Key Points
Platelet DREAM is required for platelet thrombus formation at the site of arteriolar injury in mice.
Platelet DREAM plays an important role in cell activation by regulation of PI3K class Iβ activity.
Abstract
Downstream regulatory element antagonist modulator (DREAM), a transcriptional repressor, is known to modulate pain responses. However, it is unknown whether DREAM is expressed in anucleate platelets and plays a role in thrombogenesis. By using intravital microscopy with DREAM-null mice and their bone marrow chimeras, we demonstrated that both hematopoietic and nonhematopoietic cell DREAMs are required for platelet thrombus formation following laser-induced arteriolar injury. In a FeCl3-induced thrombosis model, we found that compared with wild-type (WT) control and nonhematopoietic DREAM knockout (KO) mice, DREAM KO control and hematopoietic DREAM KO mice showed a significant delay in time to occlusion. Tail bleeding time was prolonged in DREAM KO control mice, but not in WT or DREAM bone marrow chimeric mice. In vivo adoptive transfer experiments further indicated the importance of platelet DREAM in thrombogenesis. We found that DREAM deletion does not alter the ultrastructural features of platelets but significantly impairs platelet aggregation and adenosine triphosphate secretion induced by numerous agonists (collagen-related peptide, adenosine 5′-diphosphate, A23187, thrombin, or U46619). Biochemical studies revealed that platelet DREAM positively regulates phosphoinositide 3-kinase (PI3K) activity during platelet activation. Using DREAM-null platelets and PI3K isoform-specific inhibitors, we observed that platelet DREAM is important for α-granule secretion, Ca2+ mobilization, and aggregation through PI3K class Iβ (PI3K-Iβ). Genetic and pharmacological studies in human megakaryoblastic MEG-01 cells showed that DREAM is important for A23187-induced Ca2+ mobilization and its regulatory function requires Ca2+ binding and PI3K-Iβ activation. These results suggest that platelet DREAM regulates PI3K-Iβ activity and plays an important role during thrombus formation.
Introduction
Downstream regulatory element antagonist modulator (DREAM/calsenilin/KChIP3) was identified as a neuronal Ca2+-sensing protein that binds to the downstream regulatory element (DRE) motif on DNA and modulates pain by repressing transcription of the prodynorphin gene.1,2 Among 4 EF-hand motifs, studies suggested that E186 at EF-3 and E234 at EF-4 binds to Ca2+ with a Kd of 1 µM, whereas D150 at EF-2 binds to Mg2+ under physiological conditions.3,4 EF-1 is nonfunctional and does not bind Ca2+.3 During cell activation, DREAM binds to intracellular Ca2+ and is dissociated from the DRE motif, thereby translocating out of the nucleus and allowing gene transcription.1,5 In addition to its binding to DNA, cytosolic DREAM also interacts with numerous proteins, including calmodulin and presenilin.6,7 Although DREAM is predominantly expressed in neuronal cells in which it regulates synaptic plasticity8 and apoptosis,9,10 it is also expressed in other cell types including leukocytes and endothelial cells.11,12 However, it is unknown whether DREAM is expressed in anucleate platelets and plays a role in thrombosis and hemostasis.
Following vascular injury, platelets adhere to activated endothelial cells and/or subendothelial matrix proteins such as von Willebrand factor and collagen via the glycoprotein Ib/IX/V (GPIb/IX/V) complex and GPVI, respectively.13 Although the interaction of each receptor with a ligand or agonist induces a distinct signaling pathway, downstream signaling requires an increase in cytosolic Ca2+ concentration and activation of protein kinases.14,15 Activated platelets then release thromboxane A2 and granular molecules, such as adenosine 5′-diphosphate (ADP), thereby amplifying intracellular signaling and inducing full activation of αIIbβ3 integrin for platelet-platelet aggregation. Because dysregulation of signaling events can lead to thrombosis or bleeding disorders, it is of great importance to precisely understand the molecular mechanism governing platelet activation.
Many studies demonstrated that phosphoinositide 3-kinase (PI3K) and its downstream molecule, AKT, are activated by numerous platelet receptors including GPVI, G-protein–coupled receptors, and αIIbβ3 integrin and could be attractive targets for the treatment of thrombotic diseases.16,17 PI3Ks are divided into 4 distinct classes (IA, IB, II, and III). Class IA PI3Ks are composed of a regulatory (p85α, p55α, p50α, p85β, or p55γ) and a catalytic subunit (p110α, p110β, or p110δ), whereas class IB PI3K consists of a regulatory (p101 or p84) and a catalytic subunit (p110γ).18 Class I PI3Ks mainly generate phosphatidylinositol-3,4,5-triphosphate (PI(3,4,5)P3) by phosphorylating PI(3,4)P2. Class II and III PI3Ks produce PI(3,4)P2 from PI(3)P and PI(3)P from phosphoinositide, respectively. In particular, class I PI3Ks are expressed in platelets, and their roles have been studied.18,19 However, it remains poorly understood how PI3K is activated following agonist stimulation.
In the present study, we demonstrate that DREAM plays a critical role in platelet activation and thrombogenesis. DREAM is important for platelet activation and aggregation induced by numerous agonists. Using DREAM−/− (knockout [KO]) platelets and isoform-specific PI3K inhibitors, we show that platelet DREAM acts as a novel regulator of PI3K class Iβ (PI3K-Iβ) activation. Further, studies using DREAM knockdown/overexpression and PI3K inhibitors in human megakaryoblastic cells suggest that DREAM positively regulates cell activation through Ca2+ binding and PI3K-Iβ activity. Our findings demonstrate for the first time that DREAM plays an important role in thrombosis and hemostasis in mice.
Materials and methods
Mice
Wild-type (WT) mice (C57BL/6) were obtained from The Jackson Laboratory. DREAM KO mice were obtained from Josef Penninger (Institute of Molecular Biotechnology, Vienna, Austria)2 and backcrossed for 10 generations to C57BL/6 mice. Except for intravital microscopy, age-matched male and female mice were used in all studies. The University of Illinois Institutional Animal Care and Use Committee approved all animal care and experimental procedures.
Intravital microscopy
Real-time fluorescence intravital microscopy was performed as we described.20 Briefly, WT and DREAM KO male mice (6-8 weeks old) were anesthetized by intraperitoneal injection of ketamine and xylazine, and the cremaster muscle was exposed. In some experiments, bone marrow chimeric male mice were used (14-16 weeks old). Platelet thrombus formation was visualized by infusion of DyLight 649–labeled anti-mouse CD42c antibodies (0.1 µg/g body weight [BW]) into mice. Six to 8 thrombi were generated in different arterioles with a diameter of 30 to 45 µm per mouse and recorded using an Olympus BX61W microscope with a 60 × 1.0 numerical aperture water-immersion objective and a Hamamatsu C9300 high-speed camera through an intensifier (Video Scope International). The kinetics of platelet accumulation were monitored in the integrated median fluorescence intensities and plotted as a function of time. Data were analyzed using Slidebook (v6.0; Intelligent Imaging Innovations).
For the FeCl3-induced thrombosis model, the cremaster muscle was exposed and DyLight 649–labeled anti-mouse CD42c antibodies were infused into mice as described in the previous paragraph. A filter paper (3 × 3 mm) was soaked in 9% FeCl3 solution and applied to a main cremaster arteriole with a diameter of 50 to 80 µm for 3 minutes. Time to vessel occlusion was measured when blood flow completely stopped for at least 1 minute. Time to occlusion exceeding 30 minutes was noted as 30 minutes. In adoptive transfer experiments, WT and DREAM KO platelets were isolated and labeled with a CellTracker Red CMTPX dye (excitation/emission, 577/602 nm). The recipient WT mice were pretreated with an anti-CD42b antibody (R300, 0.5 µg/g BW) to deplete endogenous platelets.21 Thirty minutes after antibody treatment, the labeled WT or KO platelets, 108 in 100 µL of saline, were infused into the thrombocytopenic WT mice. Three thrombi were generated following laser injury. Due to the depletion of infused platelets in the mouse, the same labeled platelets were reinfused to generate 3 additional thrombi. Accumulation of infused platelets was monitored and analyzed as described in the previous paragraph. To further examine the clearance kinetics of infused WT and DREAM KO platelets, CellTracker Red CMTPX-labeled platelets were infused into WT mice pretreated with R300 as described earlier in this paragraph. Blood was collected at 0, 2, 5, 10, and 30 minutes after infusion, followed by flow cytometry.
Other experiments
Experimental procedures, including bone marrow transplantation, quantitative reverse transcription–polymerase chain reaction (RT-PCR), flow chamber assay, platelet aggregation assay, transmission electron microscopy, immunoblotting, immunoprecipitation, flow cytometry, PI3K activity assay, Ca2+ mobilization, bleeding time, and DREAM small interefering RNA (siRNA) knockdown, are described in supplemental Methods (available on the Blood Web site).20,22,23
Statistics
Data analysis was performed using GraphPad Prism 6. Statistical significance was assessed by analysis of variance (ANOVA) and the Tukey test, or the Dunn test for comparison of multiple groups. Also, the Student t test or the Mann-Whitney U test was used for comparison of 2 groups. A value of P < .05 was considered significant.
Results
DREAM plays a critical role in platelet thrombus formation and hemostasis following vascular injury in mice
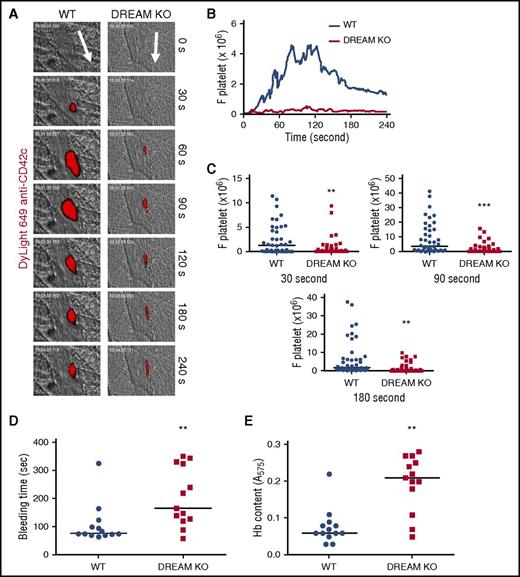
To investigate the role of DREAM during thrombus formation, we first performed intravital microscopy in a mouse model of laser-induced cremaster arteriolar thrombosis.20 We found that compared with WT mice, DREAM KO mice showed a significant reduction in platelet adhesion and accumulation at the site of arteriolar injury (Figure 1A-C; supplemental Videos 1 and 2). DREAM deletion did not affect blood counts (Table 1). We also examined the contribution of DREAM to hemostatic function. Compared with WT mice, DREAM KO mice displayed prolonged bleeding times following tail transection (Figure 1D). As quantified by hemoglobin content, DREAM deletion significantly increased blood loss from the site of tail amputation (Figure 1E). Taken together, these results indicate that DREAM is required for thrombogenesis and hemostasis in mice.
DREAM is required for platelet thrombus formation and hemostasis at the site of vascular injury in mice. Fluorescence intravital microscopy was performed as described in “Methods.” Following laser-induced injury of cremaster muscle arterioles, platelet accumulation was visualized by infusion of DyLight 649–labeled anti-mouse CD42c antibodies into WT or DREAM KO mice. (A) Representative images are shown over the course of 240 seconds after injury. Arrows show direction of blood flow. (B) The integrated median fluorescence intensities of anti-CD42c antibodies (F platelet) were obtained from 42 to 44 thrombi in 6 WT or 6 KO mice and are presented as a function of time. (C) Fluorescence intensities of anti-CD42c antibodies in WT and DREAM KO mice were compared at 30, 90, and 180 seconds after arteriolar injury. (D) Tail bleeding time was monitored by cutting 5 mm of the tail of WT (●) and DREAM KO (▪) mice (n = 13 mice). (E) Blood loss during the bleeding time assay was determined by measuring the absorbance at 575 nm of hemoglobin (Hb). **P < .01 and ***P < .001 vs WT mice after the Mann-Whitney U test. Horizontal bars represent the median value of the fluorescence intensities of anti-CD42c antibodies, bleeding times, or Hb content.
DREAM is required for platelet thrombus formation and hemostasis at the site of vascular injury in mice. Fluorescence intravital microscopy was performed as described in “Methods.” Following laser-induced injury of cremaster muscle arterioles, platelet accumulation was visualized by infusion of DyLight 649–labeled anti-mouse CD42c antibodies into WT or DREAM KO mice. (A) Representative images are shown over the course of 240 seconds after injury. Arrows show direction of blood flow. (B) The integrated median fluorescence intensities of anti-CD42c antibodies (F platelet) were obtained from 42 to 44 thrombi in 6 WT or 6 KO mice and are presented as a function of time. (C) Fluorescence intensities of anti-CD42c antibodies in WT and DREAM KO mice were compared at 30, 90, and 180 seconds after arteriolar injury. (D) Tail bleeding time was monitored by cutting 5 mm of the tail of WT (●) and DREAM KO (▪) mice (n = 13 mice). (E) Blood loss during the bleeding time assay was determined by measuring the absorbance at 575 nm of hemoglobin (Hb). **P < .01 and ***P < .001 vs WT mice after the Mann-Whitney U test. Horizontal bars represent the median value of the fluorescence intensities of anti-CD42c antibodies, bleeding times, or Hb content.
The number of circulating blood cells in WT and DREAM KO mice
| . | WBC, 109/L . | NE, 109/L . | LY, 109/L . | MO, 109/L . | RBC, 109/L . | PLT, 109/L . | MPV, fL . |
|---|---|---|---|---|---|---|---|
| WT | 5.0 ± 1.6 | 0.7 ± 0.2 | 3.3 ± 1.8 | 0.1 ± 0.0 | 9.0 ± 1.2 | 1138 ± 167 | 7.3 ± 1.6 |
| DREAM KO | 3.7 ± 1.5 | 0.5 ± 0.2 | 1.7 ± 0.7 | 0.1 ± 0.0 | 9.2 ± 1.0 | 1154.3 ± 159 | 8.3 ± 0.3 |
| . | WBC, 109/L . | NE, 109/L . | LY, 109/L . | MO, 109/L . | RBC, 109/L . | PLT, 109/L . | MPV, fL . |
|---|---|---|---|---|---|---|---|
| WT | 5.0 ± 1.6 | 0.7 ± 0.2 | 3.3 ± 1.8 | 0.1 ± 0.0 | 9.0 ± 1.2 | 1138 ± 167 | 7.3 ± 1.6 |
| DREAM KO | 3.7 ± 1.5 | 0.5 ± 0.2 | 1.7 ± 0.7 | 0.1 ± 0.0 | 9.2 ± 1.0 | 1154.3 ± 159 | 8.3 ± 0.3 |
Blood cells from WT and DREAM KO mice were counted using an automated hematology analyzer (ADVIA 120; Siemens AG). Data represent the mean ± SD (n = 6 mice per group).
LY, lymphocyte; MO, monocyte; MPV, mean platelet volume; NE, neutrophil; PLT, platelet; RBC, red blood cell; WBC, white blood cell.
Platelet DREAM is important for thrombus formation following arteriolar injury
To further dissect the role of hematopoietic and nonhematopoietic DREAMs during thrombus formation, control and DREAM bone marrow chimeric mice were generated. Blood counts did not differ between the 4 groups (Table 2). Using intravital microscopy, we observed that compared with control WT mice, hematopoietic, nonhematopoietic, and control DREAM KO mice showed a remarkable reduction in platelet thrombus formation (Figure 2A-C), suggesting the importance of both hematopoietic and nonhematopoietic DREAMs in laser-induced arteriolar thrombosis. The thrombus size was significantly smaller in hematopoietic or control DREAM KO mice, compared with nonhematopoietic DREAM KO mice. Although it remains unclear how nonhematopoietic cell DREAM regulates thrombogenesis, our results highlight the contribution of blood-derived DREAM to platelet thrombus formation.
The number of circulating blood cells in control and DREAM bone marrow chimeric mice
| . | WBC, 109/L . | NE, 109/L . | LY, 109/L . | MO, 109/L . | RBC, 109/L . | PLT, 109/L . | MPV, fL . |
|---|---|---|---|---|---|---|---|
| WT | 4.2 ± 1.3 | 0.7 ± 0.2 | 3.2 ± 1.5 | 0.1 ± 0.0 | 9.0 ± 1.3 | 1088 ± 167 | 7.6 ± 1.6 |
| HP DREAM KO | 3.1 ± 0.7 | 0.7 ± 0.2 | 3.0 ± 0.5 | 0.1 ± 0.0 | 10.5 ± 1.4 | 1011 ± 178 | 7.4 ± 1.8 |
| Non-HP DREAM KO | 3.3 ± 0.5 | 1.0 ± 0.3 | 3.8 ± 0.8 | 0.1 ± 0.0 | 9.9 ± 1.0 | 1054 ± 159 | 8.3 ± 1.3 |
| DREAM KO | 3.2 ± 0.6 | 0.6 ± 0.2 | 2.7 ± 0.7 | 0.1 ± 0.0 | 9.5 ± 1.0 | 889 ± 110 | 7.3 ± 1.5 |
| . | WBC, 109/L . | NE, 109/L . | LY, 109/L . | MO, 109/L . | RBC, 109/L . | PLT, 109/L . | MPV, fL . |
|---|---|---|---|---|---|---|---|
| WT | 4.2 ± 1.3 | 0.7 ± 0.2 | 3.2 ± 1.5 | 0.1 ± 0.0 | 9.0 ± 1.3 | 1088 ± 167 | 7.6 ± 1.6 |
| HP DREAM KO | 3.1 ± 0.7 | 0.7 ± 0.2 | 3.0 ± 0.5 | 0.1 ± 0.0 | 10.5 ± 1.4 | 1011 ± 178 | 7.4 ± 1.8 |
| Non-HP DREAM KO | 3.3 ± 0.5 | 1.0 ± 0.3 | 3.8 ± 0.8 | 0.1 ± 0.0 | 9.9 ± 1.0 | 1054 ± 159 | 8.3 ± 1.3 |
| DREAM KO | 3.2 ± 0.6 | 0.6 ± 0.2 | 2.7 ± 0.7 | 0.1 ± 0.0 | 9.5 ± 1.0 | 889 ± 110 | 7.3 ± 1.5 |
Blood cells from WT control, DREAM KO control, and their bone marrow chimeric mice (HP and Non-HP DREAM KO) were counted using an automated hematology analyzer (ADVIA 120; Siemens AG). Data represent the mean ± SD (n = 6 mice per group).
HP, hematopoietic; Non-HP, nonhematopoietic. Other abbreviations are explained in Table 1.
Both hematopoietic and nonhematopoietic cell DREAMs participate in thrombosis and hemostasis, and platelet DREAM is important for thrombogenesis. WT or DREAM KO transplant control mice and DREAM bone marrow chimeras (hematopoietic [HP] or non-HP DREAM KO) were used for intravital microscopy as described in Figure 1. (A) Representative images. Arrows show direction of blood flow. (B-C) F platelet and fluorescence intensities of anti-CD42c antibodies in 4 different groups are presented as described in Figure 1 (32-38 thrombi in 5 mice of each group). **P < .01 and ***P < .001 vs WT control mice and #P < .05, ##P < .01, and ###P < .001 between 2 groups after the Mann-Whitney U test. (D) Cremaster arteriolar thrombosis was induced by FeCl3 injury. Time to occlusion was plotted (n = 8-10). (E-F) Tail bleeding time and blood loss were measured as described in Figure 1D-E (n = 8). (G-I) Fluorescently labeled WT or DREAM KO platelets, 108 in 100 µL of saline, were infused into thrombocytopenic WT mice. Three thrombi were generated following laser injury. The same labeled platelets were reinfused into the mouse to generate 3 additional thrombi. Accumulation of infused platelets was monitored and analyzed as described above. Horizontal bars represent the median value of the fluorescence intensities of anti-CD42c antibodies (C,I), time to occlusion (D), bleeding times (E), or Hb content (F). *P < .05 and ***P < .001 vs WT platelets after the Mann-Whitney U test (n = 4-5 mice for each group) or after the Dunn test (D-F).
Both hematopoietic and nonhematopoietic cell DREAMs participate in thrombosis and hemostasis, and platelet DREAM is important for thrombogenesis. WT or DREAM KO transplant control mice and DREAM bone marrow chimeras (hematopoietic [HP] or non-HP DREAM KO) were used for intravital microscopy as described in Figure 1. (A) Representative images. Arrows show direction of blood flow. (B-C) F platelet and fluorescence intensities of anti-CD42c antibodies in 4 different groups are presented as described in Figure 1 (32-38 thrombi in 5 mice of each group). **P < .01 and ***P < .001 vs WT control mice and #P < .05, ##P < .01, and ###P < .001 between 2 groups after the Mann-Whitney U test. (D) Cremaster arteriolar thrombosis was induced by FeCl3 injury. Time to occlusion was plotted (n = 8-10). (E-F) Tail bleeding time and blood loss were measured as described in Figure 1D-E (n = 8). (G-I) Fluorescently labeled WT or DREAM KO platelets, 108 in 100 µL of saline, were infused into thrombocytopenic WT mice. Three thrombi were generated following laser injury. The same labeled platelets were reinfused into the mouse to generate 3 additional thrombi. Accumulation of infused platelets was monitored and analyzed as described above. Horizontal bars represent the median value of the fluorescence intensities of anti-CD42c antibodies (C,I), time to occlusion (D), bleeding times (E), or Hb content (F). *P < .05 and ***P < .001 vs WT platelets after the Mann-Whitney U test (n = 4-5 mice for each group) or after the Dunn test (D-F).
We also used DREAM bone marrow chimeric mice in a FeCl3-induced cremaster arteriolar thrombosis model. When a filter paper soaked in 9% FeCl3 solution was applied to a main cremaster arteriole for 3 minutes, the median time to occlusion in WT and DREAM KO control mice were 4.8 and 22.1 minutes, respectively (P < .05, Figure 2D). Compared with control WT mice, hematopoietic DREAM KO mice displayed a significant prolongation of time to occlusion (24.2 minutes), whereas nonhematopoietic DREAM KO mice had no prolongation (2.4 minutes). These results suggest that hematopoietic cell DREAM is critical for FeCl3-induced thrombus formation. In addition, because unlike laser-induced injury, FeCl3-induced injury can disrupt the endothelium,24 our finding that nonhematopoietic DREAM deletion had no effect on FeCl3-induced thrombosis suggests the importance of endothelial cell DREAM in arterial thrombosis.
Because DREAM KO mice showed an increase in bleeding time and blood loss following tail amputation, we further examined the importance of hematopoietic or nonhematopoietic DREAM in hemostasis. Compared with WT control mice, DREAM KO control, but not hematopoietic and nonhematopoietic DREAM KO, mice significantly prolonged bleeding time and increased blood loss at the site of tail amputation (Figure 2E-F). Our results suggest that both hematopoietic and nonhematopoietic DREAMs work together for hemostasis or that either is sufficient for maintaining hemostatic function.
Due to the lack of platelet-specific DREAM-null mice, we performed 2 different experiments to confirm the role of platelet DREAM in thrombogenesis. First, we depleted Gr-1+ leukocytes by infusion of anti-Gr-1 antibodies and repeated intravital microscopy.25 After depletion of the leukocytes, platelet adhesion and accumulation were still defective in DREAM KO mice, compared with WT mice (supplemental Figure 1A-B). As analyzed by flow cytometry using mouse blood drawn after intravital microscopy, the number of polymorphonuclear cells was decreased to 70% of the control and Ly6G+ neutrophils were markedly depleted in WT and DREAM KO mice treated with the anti-Gr-1 antibody (supplemental Figure 1C-F). These results suggest the minimal role of leukocyte DREAM in thrombogenesis. Next, adoptive transfer experiments were carried out using isolated platelets. To eliminate the contribution of endogenous platelets to thrombus formation, WT mice were pretreated with anti-CD42b antibodies to deplete platelets.21 We observed that antibody treatment dose-dependently reduced the number of circulating platelets with >90% depletion within 30 minutes at 0.5 µg/g BW (supplemental Figure 2). After pretreatment with R300 (0.5 µg/g BW), fluorescently labeled WT or DREAM KO platelets were infused into the thrombocytopenic mice, followed by intravital microscopy. Compared with infused WT platelets, infused DREAM KO platelets displayed a significant reduction in the thrombus size at the site of vascular injury (Figure 2G-I). In a control experiment, infused WT and DREAM KO platelets were equally cleared in WT mice pretreated with R300 (supplemental Figure 3). These results indicate that platelet DREAM is critical for thrombus formation.
DREAM regulates platelet aggregation and ATP secretion following stimulation with numerous agonists
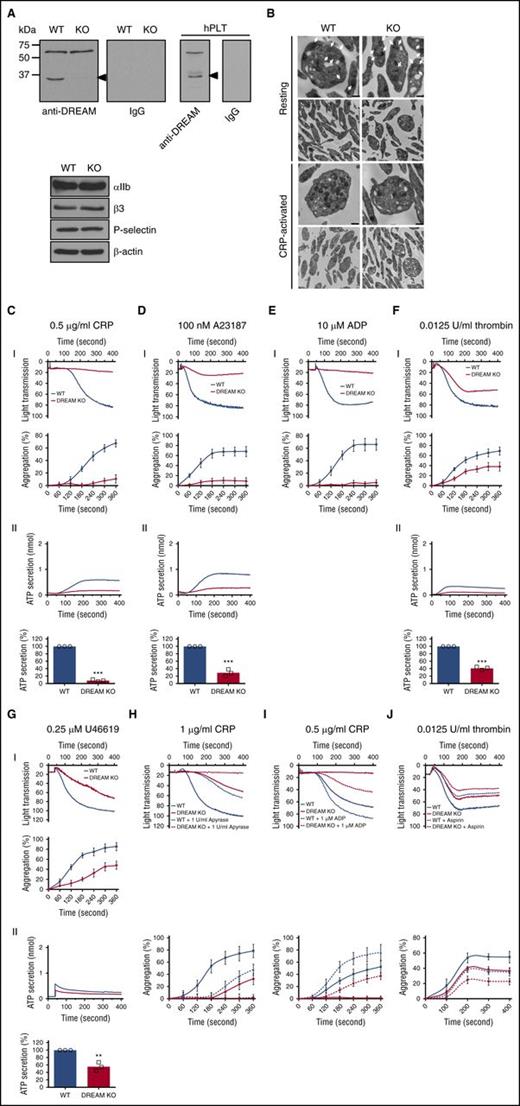
To study the role of DREAM in regulating platelet function, we first confirmed that platelets express DREAM. Immunoblotting analysis showed that DREAM was expressed in gel-filtered mouse and human platelets (Figure 3A). The 35-kDa band was not detected in DREAM KO platelets. Importantly, DREAM deletion did not alter the expression of αIIb, β3, and P-selectin (Figure 3A). Moreover, transmission electron microscopy revealed similar ultrastructures between resting and collagen-related peptide (CRP)-activated WT and DREAM-null platelets (Figure 3B). As extrapolated from the thickness of the sections (80 nm), the number of α-granules was similar between resting WT and KO platelets (59.4 ± 4 and 61.4 ± 6 [mean ± standard deviation (SD)] in 1 WT and KO platelet, respectively), although we could not precisely count dense granules due to the limited number (4-6) in a single platelet. Quantitative RT-PCR analysis showed that DREAM messenger RNA (mRNA) was present in platelets (supplemental Figure 4A-B). Equal amounts of mRNA of the leukocyte-specific β2 integrin subunit were detected in WT and DREAM-null neutrophils but not platelets (supplemental Figure 4C-D).
Platelet DREAM regulates aggregation and ATP secretion following stimulation with numerous agonists. (A) Lysates of gel-filtered mouse and human platelets (hPLT) were immunoblotted. Representative blots were obtained from 3 independent experiments. (B) WT and DREAM KO platelets were treated with or without 0.5 µg/mL CRP, followed by transmission electron micrographs. Original magnification was ×4800 and extension was ×13 000. The arrows show α-granules. Bar, 500 nm. (C-G) Platelet aggregation and ATP secretion were induced by 0.5 µg/mL CRP, 100 nM A23187, 10 µM ADP, 0.0125 U/mL thrombin, and 0.25 µM U46619. In the ADP-induced aggregation assay, 30 µg/mL human FG was added to the platelet suspension before ADP stimulation. (I) Platelet aggregation and quantitative graphs. (II) ATP secretion was monitored with platelet aggregation with a luciferin/luciferase reagent. Data represent the mean ± SD (n = 3). (H-I) WT and DREAM KO platelets were pretreated with (H) 1 U/mL apyrase or (I) 1 µM ADP, and aggregation was induced by CRP, respectively. (J) WT and DREAM KO platelets were pretreated with 1 mM aspirin, followed by stimulation with 0.0125 U/mL thrombin. Platelet aggregation and quantitative graphs are shown. Data represent the mean ± SD (n = 3). **P < .01 and ***P < .001 vs WT platelets after the Student t test.
Platelet DREAM regulates aggregation and ATP secretion following stimulation with numerous agonists. (A) Lysates of gel-filtered mouse and human platelets (hPLT) were immunoblotted. Representative blots were obtained from 3 independent experiments. (B) WT and DREAM KO platelets were treated with or without 0.5 µg/mL CRP, followed by transmission electron micrographs. Original magnification was ×4800 and extension was ×13 000. The arrows show α-granules. Bar, 500 nm. (C-G) Platelet aggregation and ATP secretion were induced by 0.5 µg/mL CRP, 100 nM A23187, 10 µM ADP, 0.0125 U/mL thrombin, and 0.25 µM U46619. In the ADP-induced aggregation assay, 30 µg/mL human FG was added to the platelet suspension before ADP stimulation. (I) Platelet aggregation and quantitative graphs. (II) ATP secretion was monitored with platelet aggregation with a luciferin/luciferase reagent. Data represent the mean ± SD (n = 3). (H-I) WT and DREAM KO platelets were pretreated with (H) 1 U/mL apyrase or (I) 1 µM ADP, and aggregation was induced by CRP, respectively. (J) WT and DREAM KO platelets were pretreated with 1 mM aspirin, followed by stimulation with 0.0125 U/mL thrombin. Platelet aggregation and quantitative graphs are shown. Data represent the mean ± SD (n = 3). **P < .01 and ***P < .001 vs WT platelets after the Student t test.
In aggregation assays, compared with WT platelets, DREAM-null platelets showed significant defects in both aggregation and adenosine triphosphate (ATP) secretion induced by CRP (0.5 µg/mL), A23187 (100 nM), ADP (10 µM), thrombin (0.0125 U/mL), or U46619 (0.25 µM) (Figure 3C-G). Higher concentrations of those agonists, however, attenuated or nullified the defect in aggregation and ATP secretion of DREAM KO platelets (supplemental Figure 5). Because secreted ADP in part mediates platelet aggregation induced by low concentrations of agonists,26 we examined whether DREAM modulates CRP-mediated platelet aggregation through ADP secretion. Pretreatment of WT platelets with apyrase, an ADP scavenger, partially inhibited aggregation induced by CRP (Figure 3H). Apyrase treatment further blocked DREAM-null platelet aggregation induced by 1 µg/mL CRP (Figure 3H), a concentration at which platelet aggregation was partially reduced by DREAM deletion (supplemental Figure 5A). Conversely, when WT platelets were pretreated with 1 μM ADP and stimulated with 0.5 µg/mL CRP, platelet aggregation was further enhanced (Figure 3I). The aggregation defect in DREAM-null platelets was only partially rescued by 1 µM ADP. These results suggest that DREAM regulates platelet aggregation through a signaling pathway induced by both CRP and ADP.
Because thromboxane A2 (TxA2) produced by a low dose of thrombin is important for mediating platelet aggregation,27 we also examined whether DREAM deletion affects TxA2-mediated aggregation. We found that aspirin (1 mM) inhibited WT platelet aggregation induced by 0.0125 U/mL thrombin and the inhibitory effect was further potentiated in DREAM KO platelets (Figure 3J). These results imply that TxA2 and DREAM induce platelet aggregation via a distinct signaling pathway.
Platelet DREAM is important for PI3K activation through GPVI signaling
Because DREAM deletion caused significant defects in platelet aggregation and ATP secretion following CRP stimulation, we investigated how DREAM regulates CRP-induced GPVI signaling that activates numerous kinases, including Src family kinases, Syk, and PI3K, and phospholipase Cγ2 (PLCγ2).16,28 To match the experimental condition with the aggregation assays, platelets were activated with 0.5 μg/mL CRP under stirring conditions in an aggregometer and lysed immediately at the indicated time points. We found that Syk, linker for activation of T cells (LAT), PI3K p85α/β, and PLCγ2 in WT platelets were rapidly phosphorylated after CRP stimulation, whereas AKT phosphorylation was slightly delayed. DREAM deletion significantly reduced the phosphorylation levels of PI3K p85α/β, PLCγ2, and AKT, but not Syk and LAT that are the upstream molecules of PI3K and PLCγ2, following CRP stimulation (Figure 4A). Because LAT serves as a platform for several signaling molecules, including PI3K, in GPVI signaling,29,30 we determined whether DREAM affects the LAT-PI3K interaction. Platelets were treated with CRP for 2 minutes with stirring, followed by immunoprecipitation. We found that binding of LAT to phosphorylated PI3K (p-PI3K) p85α/β was increased in CRP-activated WT platelets, whereas the binding was abrogated in activated DREAM KO platelets (Figure 4B). Moreover, p-PI3K p85α/β was coimmunoprecipitated with DREAM in WT platelets and the interaction was significantly enhanced following CRP stimulation (Figure 4C). Conversely, DREAM was coimmunoprecipitated with PI3K p85α/β, and the binding drastically increased in response to CRP treatment (Figure 4D). Similar results were obtained with human platelets (Figure 4E-F). Together, these results suggest that DREAM directly or indirectly binds to PI3K during platelet activation and thus regulates GPVI-mediated PI3K activation.
Platelet DREAM plays a critical role in PI3K activation through GPVI-, integrin-, or A23187-mediated signaling. (A) WT and DREAM KO platelets were stimulated by 0.5 µg/mL CRP for 1, 2, and 5 minutes under stirring conditions (1000 rpm) in an aggregometer. An equal amount of cell lysate protein (50 µg) was immunoblotted, followed by densitometry (arbitrary unit [AU], n = 5). (B-D) WT and DREAM KO platelets were stimulated with 0.5 µg/mL CRP for 2 minutes under stirring conditions. The lysates were immunoprecipitated with control IgG or antibodies against (B) LAT, (C) DREAM, or (D) total PI3K p85 (t-PI3K p85) followed by immunoblotting and densitometry (n = 4). (E-F) Human platelets were stimulated with 0.5 µg/mL CRP for 2 minutes under stirring conditions, followed by coimmunoprecipitation as shown in panels C and D. (G-H) WT and DREAM KO platelets were stimulated by 10 µM ADP for 1, 2, or 5 minutes in the (G) absence or (H) presence of 30 µg/mL FG under stirring conditions (n = 4). (I-J) WT and DREAM KO platelets were pretreated (I) without or (J) with 20 µg/mL eptifibatide following stimulation with 100 nM A23187 for 1, 2, or 5 minutes with stirring. An equal amount of lysate protein was immunoblotted, followed by densitometry (n = 4). *P < .05 and **P < .01 vs WT platelets at each time point (or resting platelets for panels C-F) after the Student t test.
Platelet DREAM plays a critical role in PI3K activation through GPVI-, integrin-, or A23187-mediated signaling. (A) WT and DREAM KO platelets were stimulated by 0.5 µg/mL CRP for 1, 2, and 5 minutes under stirring conditions (1000 rpm) in an aggregometer. An equal amount of cell lysate protein (50 µg) was immunoblotted, followed by densitometry (arbitrary unit [AU], n = 5). (B-D) WT and DREAM KO platelets were stimulated with 0.5 µg/mL CRP for 2 minutes under stirring conditions. The lysates were immunoprecipitated with control IgG or antibodies against (B) LAT, (C) DREAM, or (D) total PI3K p85 (t-PI3K p85) followed by immunoblotting and densitometry (n = 4). (E-F) Human platelets were stimulated with 0.5 µg/mL CRP for 2 minutes under stirring conditions, followed by coimmunoprecipitation as shown in panels C and D. (G-H) WT and DREAM KO platelets were stimulated by 10 µM ADP for 1, 2, or 5 minutes in the (G) absence or (H) presence of 30 µg/mL FG under stirring conditions (n = 4). (I-J) WT and DREAM KO platelets were pretreated (I) without or (J) with 20 µg/mL eptifibatide following stimulation with 100 nM A23187 for 1, 2, or 5 minutes with stirring. An equal amount of lysate protein was immunoblotted, followed by densitometry (n = 4). *P < .05 and **P < .01 vs WT platelets at each time point (or resting platelets for panels C-F) after the Student t test.
Platelet DREAM modulates PI3K activation through ADP-induced platelet aggregation
Because DREAM deletion impaired ADP-induced platelet aggregation and ADP-induced signaling activates Src kinase and PI3K-Iβ/γ via P2Y1 and P2Y12, respectively,31,32 we studied whether platelet DREAM modulates PI3K activation following ADP stimulation. The phosphorylation levels of Src, PI3K p85α/β, and AKT were elevated in ADP-activated WT platelets with stirring, but the phosphorylation pattern was similar between WT and DREAM KO platelets (Figure 4G), suggesting that DREAM does not directly regulate ADP-induced signaling pathways. Because ADP has little effect on platelet aggregation in the absence of fibrinogen (FG) and FG-αIIbβ3 interaction also activates PI3K-Iβ through Src,33,34 we examined the role of DREAM in inducing PI3K phosphorylation by ADP-mediated platelet aggregation in the presence of FG. Interestingly, DREAM deletion resulted in a significant reduction in the phosphorylation of PI3K p85α/β and AKT, but not Src (Figure 4H), suggesting that DREAM is important for PI3K activation through αIIbβ3-mediated platelet aggregation.
Platelet DREAM is important for PI3K activation through Ca2+-induced signaling
Our results suggested that platelet DREAM regulates A23187-induced signaling. It was reported that A23187-induced Ca2+ release enhances the activity of Src family kinases and PI3K in platelets.35 Consistently, we further found that the phosphorylation levels of Src, PI3K p85α/β, PLCγ2, and AKT were enhanced in WT platelets after stimulation with 100 nM A23187 under stirring conditions (Figure 4I). Interestingly, DREAM deletion significantly diminished the phosphorylation of PI3K p85α/β and AKT, but not Src and PLCγ2, following A23187 treatment. When platelets were pretreated with eptifibatide, an antagonist for αIIbβ3 integrin, to block outside-in signaling induced by A23187-mediated aggregation, Src was rapidly phosphorylated, whereas phosphorylation of PI3K p85α/β, PLCγ2, and AKT was delayed to 2 to 5 minutes (Figure 4J). DREAM deletion significantly reduced the phosphorylation of PI3K p85α/β and AKT following A23187 treatment. These results suggest that platelet DREAM positively regulates PI3K phosphorylation induced by A23187-mediated Ca2+ signaling.
DREAM regulates platelet activation through PI3K-Iβ activity
Previous studies showed that all class I PI3Ks play nonredundant roles in platelet function including granule secretion, Ca2+ mobilization, sustained activation of αIIbβ3 integrin, and thrombus stability.18,36,37 We found that CRP-induced P-selectin exposure and αIIbβ3 activation were significantly impaired by DREAM deletion (Figure 5A-B). To investigate which PI3K isoform(s) is regulated by DREAM, we used pan (LY294002 and wortmannin) and isoform-selective PI3K inhibitors (PIK75 for class Iα and TGX221 for class Iβ)36,38 in DREAM KO platelets. When stimulated with 0.5 µg/mL CRP, pan inhibitors, compared with vehicle control, perturbed P-selectin exposure without significant differences between WT and KO platelets (Figure 5C). Interestingly, PIK75 exhibited a similar inhibitory effect as pan inhibitors in activated DREAM KO platelets, whereas TGX221 did not show additive effects in the KO platelets, implying that platelet DREAM regulates α-granule secretion through PI3K-Iβ activation.
DREAM is important for platelet activation through PI3K-Iβ activity. (A-C) WT and DREAM KO platelets were pretreated (A-B) without or (C) with 0.05% dimethyl sulfoxide (DMSO) (vehicle), 50 µM LY294002 (LY), 0.1 µM wortmannin (Wort), or an isoform-specific inhibitor for PI3K-Iα (0.5 µM PIK75) or PI3K-Iβ (0.5 µM TGX221), followed by stimulation with 0.5 (for panel C), 1, or 2 µg/mL CRP. P-selectin exposure or αIIbβ3 integrin activation was analyzed by flow cytometry. The data are presented as the geometric mean fluorescence intensity (MFI) value (n = 3). (D-E) WT and DREAM KO platelets were incubated with a Ca2+ dye and treated with 0.5 µg/mL CRP or 100 nM A23187 in the absence of exogenous Ca2+ and the presence of 1 mM EGTA. Cytosolic Ca2+ levels were measured and quantified by area under the curve (AUC) (n = 3). (F-G) WT and DREAM KO platelets were incubated with vehicle or a PI3K inhibitor, followed by the Ca2+ assay as described above (n = 4). *P < .05, **P < .01, and ***P < .001 vs WT platelets after Student t test (for panels A-B and D-E) or vehicle-treated WT (white bars) or DREAM KO platelets (gray bars) after ANOVA and the Tukey test (for C and F-G). #P < .05 and ##P < .01 between 2 groups. (H-I) WT and DREAM KO platelets were pretreated with vehicle or a PI3K inhibitor as described above, followed by stimulation with 1 µg/mL CRP or 100 nM A23187. (J-K) WT and DREAM KO platelets were pretreated with vehicle or a PI3K inhibitor and then incubated (J) without or (K) with 1 mM aspirin, followed by stimulation with 0.0125 U/mL thrombin. Representative results of aggregation are presented (n = 3).
DREAM is important for platelet activation through PI3K-Iβ activity. (A-C) WT and DREAM KO platelets were pretreated (A-B) without or (C) with 0.05% dimethyl sulfoxide (DMSO) (vehicle), 50 µM LY294002 (LY), 0.1 µM wortmannin (Wort), or an isoform-specific inhibitor for PI3K-Iα (0.5 µM PIK75) or PI3K-Iβ (0.5 µM TGX221), followed by stimulation with 0.5 (for panel C), 1, or 2 µg/mL CRP. P-selectin exposure or αIIbβ3 integrin activation was analyzed by flow cytometry. The data are presented as the geometric mean fluorescence intensity (MFI) value (n = 3). (D-E) WT and DREAM KO platelets were incubated with a Ca2+ dye and treated with 0.5 µg/mL CRP or 100 nM A23187 in the absence of exogenous Ca2+ and the presence of 1 mM EGTA. Cytosolic Ca2+ levels were measured and quantified by area under the curve (AUC) (n = 3). (F-G) WT and DREAM KO platelets were incubated with vehicle or a PI3K inhibitor, followed by the Ca2+ assay as described above (n = 4). *P < .05, **P < .01, and ***P < .001 vs WT platelets after Student t test (for panels A-B and D-E) or vehicle-treated WT (white bars) or DREAM KO platelets (gray bars) after ANOVA and the Tukey test (for C and F-G). #P < .05 and ##P < .01 between 2 groups. (H-I) WT and DREAM KO platelets were pretreated with vehicle or a PI3K inhibitor as described above, followed by stimulation with 1 µg/mL CRP or 100 nM A23187. (J-K) WT and DREAM KO platelets were pretreated with vehicle or a PI3K inhibitor and then incubated (J) without or (K) with 1 mM aspirin, followed by stimulation with 0.0125 U/mL thrombin. Representative results of aggregation are presented (n = 3).
Because only PI3K-Iα and PI3K-Iβ are important for Ca2+ mobilization in GPVI-mediated signaling,37-39 we examined whether DREAM deletion affects Ca2+ mobilization in the absence of extracellular Ca2+ and in the presence of 1 mM EGTA. Compared with WT platelets, DREAM-null platelets showed a moderate but significant reduction in Ca2+ mobilization following CRP or A23187 stimulation (Figure 5D-E), suggesting that platelet DREAM regulates Ca2+ mobilization. We further studied whether DREAM modulates Ca2+ mobilization through a specific isoform of PI3K. Compared with vehicle control, treatment with 50 µM LY294002 or 0.1 µM wortmannin significantly impaired Ca2+ release in WT platelets after CRP or A23187 stimulation (Figure 5F-G). Although Ca2+ release was reduced in activated WT platelets after treatment with 0.5 µM PIK75 or TGX221, compared with vehicle control, the inhibitory effect of pan inhibitors was significantly greater than that of PIK75 or TGX221. These results suggest that both PI3K-Iα and PI3K-Iβ are important for CRP- or A23187-induced Ca2+ mobilization in platelets. When pan inhibitors were used in DREAM KO platelets, CRP- or A23187-induced Ca2+ release was further inhibited in comparison with vehicle-treated KO platelets (Figure 5F-G). Interestingly, PIK75 exhibited a similar inhibitory effect as the pan inhibitor in DREAM-null platelets, whereas TGX221 had no additional effect on Ca2+ release. It was reported that A23187-induced Ca2+ release increases TxA2 production in platelets.35 To address the possibility that A23187-induced Ca2+ mobilization is due to TxA2 production, we repeated the assay in the presence or absence of 1 mM aspirin. We found that DREAM KO and aspirin had an additive effect on A23187-induced Ca2+ release (supplemental Figure 6), showing that DREAM is unlikely to affect TxA2 production. Taken together, these results suggest that platelet DREAM regulates CRP- or A23187-induced Ca2+ mobilization through PI3K-Iβ activation.
To further determine whether DREAM directly binds to PI3K-Iβ, we used purified glutathione S-transferase–tagged human DREAM and human PI3K p110β/His-tagged p85α (supplemental Figure 7A). When DREAM was pulled down with glutathione-conjugated beads, PI3K p85α was coprecipitated (supplemental Figure 7B). We also examined whether DREAM affects PI3K activity in vitro. Incubation of PI3K-Iβ with DREAM significantly enhanced the enzyme activity (supplemental Figure 7C), whereas bovine serum albumin had no effect. Although the precise binding region between DREAM and PI3K remains to be determined, these results suggest that DREAM directly binds to PI3K-Iβ, enhancing its activity.
We also assessed whether DREAM modulates platelet aggregation through PI3K-Iβ. Pretreatment of WT and DREAM KO platelets with LY294002 or wortmannin showed similar inhibitory effects following CRP or A23187 stimulation (Figure 5H-I). Treatment with PIK75 partially inhibited aggregation of activated WT and DREAM KO platelets, whereas TGX221 treatment impaired aggregation of WT, but not KO, platelets (Figure 5H-I). Similar results were obtained in aggregation of WT and DREAM KO platelets induced by 0.0125 U/mL thrombin (Figure 5J). In addition, all PI3K inhibitors and aspirin additively inhibited aggregation of both WT and DREAM KO platelets (Figure 5K). These data suggest that DREAM regulates platelet aggregation through PI3K-Iβ activation and support our conclusion that DREAM and TxA2 could signal distinctly following stimulation with 0.0125 U/mL thrombin.
DREAM modulates Ca2+ mobilization in MEG-01 cells and the regulatory function is mediated by Ca2+ binding and PI3K-Iβ
Because no DREAM inhibitor is available, we used genetic approaches in a human megakaryoblastic cell line, MEG-01, to further determine the role of DREAM in PI3K signaling in human cells. We found that treatment of MEG-01 cells with 2 different siRNAs knocked DREAM down by >90%, compared with control siRNA (Figure 6A-B). Importantly, DREAM siRNAs did not alter the expression of αIIb, β3, PI3K p85, and AKT. Because CRP did not induce Ca2+ mobilization in MEG-01 cells (data not shown), MEG-01 cells were treated with A23187, followed by measurement of the cytosolic Ca2+ level as an indicator of cell activation. DREAM knockdown reduced A23187-induced Ca2+ release in MEG-01 cells (Figure 6C). To rule out the possibility of off-target effects of DREAM siRNAs, we performed rescue experiments using an expression vector containing siRNA #4–resistant DREAM. Because DREAM function is regulated by Ca2+ binding via E186 in the EF-3 and E234 in the EF-4 domains,1,3 we also designed vectors expressing siRNA #4–resistant EF-hand mutants (E186Q, E234Q, or both) (Figure 6D). Immunoblotting showed that both WT and mutant DREAM were equally overexpressed (Figure 6E). Although WT DREAM rescued the defect in A23187-induced Ca2+ release in siRNA #4–transfected MEG-01 cells, mutant DREAM had no rescue effect (Figure 6F). No difference was observed by single or double EF-hand mutant DREAM, suggesting that the regulatory function of MEG-01 cell DREAM on Ca2+ mobilization requires Ca2+ binding at either the EF-3 or EF-4 domain.
DREAM regulates Ca2+ mobilization in MEG-01 cells and its regulatory function requires Ca2+ binding and PI3K-Iβ activity. Control or DREAM siRNA (#3 or #4) was transfected into MEG-01 cells. (A-B) After 48 hours, cell lysates were immunoblotted, followed by densitometry (arbitrary unit [AU], n = 4). (C) After 48 hours, cells were incubated with a Ca2+ dye and treated with 100 nM A23187, followed by measurement of cytosolic Ca2+ levels (n = 4). *P < .05 vs control after the Student t test. (D) A schematic of DREAM siRNA-resistant wt and EF-hand mutant DREAM. (E) DREAM siRNA #4 was transfected into MEG-01 cells. After 4 hours, cells were transfected with a vector expressing siRNA #4–resistant wt or EF-hand mutant DREAM. After 48 hours, DREAM overexpression was verified by immunoblotting (n = 4). (F) The cells described in panel E were used for Ca2+ assays. **P < .01 and ***P < .001 vs control after the Student t test (for siRNA#4) or wt DREAM-overexpressing cells (for EF-hand mutant groups) after ANOVA and the Tukey test. ##P < .01 between 2 groups. (G) Control or DREAM siRNA #4 was transfected into MEG-01 cells. After 48 hours, cells were incubated with a Ca2+ dye and treated with 0.05% DMSO, 25 µM LY294002 (LY), 0.1 µM wortmannin (Wort), 0.5 µM PIK75, or 0.5 µM TGX221. Ca2+ release was measured following stimulation with 100 nM A23187 (n = 4). (H) Control or DREAM knockdown MEG-01 cells were pretreated with vehicle or a PI3K inhibitor and stimulated with A23187 for 5 minutes. Immunoblotting was performed as described in “Methods” (n = 3). *P < .05, **P < .01, and ***P < .001 vs control (white bars in panel G) or DREAM siRNA-treated (gray bars in panel G) cells (vehicle group), or control siRNA-treated, stimulated cells (for panel H) after ANOVA and the Tukey test. #P < .05 and ###P < .001 between 2 groups.
DREAM regulates Ca2+ mobilization in MEG-01 cells and its regulatory function requires Ca2+ binding and PI3K-Iβ activity. Control or DREAM siRNA (#3 or #4) was transfected into MEG-01 cells. (A-B) After 48 hours, cell lysates were immunoblotted, followed by densitometry (arbitrary unit [AU], n = 4). (C) After 48 hours, cells were incubated with a Ca2+ dye and treated with 100 nM A23187, followed by measurement of cytosolic Ca2+ levels (n = 4). *P < .05 vs control after the Student t test. (D) A schematic of DREAM siRNA-resistant wt and EF-hand mutant DREAM. (E) DREAM siRNA #4 was transfected into MEG-01 cells. After 4 hours, cells were transfected with a vector expressing siRNA #4–resistant wt or EF-hand mutant DREAM. After 48 hours, DREAM overexpression was verified by immunoblotting (n = 4). (F) The cells described in panel E were used for Ca2+ assays. **P < .01 and ***P < .001 vs control after the Student t test (for siRNA#4) or wt DREAM-overexpressing cells (for EF-hand mutant groups) after ANOVA and the Tukey test. ##P < .01 between 2 groups. (G) Control or DREAM siRNA #4 was transfected into MEG-01 cells. After 48 hours, cells were incubated with a Ca2+ dye and treated with 0.05% DMSO, 25 µM LY294002 (LY), 0.1 µM wortmannin (Wort), 0.5 µM PIK75, or 0.5 µM TGX221. Ca2+ release was measured following stimulation with 100 nM A23187 (n = 4). (H) Control or DREAM knockdown MEG-01 cells were pretreated with vehicle or a PI3K inhibitor and stimulated with A23187 for 5 minutes. Immunoblotting was performed as described in “Methods” (n = 3). *P < .05, **P < .01, and ***P < .001 vs control (white bars in panel G) or DREAM siRNA-treated (gray bars in panel G) cells (vehicle group), or control siRNA-treated, stimulated cells (for panel H) after ANOVA and the Tukey test. #P < .05 and ###P < .001 between 2 groups.
To further examine whether DREAM regulates A23187-induced Ca2+ mobilization through PI3K-Iβ, we used PI3K inhibitors in MEG-01 cells transfected with DREAM siRNA. Compared with vehicle control, treatment with pan inhibitors significantly reduced Ca2+ release in control MEG-01 cells following A23187 treatment (Figure 6G). Ca2+ release was significantly impaired in control siRNA-transfected MEG-01 cells after treatment with PIK75 or TGX221, compared with vehicle control, but the inhibitory effect was significantly weaker than that of pan inhibitors. When pan inhibitors were used in DREAM knockdown MEG-01 cells, A23187-induced Ca2+ release was further inhibited in comparison with vehicle-treated cells (P < .05). Treatment of DREAM knockdown MEG-01 cells with PIK75 inhibited Ca2+ release to the level similar to pan inhibitors. In contrast, TGX221 treatment had no additive effect (Figure 6G). These results suggest that like platelet DREAM, MEG-01 cell DREAM also regulates A23187-induced Ca2+ mobilization through PI3K-Iβ.
Because A23187-induced Ca2+ release enhanced PI3K and AKT phosphorylation in platelets, we further investigated how DREAM knockdown and PI3K inhibitors affect intracellular signaling. We found that the phosphorylation levels of PI3K and AKT were enhanced in control MEG-01 cells following A23187 stimulation and that DREAM knockdown significantly reduced A23187-induced PI3K/AKT phosphorylation (Figure 6H-I). Treatment of DREAM knockdown cells with LY294002 or wortmannin abrogated phosphorylation of PI3K and AKT following A23187 stimulation. Treatment of DREAM knockdown cells with PIK75 completely inhibited PI3K and AKT phosphorylation. In contrast, treatment with TGX221 had no additive effect. These data show that DREAM regulates PI3K-Iβ activation in A23187-stimulated MEG-01 cells.
Discussion
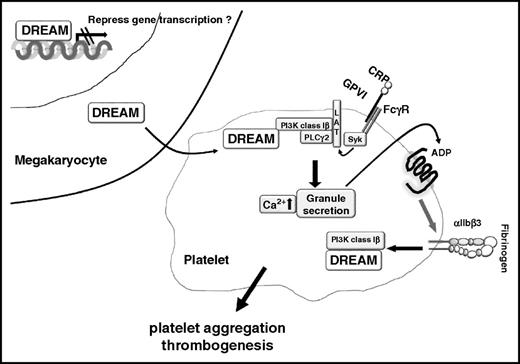
Previous studies showed that DREAM transcriptionally regulates numerous physiological processes including pain,1,2 acute inflammation,12 and adaptive immunity.11 In addition, DREAM plays a nontranscriptional role in Alzheimer disease by interaction with presenilin and regulation of its function.6 In the present study, we demonstrated that hematopoietic and nonhematopoietic DREAM are critical for thrombogenesis at the site of vascular injury. Although it is unknown how nonhematopoietic cell DREAM participates in thrombosis and hemostasis, adoptive transfer experiments clearly showed the importance of platelet DREAM in thrombogenesis. Importantly, we have identified platelet DREAM as a novel regulator for PI3K-Iβ activation, thereby controlling granule secretion, Ca2+ mobilization, and aggregation. Thus, this is the first study to uncover the novel role for DREAM in platelet activation and thrombus formation.
Growing evidence shows that anucleate platelets are able to synthesize proteins using mRNAs and microRNAs under disease conditions.40,41 Some transcription factors in megakaryocytes may be transferred to proplatelet tips during platelet production, even though it remains unclear which transcription factors are expressed in platelets and what their roles are. A recent study suggested that platelets express STAT3 and that platelet STAT3 plays a nontranscriptional role in serving as a scaffold protein for the interaction between Syk and PLCγ2 following collagen stimulation.42 We found that platelets express DREAM mRNA and protein and that platelet DREAM deletion does not alter the expression of other tested proteins and the ultrastructure. Although we cannot exclude the possibility that transcriptional changes in DREAM-null megakaryocytes might produce dysfunctional platelets, our results imply that DREAM may regulate platelet function independently of its transcriptional repressor activity. Future studies are required to examine the potential transcriptional regulation by megakaryocyte DREAM.
CRP-mediated GPVI signaling induces Tyr phosphorylation of the Fc receptor γ-chain by Fyn and Lyn, followed by Syk activation.28 Subsequently, activated Syk induces the complex formation of LAT, Src homology 2 domain containing leukocyte protein of 76 kDa, PI3K, PLCγ2, and Bruton tyrosine kinase, leading to their activation.28,29 In particular, PIP3 produced by class I PI3Ks is necessary for the membrane translocation and full activation of PLCγ2 in GPVI-mediated signaling.43,44 We found that platelet DREAM is important for the phosphorylation of PI3K, PLCγ2, and AKT, but not Syk and LAT, following CRP stimulation and that the binding of p-PI3K to LAT is abrogated in CRP-stimulated DREAM KO platelets. Furthermore, our results have shown that platelet DREAM regulates PI3K phosphorylation through integrin signaling. Thus, these results highlight the contribution of platelet DREAM to the PI3K signalosome in GPVI- and integrin-mediated signaling, thereby inducing platelet aggregation and thrombus formation (Figure 7).
Model of a novel role for platelet DREAM. Megakaryocyte DREAM mRNA and protein are transferred to platelets during thrombopoiesis. In platelets, DREAM regulates platelet activation by modulating PI3K-Iβ activity (for example, in GPVI-mediated signaling). ADP signaling further activates αIIbβ3 integrin and amplifies integrin outside-in signaling which also activates PI3K-Iβ through DREAM.
Model of a novel role for platelet DREAM. Megakaryocyte DREAM mRNA and protein are transferred to platelets during thrombopoiesis. In platelets, DREAM regulates platelet activation by modulating PI3K-Iβ activity (for example, in GPVI-mediated signaling). ADP signaling further activates αIIbβ3 integrin and amplifies integrin outside-in signaling which also activates PI3K-Iβ through DREAM.
Studies showed that PI3K-Iα and PI3K-Iβ are required for the full activation of PLCγ2, Ca2+ mobilization, and Rap1b activation through GPVI signaling.38 We found that inhibition of PI3K-Iα, but not PI3K-Iβ, activity in CRP-stimulated DREAM KO platelets further reduces granule secretion, Ca2+ mobilization, and aggregation as seen with pan PI3K inhibitors in DREAM KO platelets and that DREAM enhances PI3K-Iβ activity by direct binding in vitro. Although we cannot eliminate the possibility that DREAM deficiency may alter other aspects of platelet function beyond the regulatory effect on PI3K-Iβ activity, our results implicate a novel role for platelet DREAM in regulating PI3K-Iβ activity. It remains controversial whether inhibition of PI3K-Iβ with TGX221 affects tail bleeding times in mice.36,45 Nevertheless, mice expressing a catalytically inactive form of PI3K-Iβ or PI3K-Iγ showed no prolongation in tail bleeding times,46 suggesting that activity of either PI3K isoform may not be involved in hemostasis. Our results show that deletion of both hematopoietic and nonhematopoietic DREAMs prolongs tail bleeding times in mice. Thus, the mechanisms by which DREAM participates in hemostasis should be further investigated.
Evidence is mounting that kinase activity and cytosolic Ca2+ reciprocally influence each other during platelet activation.16,47,48 We found that Src, PI3K, and AKT are phosphorylated by A23187-induced Ca2+ release in WT platelets and that platelet DREAM regulates phosphorylation of PI3K and AKT, but not Src. These results imply that increased cytosolic Ca2+ is able to induce PI3K phosphorylation through DREAM. Future studies are required to elucidate the molecular mechanism of reciprocal cross-talk between PI3K and cytosolic Ca2+ during platelet activation and to investigate a role for platelet DREAM in this cross-talk.
DREAM knockdown systems have been used to examine its role in cellular functions.11,49 We observed that DREAM siRNAs have no effect on the expression of several proteins including PI3K and AKT in MEG-01 cells and that as seen in DREAM-null platelets, DREAM knockdown impairs A23187-induced Ca2+ release. Although overexpression of WT, but not EF-3/4 mutant, DREAM rescued the defect, we are aware that DREAM knockdown/overexpression in MEG-01 cells could influence the transcription of genes which may modulate Ca2+ signaling. Additionally, our finding that the regulatory function of MEG-01 cell DREAM requires Ca2+ binding implicates a role for DREAM as a transcriptional repressor because Ca2+-bound DREAM is dissociated from the DRE motif, initiating gene transcription. Nevertheless, DREAM may regulate cytosolic Ca2+ levels by direct binding through EF-hand domains and transfer Ca2+ to intracellular signaling molecules, such as PI3K, which may need Ca2+ for their full activity. Our speculation on the nontranscriptional role of cytosolic DREAM in MEG-01 cells is supported by previous findings that DREAM localizes not only in the nucleus but also in the endoplasmic reticulum,6 cytoplasm,5 and plasma membrane.50 Moreover, recent studies demonstrated that Ca2+-bound, but not Ca2+-free, DREAM interacts with calmodulin and regulates its function in neuronal cells.7 Thus, our results provide evidence that DREAM may play a nontranscriptional role in regulating the function of megakaryocytes and platelets.
Early work suggested that DREAM could be a therapeutic target for pain modulation.2 Our studies presented here have defined a novel role for platelet DREAM in platelet activation and thrombogenesis by regulating PI3K-Iβ activation, which may be independent of its transcriptional function. Future studies will further determine whether DREAM could be a novel target for an antithrombotic agent.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Josef Penninger for providing DREAM KO mice.
This work was supported in part by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL109439 and HL130028 [J.C.]), American Society of Hematology Bridge Fund (J.C.), and American Heart Association postdoctoral (K.K.) and predoctoral fellowship (A.T.).
Authorship
Contribution: K.K. designed and performed research, collected and analyzed data, and wrote the manuscript; A.T. performed research, analyzed data, and wrote the manuscript; A.B. performed research; J.E.I. provided important data; and J.C. initiated and designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: J.E.I. has financial interest in and is a founder of Platelet BioGenesis. The remaining authors declare no competing financial interests.
Correspondence: Jaehyung Cho, 835 S. Wolcott Ave, E403, Chicago, IL 60612; e-mail: thromres@uic.edu.

![Figure 2. Both hematopoietic and nonhematopoietic cell DREAMs participate in thrombosis and hemostasis, and platelet DREAM is important for thrombogenesis. WT or DREAM KO transplant control mice and DREAM bone marrow chimeras (hematopoietic [HP] or non-HP DREAM KO) were used for intravital microscopy as described in Figure 1. (A) Representative images. Arrows show direction of blood flow. (B-C) F platelet and fluorescence intensities of anti-CD42c antibodies in 4 different groups are presented as described in Figure 1 (32-38 thrombi in 5 mice of each group). **P < .01 and ***P < .001 vs WT control mice and #P < .05, ##P < .01, and ###P < .001 between 2 groups after the Mann-Whitney U test. (D) Cremaster arteriolar thrombosis was induced by FeCl3 injury. Time to occlusion was plotted (n = 8-10). (E-F) Tail bleeding time and blood loss were measured as described in Figure 1D-E (n = 8). (G-I) Fluorescently labeled WT or DREAM KO platelets, 108 in 100 µL of saline, were infused into thrombocytopenic WT mice. Three thrombi were generated following laser injury. The same labeled platelets were reinfused into the mouse to generate 3 additional thrombi. Accumulation of infused platelets was monitored and analyzed as described above. Horizontal bars represent the median value of the fluorescence intensities of anti-CD42c antibodies (C,I), time to occlusion (D), bleeding times (E), or Hb content (F). *P < .05 and ***P < .001 vs WT platelets after the Mann-Whitney U test (n = 4-5 mice for each group) or after the Dunn test (D-F).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/2/10.1182_blood-2016-07-724419/4/m_blood724419f2.jpeg?Expires=1769119623&Signature=dSqEfnZcokuKnYZQHQWm-aYcI3M29th2~6IRpU35Cg8xXlTf~EKm6ozMEnRpAT6an2wTZEbos~P0fw-AAAMxKvmXZ58sOipHjixRGMWPTfJSpbZjHYXZjC9gWVN38eq2--aeuEphlJPUDuEcVMtwBAvdhG8vudhDZLk9JP0L2QPYpl7d~huBkIfkWhlGVSgiJZtZHHiA3RqxQ8ptXCtOCz~IUtxoCJQDa-nwVjiBuuQXuAV3UslVxo6NuhorsxdOWM9HxM00eouqrw8Qa9ED0Wui9BkYLXPB9zMWt7tpBNVFQo~TJP-J77PJo0IFy4Ecg4qoUKDzHjsLGHja~fp~0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Platelet DREAM plays a critical role in PI3K activation through GPVI-, integrin-, or A23187-mediated signaling. (A) WT and DREAM KO platelets were stimulated by 0.5 µg/mL CRP for 1, 2, and 5 minutes under stirring conditions (1000 rpm) in an aggregometer. An equal amount of cell lysate protein (50 µg) was immunoblotted, followed by densitometry (arbitrary unit [AU], n = 5). (B-D) WT and DREAM KO platelets were stimulated with 0.5 µg/mL CRP for 2 minutes under stirring conditions. The lysates were immunoprecipitated with control IgG or antibodies against (B) LAT, (C) DREAM, or (D) total PI3K p85 (t-PI3K p85) followed by immunoblotting and densitometry (n = 4). (E-F) Human platelets were stimulated with 0.5 µg/mL CRP for 2 minutes under stirring conditions, followed by coimmunoprecipitation as shown in panels C and D. (G-H) WT and DREAM KO platelets were stimulated by 10 µM ADP for 1, 2, or 5 minutes in the (G) absence or (H) presence of 30 µg/mL FG under stirring conditions (n = 4). (I-J) WT and DREAM KO platelets were pretreated (I) without or (J) with 20 µg/mL eptifibatide following stimulation with 100 nM A23187 for 1, 2, or 5 minutes with stirring. An equal amount of lysate protein was immunoblotted, followed by densitometry (n = 4). *P < .05 and **P < .01 vs WT platelets at each time point (or resting platelets for panels C-F) after the Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/2/10.1182_blood-2016-07-724419/4/m_blood724419f4.jpeg?Expires=1769119623&Signature=suRjfJTYEsVpqeYYGPNIzd15iJL0BO7FL3JFNo3kqqaP6sYNzdmdpPttEJYNwMFnMcmQ-XPyS0eGN6NyTZs4bveHwNp9VpJxxX~qqNcDGTuEvhn7NOceybaawyDXyhAZKJC-mMHuL7159AKVwqBvOK8SlvLDtqrKF12xza~EPwj1hiVUrC2NPhF1c9IPU2iuIIagoiTGMGWgWxP-06yF8k3ir9AAGhVRIKyzFB91pov-heecxOA~yKzrDWLSOMOgGwpDauxLZP3rtjccnvltjRv2lM~bVuXBjHz0zHo2nlzL1CGZzGro9C-oQRv6Ron9WhaIKAYrMPUh8deZvyB8Yg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. DREAM regulates Ca2+ mobilization in MEG-01 cells and its regulatory function requires Ca2+ binding and PI3K-Iβ activity. Control or DREAM siRNA (#3 or #4) was transfected into MEG-01 cells. (A-B) After 48 hours, cell lysates were immunoblotted, followed by densitometry (arbitrary unit [AU], n = 4). (C) After 48 hours, cells were incubated with a Ca2+ dye and treated with 100 nM A23187, followed by measurement of cytosolic Ca2+ levels (n = 4). *P < .05 vs control after the Student t test. (D) A schematic of DREAM siRNA-resistant wt and EF-hand mutant DREAM. (E) DREAM siRNA #4 was transfected into MEG-01 cells. After 4 hours, cells were transfected with a vector expressing siRNA #4–resistant wt or EF-hand mutant DREAM. After 48 hours, DREAM overexpression was verified by immunoblotting (n = 4). (F) The cells described in panel E were used for Ca2+ assays. **P < .01 and ***P < .001 vs control after the Student t test (for siRNA#4) or wt DREAM-overexpressing cells (for EF-hand mutant groups) after ANOVA and the Tukey test. ##P < .01 between 2 groups. (G) Control or DREAM siRNA #4 was transfected into MEG-01 cells. After 48 hours, cells were incubated with a Ca2+ dye and treated with 0.05% DMSO, 25 µM LY294002 (LY), 0.1 µM wortmannin (Wort), 0.5 µM PIK75, or 0.5 µM TGX221. Ca2+ release was measured following stimulation with 100 nM A23187 (n = 4). (H) Control or DREAM knockdown MEG-01 cells were pretreated with vehicle or a PI3K inhibitor and stimulated with A23187 for 5 minutes. Immunoblotting was performed as described in “Methods” (n = 3). *P < .05, **P < .01, and ***P < .001 vs control (white bars in panel G) or DREAM siRNA-treated (gray bars in panel G) cells (vehicle group), or control siRNA-treated, stimulated cells (for panel H) after ANOVA and the Tukey test. #P < .05 and ###P < .001 between 2 groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/2/10.1182_blood-2016-07-724419/4/m_blood724419f6.jpeg?Expires=1769119623&Signature=sg5BYLQ3j~jvkhgJ60y1MKZvzoKu33umR36tZBmhuTAEu1lfbbBu8MmVzoxVzsWsohL~vZoaltm6jX73BodYdd3Gxoe~fSRGR8Gjcs0uKTmuXNtpD4v82cZpS0Ox8fH0tnlJzj86maI~zw00RmbWisyVUAA~5bWjDeEyNIm-ovcq9CCqP7YLAmqruzT4~BBmWXVJGdAwNdqmv7MZaVIAmiBHhGS4axA1Puei8Wu2E9rfd0r~quI9g0ceg~TxiPF9Z3Zxf1Rcns1rx5XH5SMdEAmTndMpRGu5NN6hkBF0D6VHN-UX00x3Ae~monsAU2vO6qWN37MdK~ExkLnPO5DYOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal