Key Points
Expression of CagA and CagA-signaling molecules p-SHP2 and p-ERK is associated with HP dependence of gastric DLBCL.
CagA is associated with the direct lymphomagenic effect of HP on B cells of HP-dependent gastric DLBCL.
Abstract
We previously reported that early-stage gastric diffuse large B-cell lymphomas (DLBCLs), including DLBCLs with mucosa-associated lymphoid tissue (DLBCL[MALT]) and without (“pure” DLBCL) the features of MALT lymphomas, can achieve long-term complete remission after frontline Helicobacter pylori (HP) eradication (HPE). We recently reported that expression of cytotoxin-associated gene A (CagA) and CagA-signaling molecules (phospho–Src homology-2 domain-containing phosphatase [p-SHP2] and phospho–extracellular signal-regulated kinase [p-ERK]) is associated with HP dependence of gastric MALT lymphoma. However, the significance of CagA and CagA-signaling molecules in gastric DLBCL remains unexplored. The association between expression of CagA, p-SHP-2, and p-ERK in malignant B cells and tumor response to HPE was evaluated in 63 patients with stage IE/IIE1 HP-positive gastric DLBCL who received HPE as frontline treatment. We detected CagA expression in 20 of 42 DLBCL (MALT) cases (47.6%) and in 13 of 21 “pure” DLBCL cases (61.9%). CagA expression was higher in HP-dependent tumors than in HP-independent tumors (74.3% [26 of 35] vs 25.0% [7 of 28]). Patients with CagA expression responded to HPE quicker than those without expression (median time to complete remission, 4.0 months vs 5.0 months). The expression of CagA was closely associated with p-SHP-2 and p-ERK expression. Combined CagA, p-SHP-2, and p-ERK expression showed an increased positive predictive value (81.8% vs 75.9%) and an increased specificity (84.0% vs 75.0%) for HP dependence compared with CagA expression alone. Our results indicated that CagA and its signaling molecules can be detected in the malignant B cells of gastric DLBCL, and the expression of these molecules is clinically and biologically associated with HP dependence.
Introduction
According to the current World Health Organization (WHO) lymphoma classification criteria, gastric diffuse large B-cell lymphoma (DLBCL) is a heterogeneous disease entity that includes DLBCLs that show no histological evidence of mucosa-associated lymphoid tissue (MALT) lymphomas, (“pure” DLBCLs) and DLBCLs that express histological evidence of MALT lymphomas, the DLBCL(MALT)s.1-4 Considering that the distinction between DLBCL components and their origins is not as straightforward as with similar MALT lymphoma components in gastric DLBCL(MALT),5,6 the term “high-grade MALT lymphoma” is no longer considered appropriate. In 2008, the WHO lymphoma classification recommended that gastric lymphoma cases that show transformation into large-cell lymphomas should be classified as DLBCLs instead of high-grade MALT lymphomas.1-4 In this context, the occurrence of lymphoepithelial lesions composed of large lymphoid cells does not alter the diagnosis of DLBCL. However, despite this, the presence of an accompanying MALT lymphoma component in DLBCL should always be reported.3,4
Gastric DLBCL with and without histologic evidence of MALT is considered Helicobacter pylori (HP)-independent, and these tumors are conventionally treated with systemic chemotherapy.1,7-9 However, in addition to gastric DLBCL(MALT),10 we demonstrated that gastric “pure” DLBCL may also be closely related to HP.11 Most importantly, both of these types of lymphoma can possibly be cured by HP eradication (HPE) therapy.11 Other investigators have also revealed that a substantial proportion of patients with HP-positive early-stage gastric “pure” DLBCL and of those with gastric DLBCL(MALT) achieve complete remission (CR) through HPE.12-16 These findings suggest that frontline HPE may enable certain patients with gastric DLBCL, including DLBCL(MALT) and “pure” DLBCL, to avoid chemotherapy with little risk; furthermore, these findings may shed light on the precise molecular mechanisms of HP-dependent lymphomagenesis in these tumors.
Cytotoxin-associated gene A (CagA) protein, the most important HP virulence factor, consists of 3 domains and a C-terminal unstructured region.17-19 The C-terminal domain contains a 5-amino acid repetitive tandem motif, glutamic acid-proline-isoleucine-tyrosine-alanine (EPIYA).17-19 When HP contacts with B lymphocytes, CagA uses the type IV secretion system (T4SS), to pass from the HP cytosol to B lymphocytes cytoplasm.19,20 Following the translocation, CagA undergoes tyrosine phosphorylation by Src and Abl-family kinases on specific tyrosine residues within the EPIYA motif.17-19 Phosphorylated CagA then binds to the Src-homology domain 2 (SH2) of Src homology-2 domain-containing phosphatase (SHP-2) and cause aberrant activation of SHP-2.17-19 The activated SHP-2 consequently stimulates the SHP-2–linked signaling pathway of the extracellular signal-regulated kinase (ERK) and the p38 MAPK.17-19,21 In addition, CagA can activate cancer-related signaling pathways to promote tumorigenesis through tyrosine phosphorylation-dependent signaling (C-terminal Src kinase, Crk, and E-cadherin) or tyrosine phosphorylation-independent signaling (p53, Grb2, c-Met, Par-1b/MAPK2, and ZO1).19 Interaction of CagA with aforementioned signaling molecules that may cause morphological changes and enhance cell motility and proliferation of gastric epithelial cells.19 However, in an interleukin 3–dependent B-lymphoid cell model, Umehara et al revealed that CagA inhibits the apoptosis of B lymphocytes through the impairment of p53 or the downregulation of the JAK-STAT pathway.22
In contrast to the classical concept that gastric low-grade MALT lymphoma (MALT lymphoma) is indirectly caused by HP through T-cell stimulation,23-26 we reported that direct contact between HP and B cells results in CagA translocation into the B cells.27 We further confirmed that CagA is expressed in the lymphoma cells of gastric MALT lymphoma, and its expression is significantly associated with the HP dependence of these tumors.28 We also identified the close association between CagA and CagA-signaling molecules, such as phospho-SHP-2 (p-SHP-2), phospho-ERK (p-ERK), p-p38 MAPK, Bcl-2, and Bcl-xL.29 The expression of these CagA-signaling molecules is closely associated with the HP dependence of gastric MALT lymphoma.29
Our recent results indicate that, among patients with primary gastric ”pure” DLBCLs, who had been traditionally treated by frontline chemotherapy, the HP-positive group had a lower International Prognostic Index score (0-1), a lower clinical stage (I-IIE1), a better complete response to chemotherapy, and significantly better 5-year event-free survival (EFS) and overall survival (OS) than the HP-negative group.30 Furthermore, among HP-positive patients, we found that those with CagA expression showed better 5-year EFS and OS than those without. In addition, in HP-positive cases, CagA expression was closely associated with p-SHP-2 expression.30 These findings imply that HP-positive gastric “pure” DLBCLs, particularly those displaying CagA expression, are clinicopathologically and biologically distinct from HP-unrelated gastric “pure” DLBCLs. The dependence on CagA-regulated signaling pathway,18,19,27,29 such as p-SHP-2, for the growth of malignant B-cell clones may explain the tendency of CagA-positive gastric “pure” DLBCLs to remain localized and thus, a lower clinical stage of disease.30 However, whether CagA and its signaling molecules participate in lymphomagenesis of HP-positive gastric DLBCLs remains unclear.
In addition to nuclear expression of BCL10 and NF-κB,31,32 we reported that aberrant expression of BAFF was significantly associated with the HP independence of early-stage gastric DLBCL(MALT).33 We also reported that CD86 is present in gastric DLBCL(MALT) and is significantly associated with the HP dependence of these tumors.34 However, the relationship between the expression of BCL10, NF-κB, BAFF, and CD86 and the HP independence in gastric “pure” DLBCL remains unclear.
In this study, we evaluated whether the expression of CagA and its signaling molecules (p-SHP-2 and p-ERK) in the tumor cells of patients with early-stage HP-positive gastric DLBCL receiving frontline HPE treatment has clinical and biological significance. We assessed the association between expression patterns of BCL10, NF-κB, BAFF, and CD86 and HP independence of the same group of tumors. We discovered that the expressions of CagA, CagA-signaling molecules, and CD86 are closely associated with the HP dependence of gastric DLBCL. In addition, nuclear expression of BCL10 or NF-κB and BAFF overexpression are closely associated with the HP independence of gastric “pure” DLBCL as well as gastric DLBCL(MALT).
Materials and methods
Patients, treatment, and clinical and histologic evaluations
Between January 2003 and December 2014, patients with stage IE/IIE1 HP-positive gastric DLBCL, including DLBCL(MALT) and “pure” DLBCL, who received HPE as frontline treatment and had available tissue samples, were included in this study.10,11 Diagnosis of DLBCL was based on the criteria of WHO classification, namely hematological malignancies that manifest as compact clusters, confluent aggregates, or sheets of large lymphoma cells with distinctive nucleoli.1-4,25,35,36 Tumors without histological evidence of MALT lymphoma, indicating the presence of predominantly low-grade centrocyte-like cell infiltration and typical lymphoepithelial lesions (LELs), were classified as “pure” DLBCL.1-4,25,35,36 Those with histological evidence of MALT were classified as DLBCL(MALT).1-4,25,35,36 The histopathological characteristics of all tumor specimens were independently reviewed by 2 experienced hematopathologists (C.-W.L. and C.-T.S.). All specimens also underwent immunohistochemical staining of CD20, CD5, CD3, and CD43 and additional study with anticytokeratin (1:50, clone AE1/AE3; Ylem, Rome, Italy) to identify LELs in the context of the minimal MALT lymphoma components in DLBCL.10,11 In cases that reactive lymphoma-like lesions (LLL) could not be excluded by immunohistochemical staining alone,37,38 clonal analyses by immunoglobulin heavy chain (IgH) gene rearrangement,6,39 and t(14;18)(q32;q21)/IGH-BCL2 rearrangement40,41 were done (supplemental Methods, supplemental Result, and supplemental Figure 1, see supplemental Data available on the Blood Web site).
The detailed information of HPE regimen, the follow-up upper gastrointestinal endoscopic examination, and the complete remission criteria (Groupe d’Etude des Lymphomes de l’Adult [GELA] histological scoring system) of tumors are listed in supplemental Methods.10,11,42-44 All experimental protocols were approved by the Institutional Review Board (IRB) of the Research Ethical Committee of National Taiwan University Hospital (NTUH IRB number: 9361700774, 9561706029, and 201207077RIC). All experiments were conducted in accordance with the approved guidelines and regulations. The patients’ medical data were anonymized prior to access and analysis. All patients provided written informed consent to participate in and to provide tissue material for biological studies.
Immunohistochemistry and immunohistochemical scoring
Confocal laser-scanning microscopy
Statistical analysis
In the present study, the primary purpose was to evaluate whether expression of CagA and CagA-signaling molecules (p-SHP2 and p-ERK) is useful in predicting HP-dependent status in patients with stage IE/IIE1 HP-positive gastric DLBCL who received HPE as frontline treatment; the secondary purpose was to identify (1) the association between CagA expression and the time to CR after completion of HPE for HP-dependent patients with gastric DLBCL and (2) the potential biomarkers (BCL10, NF-κB (p65), BAFF, and CD86) for predicting the HP dependence of these tumors. Analyses were conducted using the follow-up data available on December 31, 2015. In the present study, positive predictive value (PPV) and specificity were calculated using a 2-way table (supplemental Table 1). The other detailed statistical methods are listed in supplemental Methods.
Results
Correlation of CagA expression with clinicopathological features and tumor response to HPE in gastric DLBCL
The analysis included 42 patients with gastric DLBCL(MALT) and 21 patients with gastric “pure” DLBCL, who received HPE as frontline treatment and had their gastric HP infection successfully eradicated. A total of 35 patients (55.6%) had HP-dependent tumors and 28 (44.4%) had HP-independent tumors. Among the gastric DLBCL(MALT) patients, 22 were HP-dependent (52.4%), whereas 13 of the gastric “pure” patients were HP-dependent (61.9%).
We observed CagA protein expression in the tumor cells of the gastric mucosa and submucosa as well as scattered expression in the adjacent tumor-free gastric mucosa of patients with HP-positive gastric DLBCL (Figure 1). Immunohistochemical analyses and hematoxylin and eosin staining indicated that the majority of the CagA-positive cells were morphologically abnormal and expressed CD20. These findings were further confirmed through confocal microscopic analyses, which indicated that CagA was predominantly expressed on the neoplastic cells of gastric DLBCL (Figure 2A-C).
Immunohistochemical analysis of CagA expression in the tumor cells of gastric DLBCL. (A) Endoscopy showing multiple irregular ulcerative masses in the cardia of the stomach of an 86-year-old woman. Right bottom inset, Endoscopic ultrasonography shows a homogenous hypoechoic mass infiltration of mucosa and submucosa (white arrow, maximal thickness [7 mm]) of the gastric wall. (B) Six months after completion of HPE, complete remission (CR) was achieved. (C) Tumor cell nuclear CagA expression in the same case; original magnification ×400. (D) Cytoplasmic expression of BCL10 in tumor cells of the same case; original magnification ×400. (E) Cytoplasmic expression of NF-κB in tumor cells of the same case; original magnification ×400. (F) No CagA expression in specimens of gastritis (6 months after completion of HPE) in the same case; original magnification ×400. (G) An HP-dependent gastric “pure” DLBCL case (time to CR after completion of HPE, 1 month) displaying nuclear CagA expression in the tumor cells of the gastric mucosa; original magnification ×400. (H) An HP-dependent gastric DLBCL(MALT) case (time to CR after completion of HPE, 3 month) displaying nuclear CagA expression in the tumor cells of the gastric mucosa; original magnification ×400.
Immunohistochemical analysis of CagA expression in the tumor cells of gastric DLBCL. (A) Endoscopy showing multiple irregular ulcerative masses in the cardia of the stomach of an 86-year-old woman. Right bottom inset, Endoscopic ultrasonography shows a homogenous hypoechoic mass infiltration of mucosa and submucosa (white arrow, maximal thickness [7 mm]) of the gastric wall. (B) Six months after completion of HPE, complete remission (CR) was achieved. (C) Tumor cell nuclear CagA expression in the same case; original magnification ×400. (D) Cytoplasmic expression of BCL10 in tumor cells of the same case; original magnification ×400. (E) Cytoplasmic expression of NF-κB in tumor cells of the same case; original magnification ×400. (F) No CagA expression in specimens of gastritis (6 months after completion of HPE) in the same case; original magnification ×400. (G) An HP-dependent gastric “pure” DLBCL case (time to CR after completion of HPE, 1 month) displaying nuclear CagA expression in the tumor cells of the gastric mucosa; original magnification ×400. (H) An HP-dependent gastric DLBCL(MALT) case (time to CR after completion of HPE, 3 month) displaying nuclear CagA expression in the tumor cells of the gastric mucosa; original magnification ×400.
Double immunolabeling of CagA expression in tumor cells of gastric “pure” DLBCL and time to CR of patients with HP-dependent gastric DLBCL. (A) Double immunolabeling of CagA and CD20 in an HP-dependent gastric “pure” DLBCL case with immunohistochemically identified CagA expression. Confocal microscopy showing that most CagA-positive cells (green fluorescence) expressed CD20 (red fluorescence). Image of nucleus (DAPI), or a merged image of CagA and CD20 expression. Left bottom inset, The white arrow represents CagA expression (nuclear or cytoplasmic expression), CD20 expression (cytoplasmic expression), nucleus (DAPI), or the merged images of CagA and CD20 expression; the yellow arrow represents no CagA expression, positive CD20 expression (cytoplasmic expression), nucleus (DAPI), or the merged images of CD20 expression alone. (B) The majority of the tumor cells (red fluorescence, CD20) contain cytoplasmic CagA (green fluorescence). The white arrow represents CagA expression, CD20 expression, nucleus (DAPI), or the merged images of CagA and CD20 expression (the confocal image was viewed at ×630). (C) The majority of the tumor cells (red fluorescence, CD20) contain nuclear or cytoplasmic CagA (green fluorescence). The white arrow represents CagA expression, CD20 expression, nucleus (DAPI), or the merged images of CagA and CD20 expression (the confocal image was viewed at ×630). (D) Time to CR was calculated from the completion of antibiotic treatment to the first evidence of CR through Kaplan-Meier analysis (CagA-positive group vs CagA-negative group, 2-sided log-rank test; P = .05).
Double immunolabeling of CagA expression in tumor cells of gastric “pure” DLBCL and time to CR of patients with HP-dependent gastric DLBCL. (A) Double immunolabeling of CagA and CD20 in an HP-dependent gastric “pure” DLBCL case with immunohistochemically identified CagA expression. Confocal microscopy showing that most CagA-positive cells (green fluorescence) expressed CD20 (red fluorescence). Image of nucleus (DAPI), or a merged image of CagA and CD20 expression. Left bottom inset, The white arrow represents CagA expression (nuclear or cytoplasmic expression), CD20 expression (cytoplasmic expression), nucleus (DAPI), or the merged images of CagA and CD20 expression; the yellow arrow represents no CagA expression, positive CD20 expression (cytoplasmic expression), nucleus (DAPI), or the merged images of CD20 expression alone. (B) The majority of the tumor cells (red fluorescence, CD20) contain cytoplasmic CagA (green fluorescence). The white arrow represents CagA expression, CD20 expression, nucleus (DAPI), or the merged images of CagA and CD20 expression (the confocal image was viewed at ×630). (C) The majority of the tumor cells (red fluorescence, CD20) contain nuclear or cytoplasmic CagA (green fluorescence). The white arrow represents CagA expression, CD20 expression, nucleus (DAPI), or the merged images of CagA and CD20 expression (the confocal image was viewed at ×630). (D) Time to CR was calculated from the completion of antibiotic treatment to the first evidence of CR through Kaplan-Meier analysis (CagA-positive group vs CagA-negative group, 2-sided log-rank test; P = .05).
We detected CagA expression (16 cases, score 2; 17 cases, score 3) in 33 of 63 patients (52.4%), whereas 30 patients had negative CagA expression (27 cases, score 0; 3 cases, score 1) (supplemental Figure 2). Among patients with positive CagA expression (scores of 2 and 3), 28 had nuclear or nuclear/cytoplasmic expression of CagA, and 5 had cytoplasmic expression of CagA. As shown in supplemental Figure 2, we found that the majority of CagA-positive cells (cytoplasmic expression, green fluorescence) were morphologically abnormal (double immunohistochemical staining, brown color) and expressed PAX5 (nuclear expression, red fluorescence; double immunohistochemical staining, red color).
Table 1 displays the demographic characteristics of the 2 groups of patients (CagA-positive vs CagA-negative) and their clinicopathological features. Age, sex, endoscopic appearance, depth of gastric wall involvement, and histologic classification revealed nonsignificant differences between the 2 groups. CagA expression was more common in tumors located in distal lesions of the stomach than in those located in proximal lesions of the stomach (P = .062, Table 1).
Clinicopathological features and CagA expression in stage IE/IIE1 gastric DLBCL patients who received HPE therapy as a frontline treatment
| Clinicopathological characteristics . | CagA expression . | P* . | |
|---|---|---|---|
| Negative, n = 30 . | Positive, n = 33 . | ||
| Age (median, range), y | 59 (21-87) | 61 (34-88) | .462† |
| Sex, male/female | 12/18 | 12/21 | .767‡ |
| Endoscopic features, n (%) | .771§ | ||
| Gastritis-like or multiple erosion on infiltrative mucosa | 9 (30.0) | 10 (30.3) | |
| Ulceration or ulcerated mass | 16 (53.3) | 19 (57.6) | |
| Erosions on giant nodular folds | 5 (16.7) | 4 (12.1) | |
| Location of tumor(s), n (%) | .062‡ | ||
| Proximal|| or ≥2 components | 14 (46.7) | 8 (24.2) | |
| Distal¶ | 16 (53.3) | 25 (75.8) | |
| Depth of gastric wall involvement, n (%)# | .311‡ | ||
| Submucosa or above | 8/28 (28.6) | 12/29 (41.4) | |
| Muscularis propria or beyond | 20/28 (71.4) | 17/29 (58.6) | |
| Histologic classification | |||
| DLBCL (MALT) | 22 (73.3) | 20 (60.6) | .285‡ |
| “Pure” DLBCL | 8 (26.7) | 13 (39.4) | |
| p-SHP2 expression, n (%)** | <.001‡ | ||
| Negative | 18/24 (75.0) | 7/29 (24.1) | |
| Positive | 6/24 (25.0) | 22/29 (75.9) | |
| p-ERK expression, n (%)** | .002‡ | ||
| Negative | 15/24 (62.5) | 6/29 (20.7) | |
| Positive | 9/24 (37.5) | 23/29 (79.3) | |
| Clinicopathological characteristics . | CagA expression . | P* . | |
|---|---|---|---|
| Negative, n = 30 . | Positive, n = 33 . | ||
| Age (median, range), y | 59 (21-87) | 61 (34-88) | .462† |
| Sex, male/female | 12/18 | 12/21 | .767‡ |
| Endoscopic features, n (%) | .771§ | ||
| Gastritis-like or multiple erosion on infiltrative mucosa | 9 (30.0) | 10 (30.3) | |
| Ulceration or ulcerated mass | 16 (53.3) | 19 (57.6) | |
| Erosions on giant nodular folds | 5 (16.7) | 4 (12.1) | |
| Location of tumor(s), n (%) | .062‡ | ||
| Proximal|| or ≥2 components | 14 (46.7) | 8 (24.2) | |
| Distal¶ | 16 (53.3) | 25 (75.8) | |
| Depth of gastric wall involvement, n (%)# | .311‡ | ||
| Submucosa or above | 8/28 (28.6) | 12/29 (41.4) | |
| Muscularis propria or beyond | 20/28 (71.4) | 17/29 (58.6) | |
| Histologic classification | |||
| DLBCL (MALT) | 22 (73.3) | 20 (60.6) | .285‡ |
| “Pure” DLBCL | 8 (26.7) | 13 (39.4) | |
| p-SHP2 expression, n (%)** | <.001‡ | ||
| Negative | 18/24 (75.0) | 7/29 (24.1) | |
| Positive | 6/24 (25.0) | 22/29 (75.9) | |
| p-ERK expression, n (%)** | .002‡ | ||
| Negative | 15/24 (62.5) | 6/29 (20.7) | |
| Positive | 9/24 (37.5) | 23/29 (79.3) | |
P: Comparison of discrete variables between CagA expression-positive and CagA expression-negative.
P values (2-sided) were calculated using the Student t test.
P values (2-sided) were calculated using the χ2 test or the Fisher exact test.
P values (2-sided) were calculated using 1-way analysis of variance.
Proximal: Middle body, upper body, fundus, or cardia.
Distal: Antrum, angle, or lower body.
Gastric wall involvement was evaluated by endoscopic ultrasonography or computed tomography in 57 patients.
Fifty-three cases with available tissue specimens for assessment of expression patterns of p-SHP-2 and p-ERK by immunohistochemical staining.
We observed CagA expression (scores of 2 and 3) in 26 of the 35 HP-dependent cases (74.3%) and in 7 of the 28 HP-independent cases (25.0%) (P < .001; Table 2). Patients with CagA expression responded to HPE quicker than those without expression (median time to CR after completion of HPE, 4.0 months vs 5.0 months, P = .05, log-rank test) (Figure 2D). Among the HP-dependent patients, the patients with nuclear or nuclear/cytoplasmic expression (23 cases) responded to HPE quicker than those with cytoplasmic expression or without any expression (12 cases) (median time to CR after completion of HPE, 4.0 months [95% CI, 3.18-4.83 months] vs 5.0 months [95% CI, 2.74-7.26 months], P = .014, log-rank test). Of the 21 patients with gastric “pure” DLBCL, CagA expression was identified in 11 of the 13 HP-dependent cases (84.6%) and in 2 of the 8 HP-independent cases (25.0%) (P = .018). Similarly, among 42 cases of gastric DLBCL(MALT), CagA expression was detected in 15 (68.2%) of the 22 HP-dependent tumors and in 5 (25.0%) of the 20 HP-independent tumors (P = .007).
Correlation of clinicopathological features and tumor response to HPE therapy of gastric DLBCL
| Clinicopathological characteristics . | Tumors response to HPE . | ||
|---|---|---|---|
| HP dependent, n = 35 . | HP independent, n = 28 . | P . | |
| Age (median, range), y | 61 (34-88) | 60 (21-87) | .891* |
| Sex, male/female | 11/24 | 13/15 | .223† |
| Endoscopic features, n (%) | .033‡ | ||
| Gastritis-like or multiple erosion on infiltrative mucosa | 13 (37.2) | 6 (21.4) | |
| Ulceration or ulcerated mass | 20 (57.1) | 15 (53.6) | |
| Erosions on giant nodular folds | 2 (5.7) | 7 (25.0) | |
| Location of tumor(s), n (%) | .025† | ||
| Proximal§ or ≥2 components | 8 (22.9) | 14 (50.0) | |
| Distal|| | 27 (77.1) | 14 (50.0) | |
| Depth of gastric wall involvement, n (%)¶ | .022† | ||
| Submucosa or above | 15/31 (48.4) | 5/26 (19.2) | |
| Muscularis propria or beyond | 16/31 (51.6) | 21/26 (80.8) | |
| Histology, n (%) | .473† | ||
| DLBCL (MALT) | 22 (62.9) | 20 (71.4) | |
| Pure DLBCL | 13 (37.1) | 8 (28.6) | |
| CagA expression, n (%) | <.001† | ||
| Negative | 9 (25.7) | 21 (75.0) | |
| Positive | 26 (74.3) | 7 (25.0) | |
| p-SHP2 expression, n (%)# | .001† | ||
| Negative | 7/28 (25.0) | 18/25 (72.0) | |
| Positive | 21/28 (75.0) | 7/25 (28.0) | |
| p-ERK expression, n (%)# | .004† | ||
| Negative | 6/28 (21.4) | 15/25 (60.0) | |
| Positive | 22/28 (78.6) | 10/25 (40.0) | |
| BCL10 expression, n (%) | <.001† | ||
| Cytoplasmic/negative | 33 (94.3) | 7 (25.0) | |
| Nuclear | 2 (5.7) | 21 (75.0) | |
| NF-κB (p65) expression, n (%) | <.001† | ||
| Cytoplasmic/negative | 31 (88.6) | 9 (32.1) | |
| Nuclear | 4 (11.4) | 19 (67.9) | |
| BAFF expressions, n (%) | <.001† | ||
| Negative | 30 (85.7) | 12 (42.9) | |
| Positive | 5 (14.3) | 16 (57.1) | |
| CD86 expressions, n (%) | <.001† | ||
| Negative | 13 (37.1) | 23 (82.1) | |
| Positive | 22 (62.9) | 5 (17.9) | |
| Clinicopathological characteristics . | Tumors response to HPE . | ||
|---|---|---|---|
| HP dependent, n = 35 . | HP independent, n = 28 . | P . | |
| Age (median, range), y | 61 (34-88) | 60 (21-87) | .891* |
| Sex, male/female | 11/24 | 13/15 | .223† |
| Endoscopic features, n (%) | .033‡ | ||
| Gastritis-like or multiple erosion on infiltrative mucosa | 13 (37.2) | 6 (21.4) | |
| Ulceration or ulcerated mass | 20 (57.1) | 15 (53.6) | |
| Erosions on giant nodular folds | 2 (5.7) | 7 (25.0) | |
| Location of tumor(s), n (%) | .025† | ||
| Proximal§ or ≥2 components | 8 (22.9) | 14 (50.0) | |
| Distal|| | 27 (77.1) | 14 (50.0) | |
| Depth of gastric wall involvement, n (%)¶ | .022† | ||
| Submucosa or above | 15/31 (48.4) | 5/26 (19.2) | |
| Muscularis propria or beyond | 16/31 (51.6) | 21/26 (80.8) | |
| Histology, n (%) | .473† | ||
| DLBCL (MALT) | 22 (62.9) | 20 (71.4) | |
| Pure DLBCL | 13 (37.1) | 8 (28.6) | |
| CagA expression, n (%) | <.001† | ||
| Negative | 9 (25.7) | 21 (75.0) | |
| Positive | 26 (74.3) | 7 (25.0) | |
| p-SHP2 expression, n (%)# | .001† | ||
| Negative | 7/28 (25.0) | 18/25 (72.0) | |
| Positive | 21/28 (75.0) | 7/25 (28.0) | |
| p-ERK expression, n (%)# | .004† | ||
| Negative | 6/28 (21.4) | 15/25 (60.0) | |
| Positive | 22/28 (78.6) | 10/25 (40.0) | |
| BCL10 expression, n (%) | <.001† | ||
| Cytoplasmic/negative | 33 (94.3) | 7 (25.0) | |
| Nuclear | 2 (5.7) | 21 (75.0) | |
| NF-κB (p65) expression, n (%) | <.001† | ||
| Cytoplasmic/negative | 31 (88.6) | 9 (32.1) | |
| Nuclear | 4 (11.4) | 19 (67.9) | |
| BAFF expressions, n (%) | <.001† | ||
| Negative | 30 (85.7) | 12 (42.9) | |
| Positive | 5 (14.3) | 16 (57.1) | |
| CD86 expressions, n (%) | <.001† | ||
| Negative | 13 (37.1) | 23 (82.1) | |
| Positive | 22 (62.9) | 5 (17.9) | |
P values (2-sided) were calculated using the Student t test.
P values (2-sided) were calculated using the χ2 test or the Fisher exact test.
P values (2-sided) were calculated using 1-way analysis of variance.
Proximal: Middle body, upper body, fundus, or cardia.
Distal: Antrum, angle, or lower body.
Gastric wall involvement was evaluated by endoscopic ultrasonography in 57 patients.
Fifty-three cases with available tissue specimens for assessment of expression patterns of p-SHP-2 and p-ERK by immunohistochemical staining.
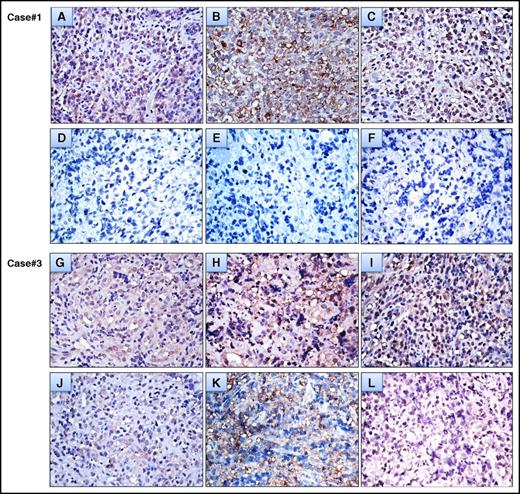
Furthermore, we assessed the serial changes of CagA expression levels in tumor cells before and after HPE treatment in 6 selected cases of HP-dependent gastric “pure” DLBCL. As shown in Figure 3, the expressions of CagA within tumor cells were significantly downregulated in tumor cells that achieved partial remission at 1 month, or at 4 months after completion of HPE (cases #2, #3, and #5). No expression of CagA was noted in gastric biopsies or in remitting tumor cells that achieved CR at 1 month, or at 4 months after completion of HPE (cases #1, #4, and #6, Figure 3). We also found that neither p-SHP-2 nor p-ERK expression was observed in remitting tumor cells one month after completion of HPE (case #1, the time to CR after completion of HPE was 4 month) (Figure 3). Similarly, p-SHP-2 and p-ERK expression were significantly downregulated in tumor cells that achieved partial remission 4 month after completion of HPE (case #3, the time to CR after completion of HPE was 10 month) (Figure 3).
Changes in the expression levels of CagA and Cag-signaling molecules in serial tumor cells before and after completion of HPE (A) A high baseline expression of CagA in case #1 tumor cells. (B) A high baseline expression of p-SHP-2 in case #1 tumor cells. (C) A high baseline expression of p-ERK in case #1 tumor cells. (D) CagA expression was no longer detectable in remitting tumor cells 1 month after completion of HPE (case #1, the time to CR after completion of HPE was 4 month). Immunohistochemical staining showed no expression of p-SHP-2 (E) and p-ERK (F) in remitting tumor cells 1 month after completion of HPE (case #1). (G) High baseline expression of CagA in case #3 tumor cells. (H) A high baseline expression of p-SHP-2 in case #3 tumor cells. (I) A high baseline expression of p-ERK in case #3 tumor cells. (J) Decreased expression of CagA in tumor cells that achieved partial remission 4 months after completion of HPE (case #3, the time to CR after completion of HPE was 10 months). Immunohistochemical staining showed decreased expression of p-SHP-2 (K) and p-ERK (L) in tumor cells that achieved partial remission 4 months after completion of HPE (case #3). All immunohistochemical images were viewed at ×400.
Changes in the expression levels of CagA and Cag-signaling molecules in serial tumor cells before and after completion of HPE (A) A high baseline expression of CagA in case #1 tumor cells. (B) A high baseline expression of p-SHP-2 in case #1 tumor cells. (C) A high baseline expression of p-ERK in case #1 tumor cells. (D) CagA expression was no longer detectable in remitting tumor cells 1 month after completion of HPE (case #1, the time to CR after completion of HPE was 4 month). Immunohistochemical staining showed no expression of p-SHP-2 (E) and p-ERK (F) in remitting tumor cells 1 month after completion of HPE (case #1). (G) High baseline expression of CagA in case #3 tumor cells. (H) A high baseline expression of p-SHP-2 in case #3 tumor cells. (I) A high baseline expression of p-ERK in case #3 tumor cells. (J) Decreased expression of CagA in tumor cells that achieved partial remission 4 months after completion of HPE (case #3, the time to CR after completion of HPE was 10 months). Immunohistochemical staining showed decreased expression of p-SHP-2 (K) and p-ERK (L) in tumor cells that achieved partial remission 4 months after completion of HPE (case #3). All immunohistochemical images were viewed at ×400.
Correlation of expression of CagA with CagA-signaling molecules in gastric DLBCL
Among the 53 patients with available tissue specimens, we observed p-SHP-2 expression in 21 of the 28 HP-dependent cases (75.0%) and in 7 of the 25 HP-independent cases (28.0%) (P = .001; Table 2). Similarly, p-ERK expression was detected in 22 of the 28 HP-dependent tumors (78.6%) and in 10 of the 25 HP-independent tumors (40.0%) (P = .004; Table 2). The expression of CagA was closely associated with p-SHP-2 (P < .001) and p-ERK (P = .002) expression (Table 1; Figure 3). Furthermore, Spearman correlation coefficient analysis indicated a strong correlation between CagA and CagA-signaling molecules (supplemental Table 2).
When used as a single marker of CagA and CagA-signaling molecules, the PPVs for CagA, p-SHP-2, and p-ERK were 75.9%, 75.0%, and 68.8%, respectively (Table 3). Among 28 HP-dependent cases, 22 tumors expressed CagA, whereas 18 tumors expressed all 3 markers: CagA, p-SHP-2, and p-ERK. Among 53 patients, combined CagA, p-SHP-2, and p-ERK expressions showed an increased PPV (18 of 22 [81.8%]) and an increased specificity (21 of 25 [84.0%]) for HP dependence compared with CagA expression alone (Table 3).
PPV of CagA and CagA-signaling molecules for HP dependence of stage IE/IIE1 gastric DLBCL
| Expression of CagA and CagA-signaling molecules in tumor tissue . | No. of gastric DLBCL patients who receive HPE as frontline treatment . | |
|---|---|---|
| HP dependent, n = 28 . | HP independent, n = 25 . | |
| CagA positive | 22 | 7 |
| CagA negative | 6 | 18 |
| PPV for CagA* = 22 of 29 (75.9%) | ||
| Specificity for CagA† = 18 of 25 (75.0%) | ||
| p-SHP-2 positive | 21 | 7 |
| PPV for p-SHP-2* = 21 of 28 (75.0%) | ||
| p-ERK positive | 22 | 10 |
| PPV for p-ERK* = 22 of 32 (68.8%) | ||
| CagA, p-SHP-2, and p-ERK are all positive | ||
| Yes | 18 | 4 |
| No | 10 | 21 |
| PPV for combined CagA, p-SHP-2, and p-ERK‡ = 18 of 22 (81.8%) | ||
| Specificity for combined CagA, p-SHP-2, and p-ERK§ = 21 of 25 (84.0%) | ||
| Expression of CagA and CagA-signaling molecules in tumor tissue . | No. of gastric DLBCL patients who receive HPE as frontline treatment . | |
|---|---|---|
| HP dependent, n = 28 . | HP independent, n = 25 . | |
| CagA positive | 22 | 7 |
| CagA negative | 6 | 18 |
| PPV for CagA* = 22 of 29 (75.9%) | ||
| Specificity for CagA† = 18 of 25 (75.0%) | ||
| p-SHP-2 positive | 21 | 7 |
| PPV for p-SHP-2* = 21 of 28 (75.0%) | ||
| p-ERK positive | 22 | 10 |
| PPV for p-ERK* = 22 of 32 (68.8%) | ||
| CagA, p-SHP-2, and p-ERK are all positive | ||
| Yes | 18 | 4 |
| No | 10 | 21 |
| PPV for combined CagA, p-SHP-2, and p-ERK‡ = 18 of 22 (81.8%) | ||
| Specificity for combined CagA, p-SHP-2, and p-ERK§ = 21 of 25 (84.0%) | ||
PPV: Number of HP-dependent cases who had CagA expression or CagA-signaling molecules expression divided by the number of the total positive cases for respective CagA expression or CagA-signaling molecule expression.
Specificity: Number of HP-independent cases who had no CagA expression/total HP-independent cases.
PPV: Number of HP-dependent cases who had all CagA, p-SHP-2, and p-ERK expression/total cases expressing all CagA, p-SHP-2, and p-ERK.
Specificity: Number of HP-independent cases who did not simultaneously express CagA, p-SHP-2, and p-ERK in tumors/total HP-independent cases.
Multivariate analyses of CagA expression, CagA-signaling, and clinicopathological factors with tumor response to HPE in gastric DLBCL
In addition to expression of CagA and CagA-signaling molecules, we correlated tumor-related and patient-related factors with tumor response to HPE in gastric DLBCL. In univariate analyses, endoscopic appearance (P = .033), lesion sites (P = .025), and endoscopic staging (P = .022) were closely associated with HP-dependent state, whereas age and sex were not associated with HP-dependent state (Table 2). For 63 patients, multivariate analysis was used to identify the expression of CagA (P = .002) as an independent predictor of HP dependence for gastric DLBCL (Table 4). By contrast, tumors limited to mucosa/submucosa (P = .077) and those located at the distal part of the stomach (P = .121) were marginally associated with the HP dependence of gastric DLBCL (Table 4).
Multiple analyses of clinicopathological features and molecular markers that predict HP dependence of gastric DLBCL using logistic regression analyses
| Characteristics . | Hazard ratio . | HP dependence . | P . |
|---|---|---|---|
| 95% CI . | |||
| Endoscopic features | |||
| Gastritis-like or multiple erosion vs ulcerated mass or nodular lesions | 1.595 | 0.323-7.870 | .566 |
| Location of tumors | |||
| Distal* vs proximal† or ≥2 components | 3.691 | 0.708-19.243 | .121 |
| Depth of gastric wall involvement | |||
| Submucosa or above vs muscularis propria or beyond | 3.832 | 0.864-16.983 | .077 |
| CagA expression | |||
| Positive vs negative | 8.425 | 2.167-32.753 | .002 |
| Characteristics . | Hazard ratio . | HP dependence . | P . |
|---|---|---|---|
| 95% CI . | |||
| Endoscopic features | |||
| Gastritis-like or multiple erosion vs ulcerated mass or nodular lesions | 1.595 | 0.323-7.870 | .566 |
| Location of tumors | |||
| Distal* vs proximal† or ≥2 components | 3.691 | 0.708-19.243 | .121 |
| Depth of gastric wall involvement | |||
| Submucosa or above vs muscularis propria or beyond | 3.832 | 0.864-16.983 | .077 |
| CagA expression | |||
| Positive vs negative | 8.425 | 2.167-32.753 | .002 |
CI, confidence interval.
Distal: Antrum, angle, or lower body.
Proximal: Middle body, upper body, fundus, or cardia.
Among the 53 patients with available information on p-SHP-2 and p-ERK, multivariate analysis showed that the expression of CagA (P = .083), the expression of p-SHP-2 (P = .109), and tumors limited to mucosa/submucosa (P = .099) were marginally associated with the HP dependence of gastric DLBCL. By contrast, the significance of the expression of p-ERK (P = .571), tumors located at the distal part of the stomach (P = .266), and gastritis-like or multiple erosion on infiltrated mucosa of endoscopic appearance (P = .957) decreased for predicting the HP dependence of gastric DLBCL.
Correlation of expression of BCL10, NF-κB (p65), BAFF, and CD86 with tumor response to HPE in patients with gastric DLBCL
In the present study, among 21 cases of gastric “pure” DLBCL, we observed nuclear BCL10 expression in 7 of the 8 HP-independent tumors (87.5%) and in 1 of the 13 HP-dependent tumors (7.7%) (P = .001). Similarly, nuclear NF-κB (p65) expression was detected in 6 of the 8 HP-independent tumors (75.0%) and in 2 of the 13 HP-dependent tumors (15.4%) (P = .018). BAFF overexpression was detected in 6 of the 8 HP-independent tumors (75.0%) and in 3 of the 13 HP-dependent tumors (23.1%) (P = .032). We observed CD86 expression in 8 of 13 HP-dependent cases (61.5%) and in 2 of 8 HP-independent cases (25%) (P = .023). For all the patients with gastric DLBCL, nuclear BCL10 expression (P < .001), nuclear NF-κB (p65) expression (P < .001), and BAFF overexpression (P < .001) were closely associated with the HP independence of gastric DLBCL, whereas CD86 expression (P < .001) was closely associated with the HP dependence of gastric DLBCL (Table 2). Furthermore, nuclear BCL10 expression was associated with nuclear NF-κB (p65) expression (P < .001) and BAFF overexpression (P = .001).
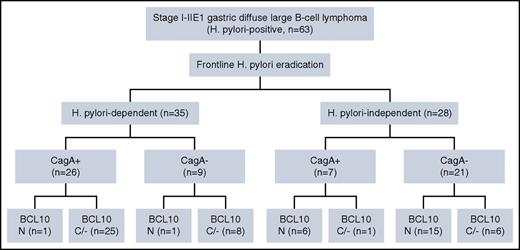
We further correlated the association between CagA and the nuclear expression of BCL10 with the tumor response to HPE in all the patients with gastric DLBCL. As displayed in Figure 4, detecting CagA expression and nuclear BCL10 expression can assist in predicting HP dependence or HP independence statuses for patients with gastric DLBCL who wish to receive HPE as frontline treatment. As shown in Table 5 and in supplemental Results, our results indicated that the combination of the depth of gastric wall involvement and HP-independence-related molecules (BCL10, NF-κB (p65), BAFF, and CD86) with CagA increased both the PPV and specificity for HP-dependent gastric DLBCL when compared with tumors expressing solely CagA.
Scheme of the relationship between HP dependence or independence and protein expression of CagA and BCL10. N indicates the number of patients in individual subgroups. +, positive expression of CagA; −, negative expression of CagA; C/−, cytoplasmic or negative expression; N, nuclear expression of BCL10.
Scheme of the relationship between HP dependence or independence and protein expression of CagA and BCL10. N indicates the number of patients in individual subgroups. +, positive expression of CagA; −, negative expression of CagA; C/−, cytoplasmic or negative expression; N, nuclear expression of BCL10.
PPV of depth of gastric wall involvement and BCL10, NF-κB (p65), BAFF, and CD86 molecules for HP dependence of stage IE/IIE1 gastric DLBCL
| Expression of CagA and CagA-signaling molecules in tumor tissue . | No. of gastric DLBCL patients who receive HPE as frontline treatment . | |
|---|---|---|
| HP dependent, n = 35 . | HP independent, n = 28 . | |
| CagA positive | 26 | 7 |
| CagA negative | 9 | 21 |
| PPV for CagA* = 26 of 33 (78.8%) | ||
| Specificity for CagA† = 21 of 27 (75.0%) | ||
| Tumor-infiltrating submucosa or above and CagA (+) (n = 57) | ||
| Yes | 11 | 1 |
| No | 20 | 25 |
| PPV for combined tumor-infiltrating submucosa or above and CagA* = 11 of 12 (91.7%) | ||
| Specificity for combined tumor-infiltrating submucosa or above and CagA† = 25 of 26 (96.2%) | ||
| CagA and CD86 are all positive | ||
| Yes | 22 | 5 |
| No | 13 | 23 |
| PPV for combined CagA and CD86* = 22 of 27 (81.5%) | ||
| Specificity for combined CagA and CD86† = 23 of 28 (82.1%) | ||
| CagA (+) and BCL10 (−) | ||
| Yes | 25 | 1 |
| No | 10 | 27 |
| PPV for combined CagA and BCL10* = 25 of 26 (96.2%) | ||
| Specificity for combined CagA and BCL10† = 27 of 28 (96.4%) | ||
| CagA (+) and NF-κB (p65) (−) | ||
| Yes | 24 | 2 |
| No | 11 | 26 |
| PPV for combined CagA and NF-κB* = 24 of 26 (92.3%) | ||
| Specificity for combined CagA and NF-κB† = 26 of 28 (92.9%) | ||
| CagA (+) and BAFF (−) | ||
| Yes | 23 | 3 |
| No | 12 | 25 |
| PPV for combined CagA and BAFF* = 23 of 26 (88.5%) | ||
| Specificity for combined CagA and BAFF† = 25 of 28 (89.3%) | ||
| Expression of CagA and CagA-signaling molecules in tumor tissue . | No. of gastric DLBCL patients who receive HPE as frontline treatment . | |
|---|---|---|
| HP dependent, n = 35 . | HP independent, n = 28 . | |
| CagA positive | 26 | 7 |
| CagA negative | 9 | 21 |
| PPV for CagA* = 26 of 33 (78.8%) | ||
| Specificity for CagA† = 21 of 27 (75.0%) | ||
| Tumor-infiltrating submucosa or above and CagA (+) (n = 57) | ||
| Yes | 11 | 1 |
| No | 20 | 25 |
| PPV for combined tumor-infiltrating submucosa or above and CagA* = 11 of 12 (91.7%) | ||
| Specificity for combined tumor-infiltrating submucosa or above and CagA† = 25 of 26 (96.2%) | ||
| CagA and CD86 are all positive | ||
| Yes | 22 | 5 |
| No | 13 | 23 |
| PPV for combined CagA and CD86* = 22 of 27 (81.5%) | ||
| Specificity for combined CagA and CD86† = 23 of 28 (82.1%) | ||
| CagA (+) and BCL10 (−) | ||
| Yes | 25 | 1 |
| No | 10 | 27 |
| PPV for combined CagA and BCL10* = 25 of 26 (96.2%) | ||
| Specificity for combined CagA and BCL10† = 27 of 28 (96.4%) | ||
| CagA (+) and NF-κB (p65) (−) | ||
| Yes | 24 | 2 |
| No | 11 | 26 |
| PPV for combined CagA and NF-κB* = 24 of 26 (92.3%) | ||
| Specificity for combined CagA and NF-κB† = 26 of 28 (92.9%) | ||
| CagA (+) and BAFF (−) | ||
| Yes | 23 | 3 |
| No | 12 | 25 |
| PPV for combined CagA and BAFF* = 23 of 26 (88.5%) | ||
| Specificity for combined CagA and BAFF† = 25 of 28 (89.3%) | ||
PPV: Number of HP-dependent cases who had combined CagA expression, and depth of gastric wall involvement or expression of various molecules, such as CD86, BCL10, NF-κB (p65), and BAFF, divided by the number of the total positive cases for respective CagA expression, depth of gastric wall involvement, or expression of various molecules, such as CD86, BCL10, NF-κB (p65), and BAFF.
Specificity: Number of HP-independent cases who had no CagA expression, or who did not simultaneously express CagA, CD86, nuclear expression of BCL10 and NF-κB (p65), or whose tumors were infiltrating muscularis propria/beyond or expressing BAFF/total HP-independent cases.
Discussion
We and other investigators have demonstrated that a high percentage of early-stage gastric DLBCL remains HP-dependent.10-16,26,44 Unlike low-grade MALT lymphoma, DLBCL may progress rapidly if unresponsive to HP.49-51 Therefore, identifying markers that may predict the HP-dependent status of gastric DLBCL is mandatory. In the present study, we demonstrated that CagA expression is closely associated with the HP dependence of not only gastric “pure” DLBCL but also of gastric DLBCL(MALT). CagA expression is significantly associated with CagA-signaling molecules, p-SHP-2, and p-ERK, and the correlation of these molecules is closely associated with the HP dependence of gastric DLBCL. These findings imply that CagA and CagA-signaling may directly affect the lymphomagenesis of these tumors.
In addition to an epidemiological study demonstrating the close association between the presence of CagA-IgG and gastric lymphoma,52 Achyut et al reported that anti-CagA antibodies and HP density were associated with risk of lymphoid follicle development in patients with gastritis.53 Epidemiological studies have reported that the occurrence of gastric MALT lymphomas in East Asia is higher than in Western countries.54-56 When examining the prevalence of CagA-positive HP infection, the rate is ∼60% in Western countries while similar studies in Eastern Asian populations suggest that it is nearly 90%.57-61 Another interesting observation reported in our previous28 and current studies is that CagA expression is more frequent in HP-dependent cases than in HP-independent cases, and that time to response to HPE is shorter in cases that express CagA. These findings are in line with the fact that Asian gastric MALT lymphoma patients have a higher response rate to HPE than Western patients (84.1% vs 73.8%; P = .0001).62
Although the rate of CagA-positive HP infection has rarely been examined in gastric DLBCL patients, Delchier et al observed that the CagA-seropositive rate was higher in patients with DLBCLs than in those with HP-positive gastric MALT lymphomas (75% [12 of 16] vs 44.8% [13 of 29], P < .05).63 Peng et al also reported that the frequency of CagA-positive strains of HP was significantly higher (P < .05) in patients with gastric DLBCL (MALT) than in those with gastric MALT lymphomas or gastritis.64 Using polymerase chain reaction analysis (supplemental Methods),60,61 we found that all 11 HP strains isolated from patients with HP-dependent gastric lymphomas (5 gastric DLBCLs and 6 gastric MALT lymphomas) are cagA gene positive (data not sown). In addition to the distinct prevalence of CagA-positive HP infection in Asian populations, East Asian CagA carries a EPIYA-D segment, which differs from the EPIYA-C motif commonly seen in Western CagA variants.18,19,65,66 CagA with EPIYA-D exhibits greater SHP-2-binding affinity and tyrosine phosphorylation activity than CagA with the EPIYA-C motif.18,19,67,68 Since we found a close association between CagA expression and p-SHP-2 in our gastric DLBCL tumor cells, most of the HP-dependent gastric DLBCLs in this study may be more closely linked to the CagA-tyrosine phosphorylation-dependent signaling pathway.
Two studies using a C57BL/6 mouse model further revealed that infection with CagA-positive HP strains can promote the development of gastric DLBCL with manifestations of MALT features.69,70 In a generated CagA transgenic mouse model, Ohnishi et al provided direct evidence of CagA acting as a bacterial oncoprotein in the growth of HP-related DLBCL through a tyrosine phosphorylation-dependent SHP-2-ERK signaling pathway.71 Using in vitro B-cell line models, we and other investigators have demonstrated that translocated CagA can stimulate proliferation and restrain the apoptosis of B cells through ERK activation, BAD phosphorylation, p53 accumulation, BAD phosphorylation, or upregulation of Bcl-2 and Bcl-XL expression.22,27,72 Our current findings revealed a close link between CagA and CagA-signaling molecules, p-SHP-2, and p-ERK in the tumor cells of gastric DLBCL and that this link appears to be closely associated with HP dependence. Our results also disclosed that the cagA gene can be detected in all gastric tumor biopsies obtained from 7 selective cases of HP-dependent gastric DLBCL whose tumor were expressing CagA (supplemental Methods; supplemental Figure 3).73 In addition, among these HP-dependent gastric DLBCL tumors, downregulated expression of CagA and CagA-signaling molecules (p-SHP-2 and p-ERK) were found in post-HPE gastric biopsy samples. These findings suggest that CagA-regulated signaling might be involved in HP-dependent lymphomagenesis of gastric DLBCL. Considering the complex interaction between micromes and the genome/epigenome,74 further studies examining the mechanism of possible CagA-directed lymphomagenesis in gastric DLBCL are required.
Although the results of our study suggest that the growth of HP-dependent gastric DLBCL cells might be dependent on the regulation of CagA and its regulated signaling, substitute mechanisms that link HP with the lymphoid neoplasm of the stomach, such as HP-specific intratumoral T cells and their communication with B cells, or antigen stimuli with tonic B-cell receptor signaling, should not be ignored.23,24,26,75 Regarding the expression of costimulatory molecules, including CD80 (B7.1), CD86 (B7.2), and their ligands in gastric lymphoma tumor cells, de Jong et al discovered a cross-link between the expression of CD86 and the HP dependence of gastric MALT lymphoma.76,77 We further reported that CD86 is present in gastric DLBCL and is significantly associated with the HP dependence of these tumors. Our findings support the notion that the growth of a substantial portion of early-stage gastric “pure” DLBCL remains dependent on the interactions of functional tumor B cells with reactive T cells. We hypothesized that a certain expression of CD86 in tumors was activated by CagA; this followed the coculturing of HP with B cells in our previous work, demonstrating that greater upregulation of CD86 was stimulated by a CagA wild-type HP strain than by a CagA-deficient HP strain.27
In this study, most of the patients with CagA-positive gastric DLBCL were HP-dependent and 7 (21.2%) were HP-independent. Of these 7 HP-independent patients, one exceptional patient was CagA-positive but showed no BCL10 expression, and the other 6 carried BCL10 nuclear expression. These findings indicated that BCL10 nuclear expression contributes to the HP-independent growth of large B-cell lymphoma cells.31-33,78 The exceptional HP-independent patient being CagA-positive without nuclear BCL10 expression might indicate that CagA is not sufficient to activate downstream signals in selected cases. However, among CagA-negative but HP-dependent tumors, having evaluated CagA-signaling molecules, we determined that 4 (66.7%) of the 6 expressed p-SHP-2, p-ERK, or both. The absence of CagA-signaling molecules in the tumor cells of the 2 cases (one case involved the expression of CD86) suggested that a significant alternative pathway associated HP with lymphomagenesis.18,19,26,65,75,79
Our results indicated that CagA and CagA-signaling molecules can be detected not only in cells of “pure” DLBCL gastric tumors, but also in cells of DLBCL(MALT) gastric tumors. Compared with the expression of CagA alone, the combined expressions of CagA, p-SHP-2, and p-ERK provide an increased PPV (81.8%) as well as an increased specificity (84.0%) for HP dependence. In addition to HP-dependent predictive markers (tumors limited to the mucosa/submucosa and CD86 expression),34,36,76,77,80 nuclear expression of BCL10 or NF-κB (p65) and BAFF overexpression can assist in predicting the HP independence of gastric DLBCL. Furthermore, our findings suggest that a combination of CagA expression, CD86 expression, and negative nuclear BCL10 expression may help predict HP dependence and allow identify perfect candidates for HPE without resorting to chemotherapy. Additional investigation of the biological significance of the direct effect of CagA on the lymphomagenesis of gastric DLBCL is warranted.
Present in poster form at the 18th annual meeting of the European Cancer Congress, Vienna, Austria, 25-29 September 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the following research grants: MOST 104-2314-B-002-189-MY3 and 105-2811-B-002-041 from the Ministry of Science and Technology, NTUH 105-S3059, NTUH 105-N3275, and NTUH 105-S3036 from National Taiwan University Hospital, and MOHW105-TDU-B-211-134005 from the Ministry of Health and Welfare, Taiwan.
Authorship
Contribution: S.-H.K., L.-T.C., and A.-L.C. contributed to the study design; S.-H.K., L.-T.C., K.-H.Y., J.-M.L., M.-S.W., and A.-L.C. treated patients; S.-H.K., L.-T.C., C.-W.L., K.-H.Y., C.-T.S., J.-M.L., M.-S.W., and A.-L.C. provided tissue sample; S.-H.K., C.-W.L., Y.-S.T., and J.-M.L. performed research; S.-H.K., L.-T.C., C.-W.L., C.-T.S., P.-N.H., M.-S.W., and A.-L.C. were involved in data analysis and interpretation; and S.-H.K., and A.L.C. wrote the manuscript, which was revised and approved by all coauthors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ann-Lii Cheng, Department of Internal Medicine and Department of Oncology, National Taiwan University Hospital, No. 7, Chung-Shan S Rd, Taipei, Taiwan; e-mail: alcheng@ntu.edu.tw.

![Figure 1. Immunohistochemical analysis of CagA expression in the tumor cells of gastric DLBCL. (A) Endoscopy showing multiple irregular ulcerative masses in the cardia of the stomach of an 86-year-old woman. Right bottom inset, Endoscopic ultrasonography shows a homogenous hypoechoic mass infiltration of mucosa and submucosa (white arrow, maximal thickness [7 mm]) of the gastric wall. (B) Six months after completion of HPE, complete remission (CR) was achieved. (C) Tumor cell nuclear CagA expression in the same case; original magnification ×400. (D) Cytoplasmic expression of BCL10 in tumor cells of the same case; original magnification ×400. (E) Cytoplasmic expression of NF-κB in tumor cells of the same case; original magnification ×400. (F) No CagA expression in specimens of gastritis (6 months after completion of HPE) in the same case; original magnification ×400. (G) An HP-dependent gastric “pure” DLBCL case (time to CR after completion of HPE, 1 month) displaying nuclear CagA expression in the tumor cells of the gastric mucosa; original magnification ×400. (H) An HP-dependent gastric DLBCL(MALT) case (time to CR after completion of HPE, 3 month) displaying nuclear CagA expression in the tumor cells of the gastric mucosa; original magnification ×400.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/2/10.1182_blood-2016-04-713719/4/m_blood713719f1.jpeg?Expires=1768268018&Signature=oDP4GK7DPir14OiCohBllIMDcA87TFHo5e~41lUBbH1aV0zAZqOoEzfBDFSVatu8J-JbEKe4YxbenlkikZhYbt7naAMXsQWxTrvzQTwzdPPTWsInHLp52Z7rIcktYo7-G8nleMgGIJvgyTzUO~yvokooqU6LNVzOKPyUiMXpY-6AmkeswVA81e9HpkQm4q21Jrm9cI9HK-WY~6NPY4970fv94kcDcgLvbmkZjBs9jSc9MpejW6~9mQWdS~Q5c3Wvl46QM1Kq79F10gBPXYH5sPToIV3eRO06CH1XDHmrhABQdZhMjc2DTEM2b18zJwp05DkHKG~glmubROlmLMq5Ow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal