable patients; disease control rate (DCR) = (CR+CRi+MLFS+PR+SD)/number of evaluable patients. (B) The best response based upon change in BM blast percentage is depicted for the 65 evaluable patients with available blast data postselinexor administration. Best response category is color-coded as indicated in the legend. The remaining 30 patients lacking post–drug blast data were assessed as SD (2), PD (14), or were NE (14).
Of the 65 patients for whom posttreatment BM blast assessments were made, a reduction in BM blast percentage for at least 1 measurement was observed in 41 patients (63%) with 20 patients (31%) showing at least a 50% reduction in BM blasts from baseline. Five patients had no detectable leukemic blasts in the BM at time of maximal benefit (Figure 3B).
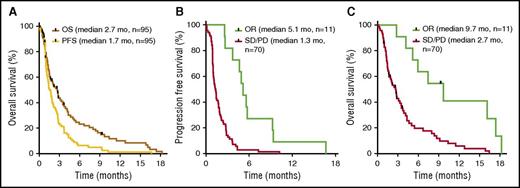
The median PFS and OS for the entire cohort of patients were 1.7 and 2.7 mo, respectively (Figure 4). OR was associated with increased PFS and OS, as patients responding to selinexor had a median PFS of 5.1 mo and OS of 9.7 mo, compared with patients who did not respond (PD/SD) (PFS, 1.3 mo; P = .008; hazard ratio [HR], 3.1) (OS, 2.7 mo; P = .01; HR, 3.1).
PFS and OS. Kaplan-Meier plots of PFS and OS. (A) For all patients, median PFS was 1.7 mo and median OS was 2.7 mo. (B) PFS for OR patients (5.1 mo) differed significantly from nonresponders (PD/SD) (1.3 mo; P = .008; HR, 3.1). (C) OS for OR patients (9.7 mo) differed significantly from nonresponders (PD/SD) (2.7 mo; P = .01; HR, 3.1). Statistical significance was determined using the Mantel-Byar method. Black marks indicate censored data at the last date a patient was known to be alive.
PFS and OS. Kaplan-Meier plots of PFS and OS. (A) For all patients, median PFS was 1.7 mo and median OS was 2.7 mo. (B) PFS for OR patients (5.1 mo) differed significantly from nonresponders (PD/SD) (1.3 mo; P = .008; HR, 3.1). (C) OS for OR patients (9.7 mo) differed significantly from nonresponders (PD/SD) (2.7 mo; P = .01; HR, 3.1). Statistical significance was determined using the Mantel-Byar method. Black marks indicate censored data at the last date a patient was known to be alive.
Best response did not correlate significantly with dose level, cytogenetic risk, age, or number of prior therapies, but patients with lower BM blasts and white blood cell (WBC) count at baseline had a significantly better response rate (supplemental Figure 1). A CR was attained in 1 patient who had FLT3-ITD, FLT-TKD, and NPM1 mutations, however, the relationship of response and mutational status could not be fully established in this trial given the small number of patients with complete molecular testing.
Discussion
Standard treatment of patients with AML who are not eligible for high-dose cytotoxic therapy includes DNA methyltransferase inhibition with either 5′azacitidine or decitabine, or low-dose cytarabine arabinoside, and supportive care. CR rates with these agents are between 10% and 20% when used as the first therapy.31 Responses with salvage therapies in this cohort of patients are abysmal with poor response to therapy and low survival rates.32,33 The need for novel therapy in AML is urgent, and inhibition of nuclear export proteins represents a completely new mechanism of interest.
This is the first trial to describe the safety, PK, PDn, and efficacy of selinexor (16.8-70 mg/m2) in patients with relapsed or refractory AML. Overall, selinexor was tolerated with generally grade 1/2 constitutional and GI toxicities, which were manageable with supportive care. Unusual toxicities such as hyponatremia and blurred vision may be a class-effect phenomenon, but they seem to be self-limiting and reversible in most patients. Likewise, the number of patients who withdrew consent (6) or who were withheld from therapy due to physician discretion (14) indicates that this novel therapy does occasionally introduce toxicities different than those associated with standard cytotoxic chemotherapy. Development of familiarity with management of toxicities is important with any new therapy, and the combination of reversible, low-grade constitutional and GI toxicities differs from that of standard cytotoxic therapy and requires alternative approaches to standard supportive care. In this and other clinical studies of selinexor, there are no data to suggest irreversibility, or accumulation of toxicity.34,35
A maximum tolerated dose was not clearly established in this patient population; however, the incidence of higher-grade GI toxicities and thrombocytopenia were more common at doses >40 mg/m2 compared with those ≤40 mg/m2. SAEs were also more frequent (12 of 15) at doses >40 mg/m2. In addition, a majority of patients that responded to treatment (8 of 11) received ≤40 mg/m2 selinexor, suggesting maximal efficacy can be achieved at intermediate doses. A similar observation was made in patients with solid tumors, as doses above 40 mg/m2 had poorer tolerability.34 Thus, when considering the totality of safety, efficacy, and PK data from NCT01607892, a 60-mg flat dose (∼35 mg/m2) of selinexor given twice weekly every other day in a 4-week cycle has been chosen for phase 2 analysis in patients with AML.
Selinexor displayed predictable PK with no evidence of drug accumulation or changes in drug metabolism with repeat dosing. Oral selinexor PK had tightly dose-dependent Cmax and AUC, and Cmax at the lowest doses tested in this study exceeded median growth inhibition (50% inhibitory concentration [IC50]) seen in vitro (supplemental Table 4). Absorption and elimination kinetics were dose-independent, and drug accumulation was not observed after multiple doses, consistent with nearly complete plasma clearance by 48 hours and supporting dosing schedules that allow ≥48 hours between doses.
Based on previously published data, we hypothesized that the in vivo antileukemic activity of selinexor is achieved through the nuclear accumulation of TSPs (p53, FOX3A) and downregulation of oncogenic FLT3 and Kit.14,18 Indeed, in this clinical trial, myeloblasts displayed increased p53 expression and downregulation of both FLT3 and Kit protein in AML blasts after administration of selinexor.
Treatment with selinexor in this heavily pretreated cohort of predominantly elderly patients with AML led to single-agent activity with an objective response rate (ORR) of 14%. Among those responders, a significant OS advantage was observed. Identifying the best AML subgroup to treat with single-agent selinexor is critical. Post hoc analysis revealed that patients with lower baseline BM blasts were more likely to respond to single-agent selinexor in this study, implying a potential role for selinexor in MDS or hypoproliferative AML.
In addition to showing robust single-agent efficacy in preclinical models of leukemia, selinexor and other SINE compounds have demonstrated synergistic activity when combined with existing AML therapies. Prolonged survival and decreased tumor burden was observed in murine models of leukemia when SINE compounds were combined with cytarabine or the hypomethylating agent, decitabine.36,37 Synergy between selinexor and topoisomerase II inhibitors was demonstrated in AML cell lines and primary AML BM aspirates of newly diagnosed and relapsed patients with AML.38 Also, work by Kojima et al reported enhanced apoptosis in numerous AML cell lines and primary AML progenitor cells cotreated with KPT-185 and the MDM2 inhibitor, Nutlin-3a, compared with each agent alone, implying a potential role of oral SINE compounds in combination with other novel agents in development.14 Likewise, early synergy seen with clinical responses of selinexor in combination with cytotoxic chemotherapy seem to edify preclinical findings that SINE compounds amplify cytotoxic chemotherapy effects in leukemia cells.39-41 The AE profile of these combinations have thus far been predictable with no new or unanticipated toxicities, and no evidence of cumulative toxicity. Current efforts are under way in selinexor phase 2 clinical trials to identify predictive biomarkers of response, including cytogenetic profiling, molecular risk assessments, and next-generation sequencing, which would facilitate patient stratification in future studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this trial and their families, the coinvestigators, nurses, and study coordinators at each of the sites. This study was sponsored by Karyopharm Therapeutics, which provided selinexor and worked with investigators to design the study, as well as collect, analyze, and interpret the data.
This work was supported by Karyopharm Therapeutics. R.G. was supported by a Scholar in Clinical Research award from the Leukemia & Lymphoma Society.
Authorship
Contribution: R.G., M.S., P.M.M.-S., T.K., B.K., and R.S. performed research, analyzed data, and reviewed the manuscript; R.B., M.A., N.G., M.G., L.S., N.W.-J., and K.Y. performed research and reviewed the manuscript; T.J.U. and R.C. analyzed data and wrote the manuscript; J.-R.S.-M. analyzed data; T.R. designed the study and reviewed the manuscript; and S.S. and M.K. designed the study, analyzed data, and reviewed the manuscript.
Conflict-of-interest disclosure: R.G. received a travel stipend from Karyopharm Therapeutics. M.S. owns stock in Karyopharm Therapeutics, has a consulting/advisory role at Amgen, CTI, Karyopharm Therapeutics, ARIAD, Gilead, and Celgene, and receives research funding from Astex, Sunesis, Takeda, and TG Therapeutics. R.B. has a consulting/advisory role at Celgene and receives research funding from Celgene, Karyopharm Therapeutics, Merck, Bristol-Myers Squibb, and Millennium/Takeda. N.G. receives honoraria from Heron, Sanofi, and Taiho and is a consultant/advisor at Heron. L.S. has a consulting/advisory role at Novartis, Bristol-Myers Squibb, Pfizer, Celgene, and JAZZ. P.M.M.-S. receives honorarium and a travel stipend from Karyopharm Therapeutics. T.J.U., J.-R.S.-M., R.C., T.R., T.K., B.K., S.S., and M.K. are employees and stockholders at Karyopharm Therapeutics. R.S. receives honoraria from Amgen, Arog, Agios, Celfor, Celgene, Sunesis, Bristol-Myers Squibb, Pfizer, Novartis, Karyopharm Therapeutics, Fujifilm, AbbVie, Roche, Genentech, and Merck, and receives research funding from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Ramiro Garzon, Department of Internal Medicine, The Ohio State University Wexner Medical Center, 460 W 12 th Ave, Columbus, OH 43210; e-mail: ramiro.garzon@osumc.edu.