Key Points
MHC-II and its master regulator CIITA are downregulated in CML stem/progenitor cells in a BCR-ABL kinase–independent manner.
JAK1/2 inhibition increased MHC-II expression, suggesting elevation of CML immunogenicity may provide a way to reduce CML persistence.
Abstract
Targeting the fusion oncoprotein BCR-ABL with tyrosine kinase inhibitors has significantly affected chronic myeloid leukemia (CML) treatment, transforming the life expectancy of patients; however the risk for relapse remains, due to persistence of leukemic stem cells (LSCs). Therefore it is imperative to explore the mechanisms that result in LSC survival and develop new therapeutic approaches. We now show that major histocompatibility complex (MHC)-II and its master regulator class II transactivator (CIITA) are downregulated in CML compared with non-CML stem/progenitor cells in a BCR-ABL kinase–independent manner. Interferon γ (IFN-γ) stimulation resulted in an upregulation of CIITA and MHC-II in CML stem/progenitor cells; however, the extent of IFN-γ-induced MHC-II upregulation was significantly lower than when compared with non-CML CD34+ cells. Interestingly, the expression levels of CIITA and MHC-II significantly increased when CML stem/progenitor cells were treated with the JAK1/2 inhibitor ruxolitinib (RUX). Moreover, mixed lymphocyte reactions revealed that exposure of CD34+ CML cells to IFN-γ or RUX significantly enhanced proliferation of the responder CD4+CD69+ T cells. Taken together, these data suggest that cytokine-driven JAK-mediated signals, provided by CML cells and/or the microenvironment, antagonize MHC-II expression, highlighting the potential for developing novel immunomodulatory-based therapies to enable host-mediated immunity to assist in the detection and eradication of CML stem/progenitor cells.
Introduction
The development of tyrosine kinase inhibitors (TKIs) to target BCR-ABL kinase has revolutionized the management of chronic phase chronic myeloid leukemia (CML), with many patients now predicted to have a normal life expectancy.1,2 Remission is maintained by continuous administration of TKI and assessed by quantification of BCR-ABL transcripts in the blood. For the 10% to 20% of patients who achieve deep and durable molecular responses, discontinuation studies have been conducted.3,4 Approximately 60% of patients maintain a major molecular response over time.5
Before TKI introduction, CML was a common indication for allogeneic stem cell transplantation. In this setting, disease remission was achieved by the combination of antileukemic chemoradiotherapy and active graft-versus-leukemia effect. The degree of immune recognition of leukemic cells by the donor immune system was such that disease relapse, if it occurred, could be managed successfully by the administration of donor lymphocytes.6 Although it is well recognized that the effect of allogeneic stem cell transplantation and graft-versus-leukemia is mainly an alloimmune effect mediated through non-disease-specific minor histocompatibility antigens, it is likely that CML cells express disease-specific antigens recognizable by the donor immune system. The role of the patient’s own immune system in recognizing BCR-ABL-expressing cells, and whether this can be boosted for beneficial effect, is currently under investigation in vaccination studies, although no convincing results have been reported.7,8 Similarly, it is not known whether immune recognition by the patient’s immune system is playing a part in maintaining remission of nonrelapsing patients in whom TKI treatment is discontinued.
Although CD8+ cytotoxic T lymphocytes are considered to play a major role in tumor immunity, CD4+ T helper cells are also important for mediating antitumor-associated immune responses, possibly through optimal induction and maintenance of cytotoxic T lymphocyte responses, interactions with effector cells, and production of antitumor-associated cytokines such as interleukin 2 (IL-2) and IFN-γ.9,10 As such, solid tumors (eg, non–small cell lung cancer, mammary adenocarcinoma, colorectal, and gastric) and hematological cancers (B-cell lymphomas) display major histocompatibility complex (MHC) class II (MHC-II) downregulation, reducing the host immune response toward the tumor; correlations have been found between higher MHC-II expression and better prognosis.11,12 Our microarray data sets comparing the expression of genes between normal and CML stem/progenitor revealed a significant downregulation in the antigen presentation (exogenous antigen) pathway in quiescent and dividing CD34+ CML cells.13 Here, we investigate the biological relevance of this finding, determining the mechanisms that underlie MHC-II downregulation in CML stem/progenitor cells and examining whether its induction could render these cells more immunogenic.
Materials and methods
Primary samples of cell culture
CD34+ cells were enriched, after informed consent, from either chronic phase samples from patients with CML at diagnosis (fresh or cryopreserved; Table 1) or allograft donors/lymphoma patients without bone marrow involvement as “non-CML” controls. The studies were approved by the West of Scotland Research Ethics Committee 4, National Health Service Greater Glasgow and Clyde (UK). Primary CML cells were cultured in serum-free medium, supplemented with Flt-3 ligand and stem cell factor (each 100 ng/mL), IL-3 and IL-6 (each 20 ng/mL; StemCell Technologies, Cambridge, UK), and G-CSF (Chugai Pharma, London, UK) overnight. Thereafter, for experimental conditions, CD34+-enriched CML cells were cultured in stem cell factor, granulocyte-macrophage colony-stimulating factor, and macrophage inflammatory protein α (all 0.2 ng/mL), G-CSF and IL-6 (both 1.0 ng/mL), and 0.05 ng/mL leukemia inhibitory factor (StemCell Technologies). IFN-γ and transforming growth factor β (TGF-β) were purchased from Peprotech EC Ltd. (London, UK), nilotinib (NIL) from Stratech Scientific Ltd. (Newmarket, UK), and imatinib mesylate (IM), dasatinib, SB-505124, and ruxolitinib (RUX) from Selleckchem (Houston, TX). Pan-MHC-II antibody (Ab; purified, clone Tü39) used for blocking experiments was purchased from BD Biosciences (Oxford, UK).
Source of clinical samples
| Sample no. . | Sample ID . | Source . | Sex . | Stage . | FISH/t(9:22) . | Figures . |
|---|---|---|---|---|---|---|
| 1 | Spirit CML 0762 | PB | M | CP | ND | 1, 2, 5 |
| 2 | CML 339 | Leukapheresis | F | CP | + | 2, 4, 5 |
| 3 | CML 340 | Leukapheresis | M | CP | +* | 4, 5 |
| 4 | CML 341 (Fresh) | Leukapheresis | M | CP | +* | 1, 2 |
| 5 | CML 378 | Leukapheresis | F | CP | + | 1, 2 |
| 6 | CML 381 | Leukapheresis | F | CP | + | 1, 2 |
| 7 | CML 385 | Leukapheresis | M | CP | +* | 1, 2 |
| 8 | CML 388 | Leukapheresis | F | CP | +* | 1, 2 |
| 9 | CML 411 (Fresh) | PB | F | CP | ND | 4, 5 |
| 10 | CML 412 (Fresh) | Leukapheresis | M | CP | ND | 2, 4, 5 |
| 11 | CML 441 | Leukapheresis | M | CP | + | 2 |
| 12 | CML 442 | PB | F | CP | + | 4, 5 |
| 13 | CML 450 | PB | M | CP | + | 5 |
| 14 | CML 452 | Leukapheresis | M | CP | + | 2 |
| 15 | CML 454 (Fresh) | Leukapheresis | M | CP | ND | 3 |
| 16 | CML 456 | Leukapheresis | F | CP | ND | 3 |
| 17 | CML 457 (Fresh) | PB | M | CP | ND | 3, 4 |
| 18 | CML 459 | Leukapheresis | M | CP | ND | 3 |
| 19 | CML 460 (Fresh) | Leukapheresis | F | CP | ND | 1, 3, 4 |
| 20 | CML 461 (Fresh) | Leukapheresis | F | CP | ND | 3, 5 |
| Sample no. . | Sample ID . | Source . | Sex . | Stage . | FISH/t(9:22) . | Figures . |
|---|---|---|---|---|---|---|
| 1 | Spirit CML 0762 | PB | M | CP | ND | 1, 2, 5 |
| 2 | CML 339 | Leukapheresis | F | CP | + | 2, 4, 5 |
| 3 | CML 340 | Leukapheresis | M | CP | +* | 4, 5 |
| 4 | CML 341 (Fresh) | Leukapheresis | M | CP | +* | 1, 2 |
| 5 | CML 378 | Leukapheresis | F | CP | + | 1, 2 |
| 6 | CML 381 | Leukapheresis | F | CP | + | 1, 2 |
| 7 | CML 385 | Leukapheresis | M | CP | +* | 1, 2 |
| 8 | CML 388 | Leukapheresis | F | CP | +* | 1, 2 |
| 9 | CML 411 (Fresh) | PB | F | CP | ND | 4, 5 |
| 10 | CML 412 (Fresh) | Leukapheresis | M | CP | ND | 2, 4, 5 |
| 11 | CML 441 | Leukapheresis | M | CP | + | 2 |
| 12 | CML 442 | PB | F | CP | + | 4, 5 |
| 13 | CML 450 | PB | M | CP | + | 5 |
| 14 | CML 452 | Leukapheresis | M | CP | + | 2 |
| 15 | CML 454 (Fresh) | Leukapheresis | M | CP | ND | 3 |
| 16 | CML 456 | Leukapheresis | F | CP | ND | 3 |
| 17 | CML 457 (Fresh) | PB | M | CP | ND | 3, 4 |
| 18 | CML 459 | Leukapheresis | M | CP | ND | 3 |
| 19 | CML 460 (Fresh) | Leukapheresis | F | CP | ND | 1, 3, 4 |
| 20 | CML 461 (Fresh) | Leukapheresis | F | CP | ND | 3, 5 |
CP, chronic phase; ND, not determined; PB, peripheral blood.
CD34+CD38− CML LSCs were also confirmed to be Ph+ by fluorescence in situ hybridization after sorting.
Microarray analysis
The microarray analysis leading to the initial identification of the MHC-II gene family (Figure 1A) was described previously.13 The experimental details for the second, larger quiescent/dividing microarray data set (Figure 1B-C) and the CML primitive/progenitor microarray data set (Figure 1C; supplemental Figure 1A-B, available on the Blood Web site) were described previously.14,15 The processing and normalization procedures for all data sets was carried out as described.16 All microarray data sets are summarized with respect to sample size, sorting strategy, and the relevant figure in supplemental Table 1; by combining these data sets, transcriptional profiles of 19 independent CML samples and 10 independent normal samples were analyzed. Where genes were represented by multiple probes, expression was summarized using the median value. Any genes not represented on all microarray chips were removed.
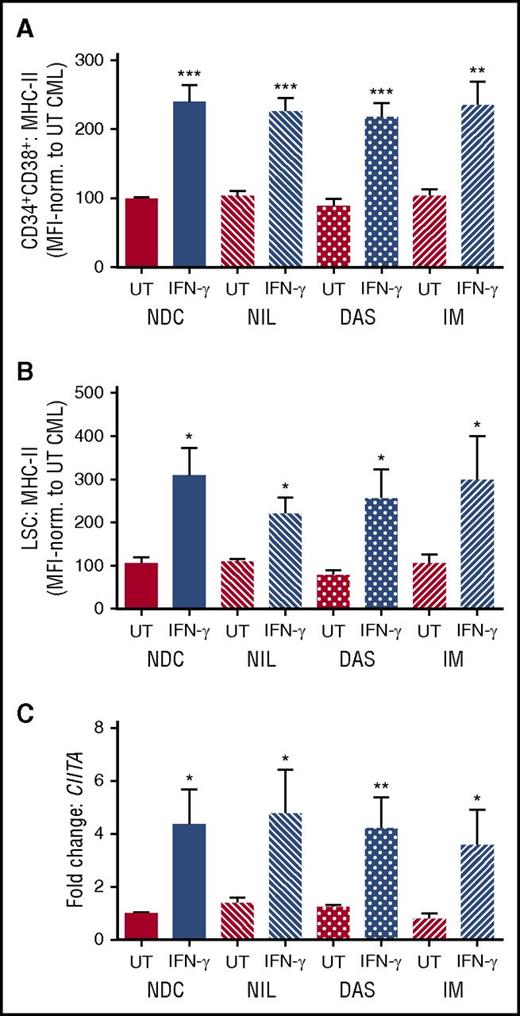
MHC-II and CIITA are selectively downregulated in CML stem/progenitor cells. (A) Ingenuity’s antigen presentation pathway overlaid with deregulation in CML vs normal G0 CD34+ cells (n = 5 CML, n = 2 non-CML). Deregulated members for MHC II-α and II-β (including HLA-DP, DQ, DR) are shown expanded in the bottom left. Significantly downregulated components highlighted in green. (B) GSEA analysis demonstrating significant (false discovery rate [FDR], ≤0.15) downregulation of the extended MHC-II gene family in a larger, complementary microarray data set of G0/quiescent (left) and dividing (right) CML and normal cells.14 (C) Heat map showing deregulation (as represented by logFC; see color scale below heat map) of the MHC-II gene family across multiple CML populations compared with corresponding non-CML cells from distinct microarray data sets, as indicated.14,15 The data derived from the original data set13 is highlighted by the orange bar above the corresponding columns. Average normalized MFI of surface MHC-II expression in primary (D) CD34+CD38+ CML and non-CML stem/progenitor cells or (E) CD34+CD38− CML LSCs and non-CML HSCs cultured for 48 hours in the presence or absence (UT) of IFN-γ (100 U/mL; n = 6 for CD34+CD38+; n = 4 for LSC; ± SEM). CD34+CD38− cells, when stringently gated, represent ∼1% to 5% of bulk CD34+ cells and overlap considerably with either Hstlo/Pylo or CD34+ CFSEmax populations described previously.13 (F) Average gene expression of CIITA in bulk CD34+ CML and non-CML stem/progenitor cells cultured for 48 hours ± IFN-γ (mean fold change; n = 6; ± SEM). Statistical significance was calculated between UT CML sample and all other samples, and if significant, it is indicated by asterisks above the bars. Additional comparisons between samples are indicated by lines.
MHC-II and CIITA are selectively downregulated in CML stem/progenitor cells. (A) Ingenuity’s antigen presentation pathway overlaid with deregulation in CML vs normal G0 CD34+ cells (n = 5 CML, n = 2 non-CML). Deregulated members for MHC II-α and II-β (including HLA-DP, DQ, DR) are shown expanded in the bottom left. Significantly downregulated components highlighted in green. (B) GSEA analysis demonstrating significant (false discovery rate [FDR], ≤0.15) downregulation of the extended MHC-II gene family in a larger, complementary microarray data set of G0/quiescent (left) and dividing (right) CML and normal cells.14 (C) Heat map showing deregulation (as represented by logFC; see color scale below heat map) of the MHC-II gene family across multiple CML populations compared with corresponding non-CML cells from distinct microarray data sets, as indicated.14,15 The data derived from the original data set13 is highlighted by the orange bar above the corresponding columns. Average normalized MFI of surface MHC-II expression in primary (D) CD34+CD38+ CML and non-CML stem/progenitor cells or (E) CD34+CD38− CML LSCs and non-CML HSCs cultured for 48 hours in the presence or absence (UT) of IFN-γ (100 U/mL; n = 6 for CD34+CD38+; n = 4 for LSC; ± SEM). CD34+CD38− cells, when stringently gated, represent ∼1% to 5% of bulk CD34+ cells and overlap considerably with either Hstlo/Pylo or CD34+ CFSEmax populations described previously.13 (F) Average gene expression of CIITA in bulk CD34+ CML and non-CML stem/progenitor cells cultured for 48 hours ± IFN-γ (mean fold change; n = 6; ± SEM). Statistical significance was calculated between UT CML sample and all other samples, and if significant, it is indicated by asterisks above the bars. Additional comparisons between samples are indicated by lines.
Gene set enrichment analysis
Gene set enrichment was performed using gene set enrichment analysis (GSEA)17 (gsea2-2.2.2.jar), as obtained from the Broad Institute (http://software.broadinstitute.org/gsea/index.jsp); q values were calculated using 10 000 permutations of the phenotype label. The MHC-II geneset analyzed by GSEA is shown in Figure 1C and is composed of the Ingenuity pathway shown in Figure 1A and “MHC class II” molecules identified by searching Metacore KB (https://portal.genego.com).
Flow cytometry
Cells were harvested and resuspended in 1 × 106 cells/100 µL fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline with 2% fetal calf serum and 0.02% sodium azide). All Abs were purchased from BD Biosciences unless otherwise stated. Cell staining was performed using 0.5 μg of each fluorochrome-conjugated Ab for 30 minutes, 4°C in dark (anti-CD34, anti-CD38, anti-HLA-DP, anti-DQ, anti-DR, anti-CD4, anti-CD69 Ab). Anti-CD8 and anti-CD107a Ab were purchased from eBioscience (Hatfield, UK). Events were acquired using a FACSCanto II cytometer and FACSDiva software (BD Biosciences), and data were analyzed using FlowJo software (Treestar Inc, Ashland, OR). To isolate CD34+CD38+ (bulk) and CD34+CD38− (leukemic stem cell [LSC]) populations, cells were sorted using a FACSAria cytometer (BD Biosciences).
RT-PCR and Fluidigm analysis
Total RNA was prepared using RNAeasy Plus extraction kit (Qiagen, Valencia, CA). RNA (1 μg) was reverse-transcribed using SuperScript reverse transcriptase and oligo dT primers (Invitrogen Life Technologies, Paisley, UK), or where mentioned, cDNA was prepared from 300 cells using the SuperScript III One-Step reverse transcription polymerase chain reaction (RT-PCR) System with Platinum Taq High Fidelity (Invitrogen). PCR analysis was performed on a 48.48 dynamic array, using the BioMarkHD System (Fluidigm, San Francisco, CA) or Applied Biosystems Prism 7900HT system (Applied Biosystems, Paisley, UK), as per manufacturers’ instructions. Relative gene expression was calculated using the 2-ΔΔCT method.
Mixed lymphocyte reactions and CD107a expression assay
Peripheral blood mononuclear cells (PBMCs; responder cells) were isolated from healthy donors by density centrifugation, using Histopaque (Sigma-Aldrich, Poole, UK). The PBMCs were incubated with Dynabeads Human T-Activator CD3/CD28 (Life Technologies), as per manufacturer’s instructions, for 48 hours in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, penicillin (100 U/mL), streptomycin (100 U/mL), and l-glutamine (2 mM) (Life Technologies). Activated PBMCs were sequestered from the beads, using a magnet and labeled with Cell Trace Violet (CTV) (Life Technologies) before coculture with stimulator/target (bulk CD34+ CML) cells. For mixed lymphocyte reactions, activated PBMCs were cocultured with stimulator cells for up to 6 days at a ratio of 3:1 responder:stimulator. Proliferation was measured as a loss of CTV mean fluorescence intensity (MFI) on analysis by flow cytometry. For CD107a expression, activated PBMCs were cocultured with bulk CD34+ CML target cells for up to 6 days at a ratio of 1.5:1 CD4+ T cells:target.
Statistical analysis
Average responses from at least 3 individual CML donors are shown (mean ± SEM). Statistical analysis was performed using GraphPad Prism 4 (GraphPad Software Inc., CA), using Student paired or unpaired t test (*P < .05; **P < .005; ***P < .001), as appropriate.
Results
MHC-II expression is downregulated in CML CD34+ stem/progenitor cells in a BCR-ABL kinase–independent manner
Microarray data sets analyzing the expression of genes in either normal or CML CD34+-quiescent (HoechstloPyronin Ylo) or dividing (Hst+Py+) cells revealed that the antigen presentation pathway was the top deregulated pathway in the CML vs normal comparison (P = 5.93 × 10−9; Figure 1A; significantly downregulated components highlighted in green).13 To validate this observation in silico, and assess more broadly whether the MHC-II gene family is suppressed in CML cells, we extended this gene list to include other relevant MHC-II molecules and identified a second, complementary data set14 in which we could perform GSEA17 of this MHC-II gene family. Limited numbers of normal samples prevented such an analysis in the original data set. This analysis confirmed significant downregulation of the MHC-II family in both quiescent and dividing CML cells (FDR, ≤0.15; Figure 1B). The same analysis was then performed in another data set transcriptionally profiling primitive (Lin−CD34+CD38−CD90+) CML cells against 3 progressively more mature cell populations, all from a single cohort of patients with CML and non-CML controls.15 Although some genes are not downregulated individually, there is a trend for downregulation of the MHC-II genes in the primitive (Lin−CD34+CD38−CD90+) and the 3 more mature (Lin−CD34+CD38+CD123+CD45RA-; Lin−CD34+CD38+CD123−CD45RA-; and Lin−CD34+CD38+CD123+CD45RAlo) cell populations (Figure 1C and supplemental Figure 1A, respectively). This represents a significant downregulation of the MHC-II gene family overall in all 4 cell populations, as confirmed by GSEA (supplemental Figure 1B). Overall, this in silico validation process considered 19 independent CML samples and 10 independent normal samples (supplemental Table 1). Analysis of costimulatory molecules indicated that although there was some variation in their expression across the populations, tumor necrosis factor (TNF) SF4 (OX-40L) and CD40 appeared to be downregulated in LSC-enriched populations compared with in non-CML populations (supplemental Figure 1C).
At the protein level we demonstrated a significant reduction in MHC-II surface expression on CML stem/progenitor cells, both in the bulk CD34+ population and in the CD34+CD38− LSC population, compared with normal hematopoietic stem cell (HSC) populations (Figure 1D-E; supplemental Figure 2). IFN-γ, an established activator of MHC-II expression18 that signals through the JAK/STAT pathway, upregulated MHC-II expression in CML stem/progenitor cells (Figure 1D-E; supplemental Figure 2). However, the level of IFN-γ-induced MHC-II upregulation in CML cells was significantly reduced compared with in non-CML HSCs (Figure 1D-E; supplemental Figure 2). Analysis of the master regulator of MHC-II expression, class II transactivator (CIITA),19 revealed a downregulation of this gene in CML stem/progenitor cells compared with in non-CML cells (Figure 1F). Supporting the MHC-II data, IFN-γ increased CIITA transcription in CML stem/progenitor cells, but not to the level observed in IFN-γ-treated non-CML cells (Figure 1F). These data indicate that CML stem/progenitor cells exhibit MHC-II downregulation, which may assist in their evasion from host immunity.
Treatment of bulk CD34+ and CD34+CD38− CML cells for 48 hours with TKIs (NIL, dasatinib, IM) had no effect on either MHC-II or CIITA expression, demonstrating that BCR-ABL activity did not significantly influence CIITA or MHC-II expression on CML stem/progenitor cells in the absence or presence of IFN-γ (Figure 2; supplemental Figure 3). This suggests that MHC-II regulation in CML is BCR-ABL kinase independent.
MHC-II and CIITA downregulation occurs independent of BCR-ABL kinase activity in CML stem/progenitor cells. Primary (A) CD34+CD38+ CML cells and (B) CD34+CD38− CML LSCs were cultured for 48 hours with TKIs (5 µM NIL, 150 nM dasatinib, 5 µM IM) or no drug control (NDC) in the presence or absence of IFN-γ. Average normalized MFI of MHC-II expression was determined using flow cytometry (n = 5; ± SEM). (C) CIITA expression levels in CD34+CD38+ CML cells were analyzed by quantitative reverse transcription polymerase chain reaction (n = 5; ± SEM; calibrated to UT NDC sample). Statistical significance was calculated between UT CML sample and all other samples and, if significant, is indicated by asterisks above the bars.
MHC-II and CIITA downregulation occurs independent of BCR-ABL kinase activity in CML stem/progenitor cells. Primary (A) CD34+CD38+ CML cells and (B) CD34+CD38− CML LSCs were cultured for 48 hours with TKIs (5 µM NIL, 150 nM dasatinib, 5 µM IM) or no drug control (NDC) in the presence or absence of IFN-γ. Average normalized MFI of MHC-II expression was determined using flow cytometry (n = 5; ± SEM). (C) CIITA expression levels in CD34+CD38+ CML cells were analyzed by quantitative reverse transcription polymerase chain reaction (n = 5; ± SEM; calibrated to UT NDC sample). Statistical significance was calculated between UT CML sample and all other samples and, if significant, is indicated by asterisks above the bars.
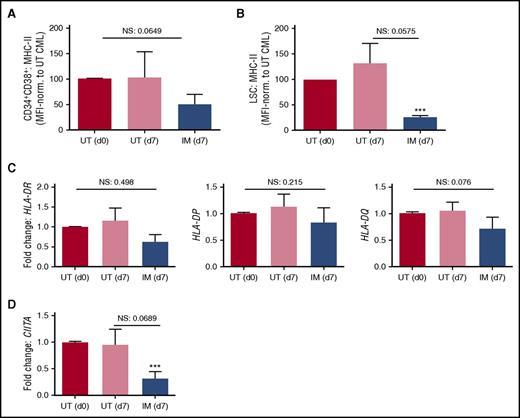
To determine whether MHC-II expression remains downregulated on extended treatment with TKI, bulk CD34+ CML stem/progenitor cells were cultured in the presence or absence of IM for 7 days before analysis of the residual cells in vitro. Over this time, IM may be expected to act either by inducing reversible changes in expression of genes that are kinase dependent or by selecting for a population of cells expressing higher or lower levels of certain genes as the result of killing more mature CD34+ cells that are sensitive to apoptosis. Surface protein and gene expression of MHC-II in day 7 cultured CML cells was normalized to untreated (UT) day 0 expression levels. Although no significant differences in MHC-II expression were observed between day 7 IM treated and UT CML cells, either on the surface of CD34+CD38+ or CD34+CD38− LSC populations or in the expression of MHC-II genes in bulk CD34+ CML cells (HLA-DR, HLA-DP, HLA-DQ; Figure 3A-C; supplemental Figure 4), there was a trend toward further downregulation of MHC-II expression in 7-day IM-treated CML cells. This was supported by a downregulation of CIITA in IM-treated cells when comparing them with UT cells on day 7 (Figure 3D). When IM-treated cells at day 7 were compared with UT cells at day 0, the observed trends reached significance for surface expression of MHC-II on CD34+CD38− LSC CML cells and for CIITA expression (Figure 3A-B,D). These data suggest that the regulation of MHC-II expression in CD34+CD38+ or CD34+CD38− LSC CML populations is BCR-ABL kinase independent, as it is not normalized after 48 hours (Figure 2) or 7 days of IM treatment (Figure 3). These data also suggest that by killing more mature cells within the CD34 compartment, IM enriches for cells with lower expression of MHC-II and CIITA, inferring that these surviving cells would be even less susceptible to immune attack.
Extended treatment of CML cells with IM does not normalize MHC-II or CIITA expression. Primary CD34+ CML were treated with 5 µM IM for 7 days. Average surface MHC-II expression levels were determined by flow cytometry, normalized to CML UT (d0) sample for (A) CD34+CD38+ CML cells and (B) CD34+CD38− CML LSCs. (C) The average expression of MHC-II encoding genes HLA-DR, HLA-DP, and HLA-DQ was determined in CD34+ CML cells by quantitative reverse transcription polymerase chain reaction (n = 7, calibrated to UT (d0) CML sample). (D) The average gene expression of CIITA was determined in CD34+ CML cells by quantitative reverse transcription polymerase chain reaction. Statistical significance was calculated between d0 UT CML sample and all other samples and, if significant, is indicated by asterisks above the bars. Additional comparisons between samples are indicated by lines. NS, not significant.
Extended treatment of CML cells with IM does not normalize MHC-II or CIITA expression. Primary CD34+ CML were treated with 5 µM IM for 7 days. Average surface MHC-II expression levels were determined by flow cytometry, normalized to CML UT (d0) sample for (A) CD34+CD38+ CML cells and (B) CD34+CD38− CML LSCs. (C) The average expression of MHC-II encoding genes HLA-DR, HLA-DP, and HLA-DQ was determined in CD34+ CML cells by quantitative reverse transcription polymerase chain reaction (n = 7, calibrated to UT (d0) CML sample). (D) The average gene expression of CIITA was determined in CD34+ CML cells by quantitative reverse transcription polymerase chain reaction. Statistical significance was calculated between d0 UT CML sample and all other samples and, if significant, is indicated by asterisks above the bars. Additional comparisons between samples are indicated by lines. NS, not significant.
MHC-II/CIITA expression is not modulated by aberrant methylation or TGF-β-mediated signaling in CML stem/progenitor cells
Aberrant DNA hypermethylation of the CIITA promoter has been identified as a mechanism for diminished IFN-γ-induced CIITA and MHC-II expression in both hematopoietic and solid tumors.20,21 Analysis of CIITA promoter methylation did not reveal any statistical differences between CML and normal stem/progenitor cells (supplemental Figure 5). Moreover, treatment of CML cells with methyltransferase inhibitor 5-azacytidine did not lead to increased MHC-II expression (supplemental Figure 6).
TGF-β has previously been shown to antagonize CIITA18,22,23 and MHC-II expression in the presence and absence of IFN-γ.19 Indeed, previous work has shown that TGF-β-mediated signals are activated in CML cells,24 and CML has been demonstrated to drive deregulated cytokine expression in mouse models.25 Initially, to analyze the effect of TGF-β on CIITA/MHC-II, we treated CML and non-CML cells with SB-505124 (TGF-βRI, ALK4, and ALK7 inhibitor26 ). Although CML stem/progenitor cells were slightly more responsive to TGF-β-mediated downregulation of MHC-II levels than non-CML cells, SB-505124 treatment did not affect MHC-II/CIITA expression on CML stem/progenitor cells (supplemental Figure 7).
Inhibition of JAK elevates MHC-II expression in CML stem/progenitor cells and increases their immunogenicity
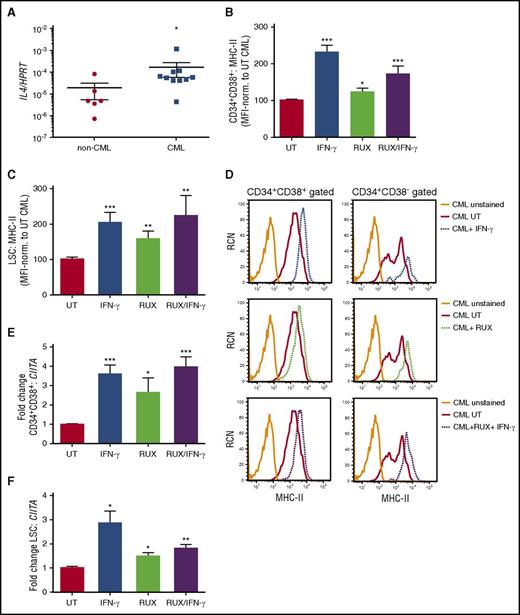
The cytokine IL-4 is also known to antagonize MHC-II and CIITA expression,18,22 suggesting that aberrant alterations in cytokine production within the niche may be responsible for their deregulation. Moreover, IL-4 has been shown to maintain survival of Ph+ cells on TKI inhibition.27 We demonstrate elevated transcript levels of IL-4 in CML compared with non-CML stem/progenitor cells (Figure 4A). As IL-4 and many cytokines signal via the JAK/STAT pathway, we analyzed the effect of cytokine-mediated signaling on CIITA/MHC-II, treating CML cells with RUX (a broad-spectrum JAK1/2 inhibitor28 ). RUX treatment of CML stem/progenitor cells significantly increased MHC-II and CIITA expression both on bulk CD34+ cells (Figure 4B,D-E) and CD34+CD38− LSC populations (Figure 4C-D,F). As IFN-γ activates the JAK/STAT pathway and RUX inhibits the same pathway, it was anticipated that RUX treatment combined with IFN-γ might reduce the level of MHC-II upregulation compared with that of IFN-γ alone. However, this reduction was not significant, suggesting that IFN-γ signaling was not fully inhibited in the presence of a JAK inhibitor (Figure 4B-F). Supporting this, analyzing genes associated with the IFN response, only the GBP1 response was partially modulated downward in CML cells when IFN-γ and RUX treatments were combined (supplemental Figure 8). These data indicate that RUX-mediated elevation in MHC-II may result from an inhibition of JAKs downstream of IL-4 receptors, thus arresting antagonistic signals and enhancing MHC-II expression.22
JAK inhibition elevates MHC-II expression in CML stem/progenitor cells. (A) Quantitative RT-PCR of RNA/cDNA generated from either non-CML or CML stem/progenitor samples for IL-4 transcripts revealed higher expression levels of IL-4 in CML cells. Data are expressed relative to the reference gene HPRT1. Primary (B) CD34+CD38+ CML cells and (C) CD34+CD38− CML LSCs were treated with IFN-γ and/or JAK inhibitor (RUX, 200 nM), as indicated. The average MHC-II expression levels were determined by flow cytometry, normalized to CML UT sample. NS, not significant. (D) Representative histograms showing MHC-II expression on CD34+CD38+ and CD34+CD38− CML cells treated with IFN-γ and/or RUX, as indicated; Primary CD34+ CML stem/progenitor cells were treated with IFN-γ and/or RUX, as indicated earlier. Thereafter, 300 cells were sorted for either (E) CD34+CD38+ CML and (F) CD34+CD38− CML LSC populations, and the average gene expression of CIITA was determined by quantitative reverse transcription polymerase chain reaction (n = 6 for CD34+CD38+, n = 3 for LSC; calibrated to UT CML sample). Statistical significance was calculated between UT CML sample and all other samples and, if significant, is indicated by asterisks above the bars.
JAK inhibition elevates MHC-II expression in CML stem/progenitor cells. (A) Quantitative RT-PCR of RNA/cDNA generated from either non-CML or CML stem/progenitor samples for IL-4 transcripts revealed higher expression levels of IL-4 in CML cells. Data are expressed relative to the reference gene HPRT1. Primary (B) CD34+CD38+ CML cells and (C) CD34+CD38− CML LSCs were treated with IFN-γ and/or JAK inhibitor (RUX, 200 nM), as indicated. The average MHC-II expression levels were determined by flow cytometry, normalized to CML UT sample. NS, not significant. (D) Representative histograms showing MHC-II expression on CD34+CD38+ and CD34+CD38− CML cells treated with IFN-γ and/or RUX, as indicated; Primary CD34+ CML stem/progenitor cells were treated with IFN-γ and/or RUX, as indicated earlier. Thereafter, 300 cells were sorted for either (E) CD34+CD38+ CML and (F) CD34+CD38− CML LSC populations, and the average gene expression of CIITA was determined by quantitative reverse transcription polymerase chain reaction (n = 6 for CD34+CD38+, n = 3 for LSC; calibrated to UT CML sample). Statistical significance was calculated between UT CML sample and all other samples and, if significant, is indicated by asterisks above the bars.
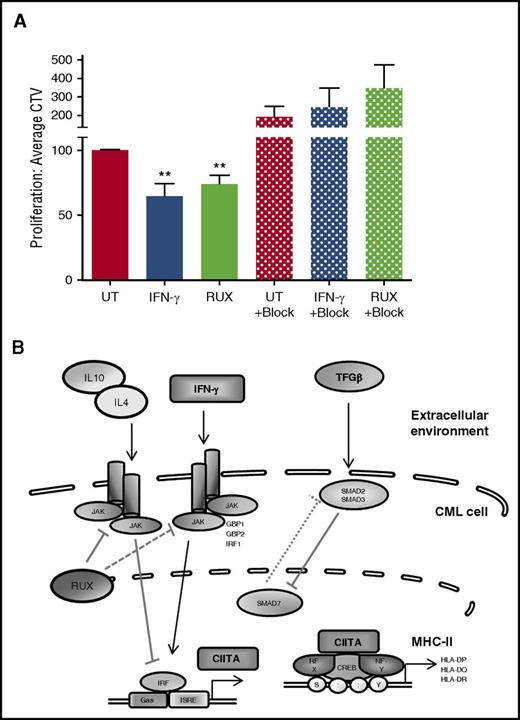
To determine whether the IFN-γ- or RUX-mediated elevation in MHC-II expression on CML cells could induce an immune response from alloreactive CD4+ T cells, we cocultured previously treated or control (UT) CML CD34+ stem/progenitor cells with CD3/CD28-activated PBMCs. No increase in expression of the cytolytic marker CD107a (LAMP1) was observed on CD4+ T cells after exposure to the CML targets, suggesting there was no induction of cytotoxic CD4+ T cells (supplemental Figure 9). However, when mixed lymphocyte reactions were conducted, IFN-γ and RUX treatments enhanced proliferation of responder CD4+CD69+ T cells, as indicated by decreased mean fluorescence intensity of CTV (Figure 5A). Importantly, the induction of proliferation was MHC-II dependent, as addition of anti-MHC-II blocking Ab blocked cellular proliferation in the cultures containing IFN-γ- and RUX-treated CML cells (Figure 5A).
Elevated MHC-II expression is associated with an increase in CD34+CML cell immunogenicity. (A) Activated and CTV labeled T cells (MNCs from healthy donors) were cocultured with bulk CD34+ CML cells for 72 hours (± treatment as indicated. Block, 10 μg/mL anti-pan HLA-class II blocking Ab). Proliferation of the responder cells was measured as a reduction in the MFI of CTV-labeled CD4+CD69+ T cells (n = 6 ± SEM; n = 3 for MHC-II blocking assays). (B) The autocrine or paracrine growth factor/cytokine signaling pathways that regulate MHC-II expression. Statistical significance was calculated between UT CML sample and all other samples and, if significant, is indicated by asterisks above the bars.
Elevated MHC-II expression is associated with an increase in CD34+CML cell immunogenicity. (A) Activated and CTV labeled T cells (MNCs from healthy donors) were cocultured with bulk CD34+ CML cells for 72 hours (± treatment as indicated. Block, 10 μg/mL anti-pan HLA-class II blocking Ab). Proliferation of the responder cells was measured as a reduction in the MFI of CTV-labeled CD4+CD69+ T cells (n = 6 ± SEM; n = 3 for MHC-II blocking assays). (B) The autocrine or paracrine growth factor/cytokine signaling pathways that regulate MHC-II expression. Statistical significance was calculated between UT CML sample and all other samples and, if significant, is indicated by asterisks above the bars.
Discussion
Eradicating CML LSCs represents a significant therapeutic challenge that will not be addressed with TKI therapy alone and requires novel treatments to enable removal of the disease reservoir to pave the way to a cure. One potential mechanism used by CML stem/progenitor cells to maintain a reservoir of LSCs is to evade the immune system by downregulating MHC-II expression. Our studies demonstrate that modulation of MHC-II is associated with an increase in CML cell immunogenicity in a JAK-dependent manner, leading us to propose a cytokine-mediated pathway for MHC-II deregulation in CML cells (Figure 5B). MHC-II and CIITA expression was independent of BCR-ABL kinase activity, as TKI treatment of CML cells did not significantly alter expression levels. Indeed, during IM exposure over the course of 7 days, the levels of MHC-II and CIITA were even further downregulated, rather than normalized. Of note, in silico analysis of costimulatory molecules revealed a mixed pattern of expression depending on the data set, although it does appear that OX40L is consistently downregulated in more quiescent CML cell subsets, compared with non-CML cells. These findings suggest that in addition to downregulating MHC-II genes, CML stem/progenitor cells may downregulate additional costimulatory molecules, further enhancing immune evasion. Exploitation of therapies that enhance CML stem cell immunogenicity to host immune cells could intensify immune responses against CML LSCs, thereby assisting in the elimination of this population, and ultimately the disease.
Although a number of mechanisms regulate MHC-II expression, one main cause for the loss of constitutive or inducible MHC-II expression in both hematopoietic and nonhematopoietic tumor cells is epigenetic silencing of the gene encoding CIITA.29,30 CIITA acts as a scaffold, interacting with DNA-binding transcription factors, regulatory factor X family, NF-Y, and CREB, which interact with and induce MHC-II expression.31,32 In addition to recruiting transcription factors, CIITA orchestrates the recruitment of histone acetyltransferases and histone methyltransferases to MHC-II gene promoters to regulate MHC-II expression.33 Although aberrant DNA hypermethylation of the CIITA promoter and breaks (nonrandom chromosomal translocations) can aid in the downregulation of MHC-II in hematopoietic malignancies,21,29,31 analyses of methylation marks on the CIITA promoter of CML LSCs revealed no difference in the CpG methylation profiles compared with non-CML stem/progenitor cells. Supporting this, 5-azacytidine treatment did not lead to increased MHC-II expression on CML cells. These data indicate that MHC-II downregulation in CML does not occur as the result of CIITA promoter hypermethylation, suggesting other molecular mechanisms are responsible for regulating CIITA/MHC-II expression in CML.
MHC-II and CIITA expression can be modulated by a number of cytokines, with TGF-β, TNF-α, IL-4, and IL-10 known to antagonize their expression,18,22,23 whereas IFN-γ upregulates expression (Figure 5B). Therefore, it is likely that the cytokine profile of the bone marrow microenvironment and/or of the leukemic cells themselves would have a significant effect on surface MHC-II expression of CML stem/progenitor cells. Indeed, CML LSCs have been demonstrated to produce higher levels of growth factors, via an autocrine loop, including elevated levels of TNF-α34 and IL4 in CD34+ CML cells. Furthermore, analysis of the bone marrow stroma in patients with CML and CML mouse models revealed elevated IL-1, IL-6, GCSF, and TNF-α, which together confer a growth advantage of CML LSCs over normal HSCs.25,35 These findings indicate that modulation of the cytokine profile in the CML bone marrow will have a significant effect not only on CML vs normal stem/progenitor cell survival, but also on CML LSC immunogenicity.
One family of protein kinases responsible for relaying signals downstream of cytokine receptors are the JAKs. BCR-ABL+ cells exhibit constitutively active JAK/STAT signaling, with JAK2 playing a central role in BCR-ABL-induced leukemogenesis.36 However, STAT5 activation is not dependent on JAK2 activity,37 and JAK2 can also act independently of STAT5 by activating Myc38 or β-catenin,39 or acting directly as a histone modifier,40 indicating that JAK2 may have pleiotropic roles in CML cells. Moreover, one of the main roles of JAK signaling is in immune cell activation/regulation.41 Here, we showed that treatment of cells with the JAK1/2 inhibitor RUX enhanced the expression of MHC-II on CML stem/progenitor cells, enhancing CD4+ T-cell proliferation. Although we demonstrated the induction of a helper T-cell response through RUX-mediated induction of MHC-II expression, CD4+ T cells have also been demonstrated to function as cytotoxic effectors against leukemic cells.42,43 On assessment of the cytolytic marker CD107a in vitro, we were unable to detect an elevation in expression, indicating that under our experimental conditions, cytotoxic CD4+ T cells were not induced. These results may reflect the limitations of the assay regarding the polyclonality of the CD4+ T-cell population, or a requirement for optimization at the level of effector/target ratio or coculture duration before assessing for the presence of cytotoxic CD4+ T cells.42,43
Recently, RUX has been shown to modulate immune responses in mouse models of graft-versus-host disease and hemophagocytic lymphohistiocytosis by dampening the hyperactive immune responses typical of these conditions.44,45 However, despite its anti-inflammatory properties, RUX was able to preserve the graft-versus-leukemia effect in graft-versus-host disease mouse models comprising in vivo tumor inoculation.46,47 Some of these selective effects were explained by differential responses of distinct T-cell subsets to RUX, particularly when used at different concentrations.45 It is therefore possible to speculate that the modulation of the immune system by RUX is cell context dependent and potentially affected by the intensity of the inflammatory responses. Moreover, using different doses of RUX might enable titration of its effects, thus maximizing its ability to enhance immunogenicity of CML cells while sparing T-cell immune function.
In conclusion, our studies suggest a key role for cytokines, such as IL-4, in reducing CML immunogenicity and offer a novel therapeutic route to aid in the elimination of CML LSCs. Because of the BCR-ABL-independent nature of MHC-II downregulation, combination therapies of JAK inhibitors with TKI could target the disease bulk and the LSCs.39,48 Indeed, recent studies demonstrate synergy between RUX and NIL, inducing apoptosis in CML LSCs.49 Our studies could suggest that LSCs remaining after dual RUX/TKI treatment may have a reduced ability to evade host immunity because of re-expression of MHC-II. Analysis of MHC-II surface expression on LSCs of patients treated with RUX in combination with NIL in current clinical trials (clinicaltrials.gov, NCT 01751425; 02253277; 01702064; 01914484) would be of great interest to test this in vivo. Boosting MHC-II expression on CML stem/progenitor cells may represent a valid therapeutic approach to reduce disease persistence, and our findings should promote the development of novel immunomodulatory-based therapies for patients with CML. These therapeutic strategies could enable CML stem cells to be more readily detected and eradicated by the host immune response, which in turn will deliver long-term remission or cure for patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients with CML and without CML and United Kingdom hematology departments, including those patients on the UK SPIRIT2 Trial Management Group for access to CML samples, Eduardo Gómez Castañeda for preparing some RNA samples, and Karen Stewart for collecting clinical information on patients.
This study was funded by project grants from Leuka and Tenovus-Scotland (Ref. S12/21). This study was supported by the Glasgow Experimental Cancer Medicine Centre, which is funded by Cancer Research UK and the Chief Scientist’s Office, Scotland. Cell sorting facilities were funded by the Kay Kendall Leukaemia Fund (KKL501) and the Howat Foundation. A.T. was funded by a Bloodwise project grant (13012). P.G. was funded by a Medical Research Council (MRC) UK clinical research training fellowship grant (G1000288). H.G.J. was funded by the Friends of Paul O’Gorman Leukemia Research Centre. F.P., L.E.M.H., and T.L.H. were supported by Cancer Research UK Programme grant (C11074/A11008). D.V. was funded by LLR project grant (14005). A.M.M. was supported by an MRC project grant (MR/K014854/1).
Authorship
Contribution: A.T. designed/performed the majority of experiments, analyzed and interpreted the data, carried out statistical analysis, and drafted the manuscript; P.G., K.K., and F.P. performed some experiments and carried out data analysis; L.E.M.H. carried out microarray and pathway analyses; J.C. carried out the cell sorting for all the experiments; A.H. processed all of the primary samples; H.G.J. supervised the studies and performed some of the experiments; D.V., T.L.H., and A.M.M. obtained funding for the study, designed the research, supervised the studies, analyzed and interpreted the data, and wrote the manuscript. All authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alison M. Michie, Paul O’Gorman Leukaemia Research Centre, Gartnavel General Hospital, 21 Shelley Rd, Glasgow G12 0ZD, United Kingdom; e-mail: alison.michie@glasgow.ac.uk; and Tessa L. Holyoake, Paul O’Gorman Leukaemia Research Centre, Gartnavel General Hospital, 21 Shelley Rd, Glasgow G12 0ZD, United Kingdom; e-mail: tessa.holyoake@glasgow.ac.uk.

![Figure 1. MHC-II and CIITA are selectively downregulated in CML stem/progenitor cells. (A) Ingenuity’s antigen presentation pathway overlaid with deregulation in CML vs normal G0 CD34+ cells (n = 5 CML, n = 2 non-CML). Deregulated members for MHC II-α and II-β (including HLA-DP, DQ, DR) are shown expanded in the bottom left. Significantly downregulated components highlighted in green. (B) GSEA analysis demonstrating significant (false discovery rate [FDR], ≤0.15) downregulation of the extended MHC-II gene family in a larger, complementary microarray data set of G0/quiescent (left) and dividing (right) CML and normal cells.14 (C) Heat map showing deregulation (as represented by logFC; see color scale below heat map) of the MHC-II gene family across multiple CML populations compared with corresponding non-CML cells from distinct microarray data sets, as indicated.14,15 The data derived from the original data set13 is highlighted by the orange bar above the corresponding columns. Average normalized MFI of surface MHC-II expression in primary (D) CD34+CD38+ CML and non-CML stem/progenitor cells or (E) CD34+CD38− CML LSCs and non-CML HSCs cultured for 48 hours in the presence or absence (UT) of IFN-γ (100 U/mL; n = 6 for CD34+CD38+; n = 4 for LSC; ± SEM). CD34+CD38− cells, when stringently gated, represent ∼1% to 5% of bulk CD34+ cells and overlap considerably with either Hstlo/Pylo or CD34+ CFSEmax populations described previously.13 (F) Average gene expression of CIITA in bulk CD34+ CML and non-CML stem/progenitor cells cultured for 48 hours ± IFN-γ (mean fold change; n = 6; ± SEM). Statistical significance was calculated between UT CML sample and all other samples, and if significant, it is indicated by asterisks above the bars. Additional comparisons between samples are indicated by lines.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/2/10.1182_blood-2016-09-742049/4/m_blood742049f1.jpeg?Expires=1769852719&Signature=2UBQjF3G-xfT671GQQYPDZbN~Ut8cpWnotZn~UHVzz4Zj4LDbWyEeZV6q85m6okGXWuopjbEBuF-lFL0~zjoFU2FZydTZcHo9U998-ZYJ4rKndCbpLeM2GVmKmtPpSUoAlCMAaSaSCdUWcuyn~LgZ--N6Ygs8XsJ4-pBFCGlOMwzsWPwk~3ucyB0qscDn4megph2-St6ud04m~PliMNge8CuQGUGJ84f6GfxYx6MRWJKxdPv9GePx91HBBTX-o0XKmjTD5KYFedo75iZUyvqoxvGv5-kBjhB3zWVnJtc2ECXrYE83LnIxN83tl1Kkjq~8td8ILyI-l5IBFaSaCjnrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)