In this issue of Blood, Garzon et al1 and Kuruvilla et al2 report on the results of phase 1 dose-escalation studies of selinexor in patients with acute myeloid leukemia (AML) and different subtypes of non-Hodgkin lymphoma (NHL), respectively. Together the studies report on 174 patients (95 with AML and 79 with NHL) treated. The drug appears to be tolerable in most patients, with mostly grade 1 or 2 adverse events. The most frequent adverse events included fatigue, anorexia, nausea, diarrhea, vomiting, and weight loss. A variety of dosing regimens were tested as described, largely in an attempt to limit the observed gastrointestinal toxicity, but none was successful, and the recommended phase 2 dosage is 60 mg flat dosing twice weekly in 4-week cycles. Pharmacokinetic studies in a cohort of patients demonstrated no dose accumulation. Grade 3 or 4 toxicities tended to be hematologic.
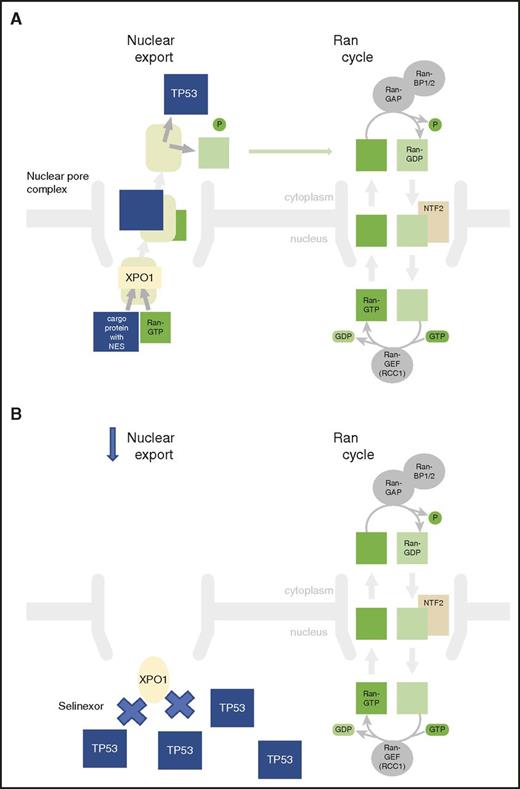
XPO1 inhibition prevents export of tumor-suppressor genes from the nucleus. (A) XPO1 mediates leucine-rich NES transport of tumor-suppressor proteins such as TP53 from the nucleus to the cytoplasm. This enhances leukemia cell survival. (B) Selinexor inhibits XPO1 function, leading to nuclear accumulation of TSPs, such as TP53, that can induce leukemia cell death.
XPO1 inhibition prevents export of tumor-suppressor genes from the nucleus. (A) XPO1 mediates leucine-rich NES transport of tumor-suppressor proteins such as TP53 from the nucleus to the cytoplasm. This enhances leukemia cell survival. (B) Selinexor inhibits XPO1 function, leading to nuclear accumulation of TSPs, such as TP53, that can induce leukemia cell death.
Selinexor is a novel, first-in-class, orally available small molecule with antineoplastic activity by selective inhibition of XPO1,3 which is also known as exportin 1 or chromosome region maintenance 1 protein (CRM1).4 XPO1 is the major karyopherin export factor for proteins from the nucleus to the cytoplasm and is overexpressed in a variety of cancer cell types. Karyopherins are a group of proteins involved in transportation proteins between the cytoplasm and the nucleus, usually through the nuclear pore, and can act as importins or exportins. XPO1 mediates leucine-rich nuclear export signal (NES)–dependent protein export, and energy is provided by the RAS-related nuclear protein also known as the GTP-binding nuclear protein Ran, which is a small G protein that is essential for the translocation of RNA and proteins through the nuclear pore complex (see figure, panel A). Overexpression of XPO1 leads to enhanced export and therefore inactivation of tumor suppressor proteins (TSPs) that detect DNA damage and therefore enhance cancer cell survival. Selinexor modifies the essential XPO1-cargo binding residue cysteine-528, thereby irreversibly inactivating XPO1-mediated nuclear export of cargo proteins, including TSPs such as TP53,5 TP21, BRCA1/2, pRB, and FOXO. This agent uses the novel approach of selective inhibition of nuclear export in an attempt to restore endogenous tumor-suppressing processes, selectively eliminating tumor cells while sparing normal cells (see figure, panel B).
There is a critical need to identify novel treatments to improve the outcome of relapsed and refractory AML6 and NHL7 and to identify better treatments in the elderly with AML and myelodysplastic syndrome.8 Novel agents showing activity in these groups of patients are therefore of great interest. It is always difficult to disassemble the impact of dosing on response in phase 1 studies, particularly when, as here, the study examines multiple dosing schedules. However, the numbers are relatively large here for a phase 1 study. Among the 95 AML patients, 81 were evaluable for response, 31% had a >50% reduction in blasts, and 14% had objective responses, including 2 complete remissions (CRs) and 2 CRs with incomplete count recovery. Correlative studies demonstrated a sustained increase in XPO1 levels in peripheral blood mononuclear cells for up to 48 hours after the first selinexor dose. By the end of cycle 1, there was a decrease in XPO1 protein, but not RNA, in AML blasts and there was an increase in protein levels of TP53 and FLT3. The lymphoma cohort was heterogeneous, but there was an expansion cohort in the diffuse large B-cell lymphoma (DLBCL) subgroup. Among 79 lymphoma patients enrolled, 70 were evaluable for response. The overall response rate was 31% and included 4 CRs in DLBCL patients and 18 partial responses, and almost half of the patients had target lesion reduction. Biopsy specimens were taken in a very small cohort of responding patient, and these showed nuclear accumulation of XPO1 cargo proteins, including TP53 and PTEN, with decreased levels of cMYC, BCL2, BCL6, and pSTAT.
Selinexor therefore appears to have activity in both AML and NHL, especially DLBCL. The observation of any CRs with single agents in phase 1 studies in these patient populations is always grounds for cautious excitement. What would be really useful is if we had some sense of being able to determine which patients will respond well to this drug. High XPO1 levels are associated with poor outcome,9 so it would be attractive to have an agent that targets this protein complex. It is not clear if the threefold reduction in XPO1 levels seen in this study is sufficient to inhibit XPO1 activity in the high-expressing poor-risk patients or if it works better in those with lower levels, where a further reduction in levels might lower XPO1 function below critical levels. The restoration of expression of TSPs, notably TP53, might potentially lead to increased sensitivity to conventional chemotherapy, and this is being explored in future. The paradoxical early increase in XPO1 levels by compensatory levels means that the timing and sequencing of agents will have to be studied carefully. Hematologic toxicity could also limit this approach.
Notwithstanding these limitations, these studies demonstrate interesting activity in high-risk patient populations and clearly merit further investigation, and phase 2 studies are under way. Biomarkers of response to identify the small subgroup of patients who can have the type of excellent responses seen here in this phase 1 study should be a priority.
Conflict-of-interest disclosure: J.G.G. has received honoraria from Janssen, Pharmacyclics, Abbvie, Roche, Celgene, Acerta, Gilead, and TG Therapeutics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal