Key Points
STRATUS (MM-010), the largest POM + LoDEX trial, confirms the regimen offers clinically meaningful benefit and is generally well tolerated.
STRATUS supports POM + LoDEX as a standard of care for patients with RRMM who have poor prognosis and high need for effective treatments.
Abstract
Patients with relapsed and/or refractory multiple myeloma (RRMM) have poor prognosis. The STRATUS study assessed safety and efficacy of pomalidomide plus low-dose dexamethasone in the largest cohort to date of patients with RRMM. Patients who failed treatment with bortezomib and lenalidomide and had adequate prior alkylator therapy were eligible. Pomalidomide 4 mg was given on days 1-21 of 28-day cycles with low-dose dexamethasone 40 mg (20 mg for patients aged >75 years) on days 1, 8, 15, and 22 until progressive disease or unacceptable toxicity. Safety was the primary end point; secondary end points included overall response rate (ORR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Among 682 patients enrolled, median age was 66 years, and median time since diagnosis was 5.3 years. Median number of prior regimens was 5. Most patients were refractory to both lenalidomide and bortezomib (80.2%). Median follow-up was 16.8 months; median duration of treatment was 4.9 months. Most frequent grade 3/4 treatment-emergent adverse events were hematologic (neutropenia [49.7%], anemia [33.0%], and thrombocytopenia [24.1%]). Most common grade 3/4 nonhematologic toxicities were pneumonia (10.9%) and fatigue (5.9%). Grade 3/4 venous thromboembolism and peripheral neuropathy were rare (1.6% each). The ORR was 32.6%, and the median DOR was 7.4 months. Median PFS and OS were 4.6 months and 11.9 months, respectively. We present the largest trial to date evaluating pomalidomide plus low-dose dexamethasone in patients with RRMM, further confirming that this regimen offers clinically meaningful benefit and is generally well tolerated. www.Clinicaltrials.gov identifier NCT01712789.
Introduction
Patients with refractory or relapsed and refractory multiple myeloma (RRMM) following treatment with newer agents (eg, lenalidomide and bortezomib) have a poor prognosis characterized by a shortened overall survival (OS).1 Thus, there is significant unmet need for alternative treatment options for this patient population. One such agent is pomalidomide, a distinct IMiD immunomodulatory agent with tumoricidal and antiangiogenic activities.2 Preclinical studies of pomalidomide have demonstrated antiproliferative and proapoptotic activity in lenalidomide-resistant myeloma cells3-5 as well as synergistic effects with dexamethasone.3,6
Two pivotal clinical studies of pomalidomide combined with low-dose dexamethasone, MM-002 (phase 2) and MM-003 (phase 3), have demonstrated the clinical benefit of this regimen in patients with RRMM.7,8 In the randomized MM-002 study, overall response rate (ORR; ≥ partial response [PR]) was significantly higher with pomalidomide plus low-dose dexamethasone compared with pomalidomide alone (33% vs 18%, P = .013).7 Median progression-free survival (PFS) was also significantly greater in the pomalidomide plus low-dose dexamethasone group (4.2 vs 2.7 months, P = .003).7 The randomized phase 3 MM-003 study demonstrated that the combination of pomalidomide plus low-dose dexamethasone led to significant improvement in PFS (4.0 vs 1.9 months, P < .0001) and OS (12.7 vs 8.1 months, P = .0285) compared with high-dose dexamethasone,8 an OS advantage that was confirmed with longer follow-up despite 56% of patients on the high-dose dexamethasone arm receiving subsequent pomalidomide.9
Based on the results from MM-002 and MM-003, pomalidomide was approved in the United States, the European Union, and other countries around the world for the treatment of patients with RRMM who have received ≥2 prior therapies (including lenalidomide and bortezomib) and progressed on the last therapy (United States and European Union), or within 60 days following completion of their last therapy (United States).10,11
To further assess the safety and efficacy of pomalidomide plus low-dose dexamethasone, a phase 3b study, MM-010 (STRATUS), was conducted in a large population of patients with RRMM. The results of this study are presented in this paper.
Methods
Study design and participants
STRATUS (MM-010) is an open-label, single-arm phase 3b study undertaken at 91 centers in 19 countries across Europe. For inclusion in the study, patients had to be at least 18 years of age, refractory to their last treatment, and have RRMM. Patients had to have received ≥2 prior treatment lines, including ≥2 cycles of lenalidomide and bortezomib (alone or in combination) and adequate prior alkylator therapy (≥4 cycles or progressive disease [PD] after ≥2 cycles or received alkylator treatment as a part of a stem cell transplant). In addition, all patients must have failed treatment with both bortezomib and lenalidomide, defined as PD on or within 60 days of treatment (refractory), PD ≤ 6 months after achieving a PR (relapsed), or intolerance to bortezomib.
Patients were ineligible if they had previously received therapy with pomalidomide or had hypersensitivity to thalidomide, lenalidomide, or dexamethasone. Peripheral neuropathy grade ≥ 2 or substantial cardiac disease (New York Heart Association class III or IV congestive heart failure, myocardial infarction within 12 months prior to enrollment, or unstable or poorly controlled angina pectoris) were exclusion criteria. Patients with the following laboratory values were also considered ineligible: absolute neutrophil count <800/μL; platelets <75 000/μL for patients in whom <50% of bone marrow nucleated cells were plasma cells or <30 000/μL for patients in whom ≥50% of bone marrow nucleated cells were plasma cells; creatinine clearance (CrCl) <45 mL/min according to the Cockcroft-Gault formula or 24-hour urine collection12 ; hemoglobin <8 g/dL; corrected serum calcium >3.5 mmol/L; total bilirubin >34.2 μmol/L; or liver enzymes >3 times the upper limit of normal.
All patients provided written informed consent prior to the start of the study. This study was approved by an institutional review board for each study site prior to initiation of any study procedures and was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice (as outlined by the International Conference on Harmonization E6 requirements).
Procedures
Patients were administered pomalidomide 4 mg on days 1-21 of a 28-day cycle. Patients also received low-dose dexamethasone 40 mg (if aged ≤75 years) or 20 mg (if aged >75 years) on days 1, 8, 15, and 22 of a 28-day cycle. Protocol guidance for dose interruptions and reductions was similar to that used in the MM-003 phase 3 study and are described elsewhere.8 Thromboprophylaxis with low-dose aspirin, low-molecular-weight heparin, or equivalent was required for all patients. Treatment was continued until PD or unacceptable toxicity. Patients who discontinued treatment entered the follow-up phase, where information on subsequent antimyeloma treatments, date of progression, survival, and second primary malignancies (SPMs) were collected every 3 months for up to 5 years after enrollment of the last patient. Adverse events (AEs) were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, v. 4.0. Serious AEs were those that resulted in death, were life-threatening, required hospitalization or prolongation of hospitalization, caused a congenital anomaly or birth defect, or constituted an important medical event.
Statistical analysis
The primary end point was safety as measured by the incidence of AEs (type, frequency, severity, and relationship to study drugs), including SPMs. Key secondary end points included pomalidomide exposure, ORR (≥ PR) based on the study investigator’s assessment and evaluated according to the International Myeloma Working Group (IMWG) response criteria,13 duration of response (DOR), PFS, OS, time to response, and time to progression. The safety population was defined as all patients who received ≥1 dose of trial treatment and was used for all safety analyses. Efficacy assessments were conducted in the intention-to-treat (ITT) population (all enrolled patients). The trial was designed to enroll patients for up to 24 months or until 720 patients were enrolled, whichever came first.
This study is registered with ClinicalTrials.gov (NCT01712789) and EudraCT (2012-001888-78).
Results
Patients
Between November 2012 and December 2014, the MM-010 study enrolled 682 patients with RRMM, of whom 6 (0.9%) did not receive study drug. With a median follow-up of 16.8 months as of the data cutoff date of May 4, 2015, 104 patients (15.2%) remained on treatment, and 572 patients (83.9%) have discontinued treatment. The primary reason for treatment discontinuation was PD (62.2%), followed by death (7.9%), AEs (5.9%), other causes (4.8%; including clinical progression without confirmed IMWG-defined PD and transition to commercial pomalidomide), withdrawal of consent (2.9%), and lost to follow-up (<1.0%).
The median age of patients in MM-010 was 66 years with a median time from initial diagnosis of 5.3 years and a median of 5 (range, 2-18) prior treatment regimens. Most patients were refractory to lenalidomide and/or bortezomib: 95.9% of patients were refractory to lenalidomide and 4.1% were relapsed; 83.7% were refractory to bortezomib, 12.0% were relapsed, and 4.3% were intolerant; and 80.2% of patients were refractory to both lenalidomide and bortezomib. International Staging System Stage III was reported for 34.6% of patients, 34.8% had CrCl < 60 mL/min, and 10.7% of patients had extramedullary disease. Few patients had moderate cytopenia at baseline; 2.9% had grade 3 neutropenia and 11.0% had grade 3/4 thrombocytopenia. Additional baseline characteristics are presented in Table 1.
Baseline characteristics
| Characteristic . | ITT population (N = 682) . |
|---|---|
| Median age, y (range) | 66 (37-88) |
| >65, n (%) | 369 (54.1) |
| >70, n (%) | 213 (31.2) |
| >75, n (%) | 87 (12.8) |
| Sex, n (%) | |
| Male | 381 (55.9) |
| Female | 301 (44.1) |
| Median time since initial diagnosis, y (range) | 5.3 (0.6-28.2) |
| ECOG performance status, n (%) | |
| 0-1 | 614 (90.0) |
| 2-3 | 68 (10.0) |
| ISS stage at study entry, n (%) | |
| I-II | 414 (60.7) |
| III | 236 (34.6) |
| Missing | 32 (4.7) |
| CrCl < 60 mL/min, n (%) | 237 (34.8) |
| Median prior regimens, n (range) | 5 (2-18) |
| >2 previous regimens, n (%) | 637 (93.4) |
| Prior dexamethasone, n (%) | 666 (97.7) |
| Prior lenalidomide, n (%) | 682 (100.0) |
| Prior bortezomib, n (%) | 682 (100.0) |
| Prior thalidomide, n (%) | 372 (54.5) |
| Prior carfilzomib, n (%) | 24 (3.5) |
| Prior stem cell transplant, n (%) | 451 (66.1) |
| Lenalidomide refractory, n (%) | 654 (95.9) |
| Bortezomib refractory, n (%) | 571 (83.7) |
| Lenalidomide and bortezomib refractory, n (%) | 547 (80.2) |
| Characteristic . | ITT population (N = 682) . |
|---|---|
| Median age, y (range) | 66 (37-88) |
| >65, n (%) | 369 (54.1) |
| >70, n (%) | 213 (31.2) |
| >75, n (%) | 87 (12.8) |
| Sex, n (%) | |
| Male | 381 (55.9) |
| Female | 301 (44.1) |
| Median time since initial diagnosis, y (range) | 5.3 (0.6-28.2) |
| ECOG performance status, n (%) | |
| 0-1 | 614 (90.0) |
| 2-3 | 68 (10.0) |
| ISS stage at study entry, n (%) | |
| I-II | 414 (60.7) |
| III | 236 (34.6) |
| Missing | 32 (4.7) |
| CrCl < 60 mL/min, n (%) | 237 (34.8) |
| Median prior regimens, n (range) | 5 (2-18) |
| >2 previous regimens, n (%) | 637 (93.4) |
| Prior dexamethasone, n (%) | 666 (97.7) |
| Prior lenalidomide, n (%) | 682 (100.0) |
| Prior bortezomib, n (%) | 682 (100.0) |
| Prior thalidomide, n (%) | 372 (54.5) |
| Prior carfilzomib, n (%) | 24 (3.5) |
| Prior stem cell transplant, n (%) | 451 (66.1) |
| Lenalidomide refractory, n (%) | 654 (95.9) |
| Bortezomib refractory, n (%) | 571 (83.7) |
| Lenalidomide and bortezomib refractory, n (%) | 547 (80.2) |
ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System.
Pomalidomide dosing and modification
The median treatment duration was 4.9 months with a median relative dose intensity of 0.901, indicating that treatment was generally well tolerated with a low-dose reduction/interruption rate and high treatment compliance (Table 2). Dose reductions and interruptions of pomalidomide due to AEs occurred in 22.0% and 66.3% of patients, respectively. The most frequent AEs leading to dose reductions of pomalidomide were neutropenia (5.9%), thrombocytopenia (4.3%), fatigue (2.5%), and pneumonia (2.4%). AEs leading to dose interruptions of pomalidomide were most commonly neutropenia (22.6%), thrombocytopenia (11.1%), and pneumonia (10.2%).
Pomalidomide dosing and most common causes of dose modification
| . | Safety population (N = 676) . |
|---|---|
| Median pomalidomide treatment duration, mo (range) | 4.9 (0.1-28.3) |
| Median pomalidomide relative dose intensity* | 0.901 |
| Overall discontinuation of pomalidomide due to AE, n (%) | 40 (5.9) |
| Due to thrombocytopenia | 5 (0.7) |
| Due to pneumonia | 4 (0.6) |
| Dose reduction of pomalidomide due to AE, n (%) | 149 (22.0) |
| Due to neutropenia | 40 (5.9) |
| Due to thrombocytopenia | 29 (4.3) |
| Due to fatigue | 17 (2.5) |
| Due to pneumonia | 16 (2.4) |
| Dose interruption of pomalidomide due to AE, n (%) | 448 (66.3) |
| Due to neutropenia | 153 (22.6) |
| Due to thrombocytopenia | 75 (11.1) |
| Due to pneumonia | 69 (10.2) |
| . | Safety population (N = 676) . |
|---|---|
| Median pomalidomide treatment duration, mo (range) | 4.9 (0.1-28.3) |
| Median pomalidomide relative dose intensity* | 0.901 |
| Overall discontinuation of pomalidomide due to AE, n (%) | 40 (5.9) |
| Due to thrombocytopenia | 5 (0.7) |
| Due to pneumonia | 4 (0.6) |
| Dose reduction of pomalidomide due to AE, n (%) | 149 (22.0) |
| Due to neutropenia | 40 (5.9) |
| Due to thrombocytopenia | 29 (4.3) |
| Due to fatigue | 17 (2.5) |
| Due to pneumonia | 16 (2.4) |
| Dose interruption of pomalidomide due to AE, n (%) | 448 (66.3) |
| Due to neutropenia | 153 (22.6) |
| Due to thrombocytopenia | 75 (11.1) |
| Due to pneumonia | 69 (10.2) |
Relative dose intensity, dose intensity/planned dose intensity.
Safety
Among the 676 treated patients, the most frequently reported grade 3/4 hematologic AEs were neutropenia (49.7%), anemia (33.0%), and thrombocytopenia (24.1%; Table 3). The incidence of grade 3/4 febrile neutropenia was 5.3%. Infections were the most frequent grade 3/4 nonhematologic AEs (28.1%, including 10.9% of patients with grade 3/4 pneumonia). Results were similar regardless of age. In a subanalysis in patients by age (≤65 vs >65 years and ≤70 vs >70 years), the most frequent grade 3/4 treatment-emergent AEs (TEAEs) across age groups were neutropenia (47% to 51%), anemia (32% to 34%), thrombocytopenia (19% to 26%), and infections (31% to 37%). Overall, pneumonia of any grade occurred in 111 patients (16.4%). The majority of pneumonia events resolved, with 13 patients (1.9%) having a fatal grade 5 event. There was only one case of noninfectious pneumonitis. Occurrence of neutropenia did not seem to affect the incidence of infections because over half of infections (any grade) occurred in the absence of neutropenia (57.2%). The rate of pomalidomide discontinuation due to infection (1.6%) was low. Granulocyte colony-stimulating factor was administered for 56.4% of patients with infections and 75.4% of patients with neutropenia. Anti-infectives were used in 95.5% of patients experiencing infections. Red blood cell transfusions and platelet transfusions were used in 48.4% and 16.1% of the entire patient population, respectively. Deep vein thrombosis and pulmonary embolism were infrequent with 1.6% of patients experiencing grade 3/4 and 3.1% of patients experiencing a venous thromboembolic event of any grade (Table 4). Fewer than 2% of patients had grade 3/4 peripheral neuropathy (PN). PN of any grade was experienced by 17.9% of patients, 43.8% of whom had PN at baseline, and the median time to PN onset of any grade was 1.7 months.
Most commonly reported TEAEs (occurring in >10% [any grade] of the safety population)
| . | Safety population (N = 676) . | |||
|---|---|---|---|---|
| AE, n (%) . | Total . | Grade 3* . | Grade 4* . | Grade 5* . |
| Neutropenia | 383 (56.7) | 200 (29.6) | 136 (20.1) | 0 |
| Anemia | 326 (48.2) | 215 (31.8) | 8 (1.2) | 0 |
| Thrombocytopenia | 234 (34.6) | 82 (12.1) | 81 (12.0) | 0 |
| Fatigue | 194 (28.7) | 38 (5.6) | 2 (0.3) | 0 |
| Pyrexia† | 194 (28.7) | 19 (2.8) | 1 (0.1) | 0 |
| Constipation | 155 (22.9) | 5 (0.7) | 0 | 0 |
| Asthenia | 153 (22.6) | 20 (3.0) | 3 (0.4) | 0 |
| Cough | 133 (19.7) | 2 (0.3) | 0 | 0 |
| Diarrhea | 113 (16.7) | 6 (0.9) | 0 | 0 |
| Dyspnea | 113 (16.7) | 18 (2.7) | 2 (0.3) | 1 (0.1) |
| Pneumonia | 111 (16.4) | 64 (9.5) | 10 (1.5) | 13 (1.9) |
| Peripheral edema | 106 (15.7) | 8 (1.2) | 0 | 0 |
| Back pain | 98 (14.5) | 15 (2.2) | 1 (0.1) | 1 (0.1) |
| Leukopenia | 93 (13.8) | 37 (5.5) | 17 (2.5) | 0 |
| Muscle spasms | 92 (13.6) | 2 (0.3) | 0 | 0 |
| Nausea | 92 (13.6) | 2 (0.3) | 0 | 0 |
| Peripheral sensory neuropathy | 73 (10.8) | 4 (0.6) | 1 (0.1) | 0 |
| Decreased appetite† | 69 (10.2) | 5 (0.7) | 0 | 0 |
| Insomnia | 69 (10.2) | 5 (0.7) | 0 | 0 |
| . | Safety population (N = 676) . | |||
|---|---|---|---|---|
| AE, n (%) . | Total . | Grade 3* . | Grade 4* . | Grade 5* . |
| Neutropenia | 383 (56.7) | 200 (29.6) | 136 (20.1) | 0 |
| Anemia | 326 (48.2) | 215 (31.8) | 8 (1.2) | 0 |
| Thrombocytopenia | 234 (34.6) | 82 (12.1) | 81 (12.0) | 0 |
| Fatigue | 194 (28.7) | 38 (5.6) | 2 (0.3) | 0 |
| Pyrexia† | 194 (28.7) | 19 (2.8) | 1 (0.1) | 0 |
| Constipation | 155 (22.9) | 5 (0.7) | 0 | 0 |
| Asthenia | 153 (22.6) | 20 (3.0) | 3 (0.4) | 0 |
| Cough | 133 (19.7) | 2 (0.3) | 0 | 0 |
| Diarrhea | 113 (16.7) | 6 (0.9) | 0 | 0 |
| Dyspnea | 113 (16.7) | 18 (2.7) | 2 (0.3) | 1 (0.1) |
| Pneumonia | 111 (16.4) | 64 (9.5) | 10 (1.5) | 13 (1.9) |
| Peripheral edema | 106 (15.7) | 8 (1.2) | 0 | 0 |
| Back pain | 98 (14.5) | 15 (2.2) | 1 (0.1) | 1 (0.1) |
| Leukopenia | 93 (13.8) | 37 (5.5) | 17 (2.5) | 0 |
| Muscle spasms | 92 (13.6) | 2 (0.3) | 0 | 0 |
| Nausea | 92 (13.6) | 2 (0.3) | 0 | 0 |
| Peripheral sensory neuropathy | 73 (10.8) | 4 (0.6) | 1 (0.1) | 0 |
| Decreased appetite† | 69 (10.2) | 5 (0.7) | 0 | 0 |
| Insomnia | 69 (10.2) | 5 (0.7) | 0 | 0 |
Patients who experienced a particular event in more than one grade were counted only once in the highest grade.
One patient experienced a TEAE of unknown grade.
AEs of interest
| AE . | Safety population (N = 676) . |
|---|---|
| Any grade, n (%) | |
| VTE* | 21 (3.1) |
| Peripheral neuropathy† | 121 (17.9) |
| Grade 3/4 AEs, n (%) | |
| VTE* | 11 (1.6) |
| Peripheral neuropathy† | 11 (1.6) |
| AE . | Safety population (N = 676) . |
|---|---|
| Any grade, n (%) | |
| VTE* | 21 (3.1) |
| Peripheral neuropathy† | 121 (17.9) |
| Grade 3/4 AEs, n (%) | |
| VTE* | 11 (1.6) |
| Peripheral neuropathy† | 11 (1.6) |
Includes the preferred terms, deep vein thrombosis and pulmonary embolism.
Includes the preferred terms, neuropathy peripheral, peripheral sensory neuropathy, paresthesia, hypoesthesia, polyneuropathy, peripheral sensorimotor neuropathy, peripheral motor neuropathy, dysesthesia, gait disturbance, neuralgia, amyotrophy, burning sensation, motor dysfunction, muscle atrophy, sensory disturbance, and toxic neuropathy.
VTE, venous thromboembolic event.
Serious AEs were observed in 425 patients (62.9%). SPMs were reported in 15 patients (5 patients had invasive solid tumors, and 10 patients had noninvasive skin cancers). The incidence rate of developing an invasive solid tumor SPM was 0.90 per 100 person-years (95% confidence interval [CI]: 0.37, 2.16). The median time to onset of the 5 solid tumor SPMs was 12.5 months (range, 5.9-20.6 months). Two of the solid tumor SPMs (squamous cell carcinoma of the tongue and gastrointestinal stromal tumor) were diagnosed less than a year following the start of study therapy. As of the data cutoff, 57.7% of the patients in the safety population had died. Of these, 68.5% of deaths (267/390 total safety population deaths) occurred after treatment discontinuation (during follow-up). The most common cause of death in the safety population was progression of multiple myeloma (plasma cell myeloma [35.2%] and plasma cell leukemia [0.4%]), followed by infections and infestations (9.3%), and general disorders (5.6%). Deaths due to other causes were infrequent. Infection was also the most common cause of hospitalization (237 of the 390 patients hospitalized [60.8%]). Six percent of patients had TEAEs leading to discontinuation of pomalidomide; thrombocytopenia (0.7%) and pneumonia (0.6%) were the most frequent TEAEs leading to pomalidomide discontinuation. Other TEAEs leading to discontinuation of pomalidomide included lung infection, general physical health deterioration, pyrexia, dyspnea, cardiac failure, confusional state, and renal failure (0.3% of patients for each).
Efficacy ORR
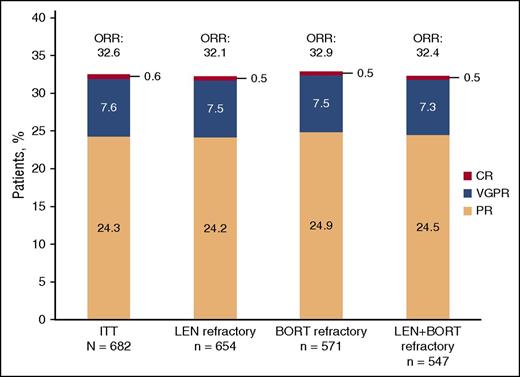
In the ITT population, the ORR was 32.6% (95% CI: 29.0, 36.2) with 7.6% of patients achieving a very good partial response and 0.6% of patients achieving a complete response (Figure 1). The median time to response was 1.9 months (range, 0.5-17.5 months), and the median DOR was 7.4 months (95% CI: 6.5, 8.7). Results were similar for patients refractory to lenalidomide, refractory to bortezomib, and refractory to both lenalidomide and bortezomib with ORRs of 32.1%, 32.9%, and 32.4%, respectively. Stable disease was observed in 49.7% (339/682) of patients. The median time to progression was 4.7 months (95% CI: 4.2, 5.6).
Investigator-assessed response to treatment using IMWG criteria. BORT, bortezomib; CR, complete response; LEN, lenalidomide; VGPR, very good partial response.
Investigator-assessed response to treatment using IMWG criteria. BORT, bortezomib; CR, complete response; LEN, lenalidomide; VGPR, very good partial response.
ORR results were also similar for patients with ≤3 vs >3 prior lines of therapy (28.5% [95% CI: 22.1, 35.6] vs 34.1% [95% CI: 29.9, 38.4]) and in patients with (CrCl < 60 mL/min) or without renal impairment (CrCl ≥ 60 mL/min) at study entry (30.8% [95% CI: 25.0, 37.1] vs 33.9% [95% CI: 29.4, 38.5]).
PFS
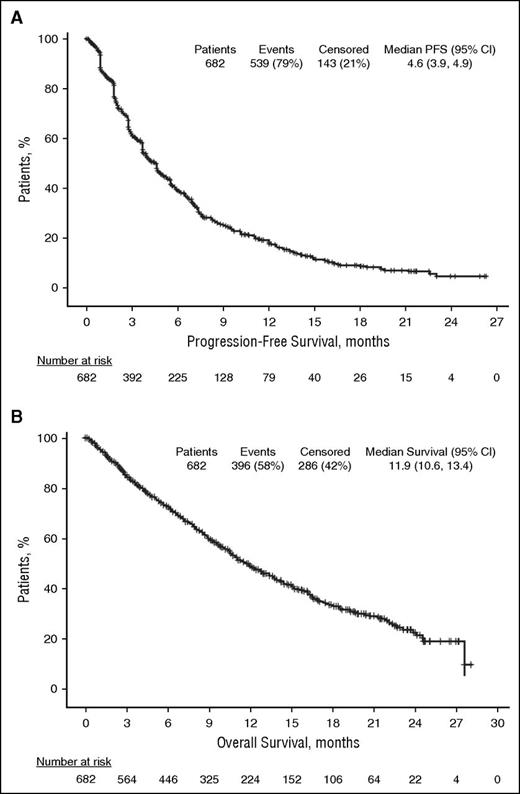
Median PFS was 4.6 months (95% CI: 3.9, 4.9) in the ITT population (Figure 2A). Similar results were obtained in patients refractory to lenalidomide (median PFS, 4.6 months [95% CI: 3.8, 4.9]), patients refractory to bortezomib (median PFS, 4.2 months [95% CI: 3.8, 4.8]), and patients refractory to both lenalidomide and bortezomib (median PFS, 4.2 months [95% CI: 3.8, 4.7]). Median PFS was also similar regardless of number of prior lines of therapy (≤3 prior lines, 3.9 months [95% CI: 3.7, 5.1] vs >3 prior lines, 4.6 months [95% CI: 4.0, 5.3]) and in patients with or without moderate renal impairment at study entry (3.8 months [95% CI: 2.9, 4.6] vs 4.7 months [95% CI: 4.2, 5.6], respectively).
OS
In the ITT population, median OS was 11.9 months (95% CI: 10.6, 13.4; Figure 2B). The median OS was similar regardless of prior refractory status with median OS of 11.9 months (95% CI: 10.6, 13.4) in patients refractory to lenalidomide, bortezomib, and both lenalidomide and bortezomib. Similar results were also found regardless of number of prior lines of therapy and moderate renal impairment at study entry. Median OS was 12.8 (95% CI: 8.9, 18.4) vs 11.9 months (95% CI: 10.6, 13.0) for patients with ≤3 vs >3 prior lines, respectively, and 10.2 (95% CI: 8.5, 12.0) vs 13.0 months (95% CI: 11.4, 14.7) in patients with or without moderate renal impairment, respectively.
Discussion
The STRATUS study is the largest study conducted to date with pomalidomide plus low-dose dexamethasone in a heavily pretreated RRMM patient population. All patients had previously been exposed to both lenalidomide and bortezomib and received a median of 5 prior antimyeloma treatment regimens. Pomalidomide plus low-dose dexamethasone was generally well tolerated. The safety profile was consistent with the profile observed in the pivotal studies of pomalidomide plus low-dose dexamethasone, and no new safety signals were identified in this large patient population.7,8 Similar to the MM-002 and MM-003 studies, the most frequent grade 3/4 AEs were hematologic with a low incidence of febrile neutropenia; discontinuations due to AEs were infrequent. Across all 3 studies, neutropenia was the most frequently observed grade 3/4 hematologic AE occurring in 41.1%, 47.7%, and 49.7% of patients enrolled in MM-002, MM-003, and the present study, respectively.7,8 The incidence of grade 3/4 pneumonia (10.9%) observed in the present study was similar to that observed in a prior pivotal phase 3 study of pomalidomide plus low-dose dexamethasone (12.7%).8 The hematologic and nonhematologic AEs tended to occur mostly in the early cycles with a diminished frequency afterward (data not shown). Dose interruptions and reductions with pomalidomide due to AEs were 66.3% and 22.0%, respectively, and occurred most often as a result of neutropenia or thrombocytopenia. However, the median relative dose intensity remained high at 0.901, indicating that most patients received close to the full dose of pomalidomide throughout the study.
The efficacy results observed with pomalidomide plus low-dose dexamethasone in this study confirm the clinical benefit (ORR, PFS, and OS) observed in other clinical trials. The ORR was 32.6% (with 7.6% of patients achieving ≥ very good partial response), which was similar to that observed in the MM-002 (ORR = 32.7%), MM-003 (31.4%), and IFM-2009-02 (34.8%) studies.7,8,14 The median DOR observed in this study (7.4 months) was consistent with that observed in the MM-002 (8.3 months), MM-003 (7.0 months), and IFM-2009-02 (6.4 months) studies.7,8,14 The observed PFS and OS of 4.6 months and 11.9 months, respectively, were similar to the outcomes observed in the MM-002 and MM-003 pivotal studies.7,8 In addition to the efficacy outcomes observed in the ITT population, ORR, PFS, and OS were similar regardless of prior treatments, including patients refractory to lenalidomide, bortezomib, or both lenalidomide and bortezomib, number of prior therapies, and presence or absence of moderate renal impairment. The results of our study compare favorably with those from a study of daratumumab monotherapy in a heavily pretreated population refractory to both lenalidomide and bortezomib.15
The >7-month difference between PFS and OS may be reflective of early identification of biochemical relapse (which precedes clinical manifestations of relapse) and subsequent salvage regimens. Thus, despite being a heavily pretreated population, patients who progress following treatment in this study may be medically fit enough to tolerate additional treatments. Indeed, the pomalidomide and low-dose dexamethasone regimen has been shown to improve and prolong health-related quality of life vs high-dose dexamethasone in the MM-003 study,16,17 which may make it possible for patients to tolerate subsequent treatments that might not otherwise have been an option.
This study confirms previous analyses demonstrating that the combination of pomalidomide and low-dose dexamethasone is a safe and effective treatment of patients with RRMM who have exhausted currently available treatment options. In addition, patients who were refractory to lenalidomide and/or bortezomib experienced similar clinical benefits, supporting the sequential use of these treatment regimens. Taken together, these data support pomalidomide plus low-dose dexamethasone as a standard of care for patients with RRMM who have poor prognosis and high need for effective treatments.
Presented at the 50th Annual American Society of Clinical Oncology Meeting (2014), the 56th American Society of Hematology Annual Meeting (2014), the 20th Congress of the European Hematology Association (2015), the 15th International Myeloma Workshop (2015), and the 57th American Society of Hematology Annual Meeting (2015).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was funded by Celgene Corporation. The authors thank the patients and families who contributed to this study, as well as the MM-010 study investigators, nurses, and study personnel. The authors received editorial assistance from Skye Geherin and Peter J. Simon funded by Celgene Corporation. Skye Geherin and Peter J. Simon provided drafts and editorial assistance to the authors during the preparation of this manuscript.
Authorship
Contribution: M.A.D., P.M., L.S., M.H.Z., J.S.-M., and N.M. contributed to the conception of the work; M.D., L.S., M.H.Z., J.S.-M., A.V., and N.M. contributed to the design of the work; K.C.W., M.A.D., P.M., M.D., H.G., A.O., M.C., S.K., L.S., M.H.Z., J.S.-M., F.d.A., C.R., E.M.O., M.K., J.H., M.H., N.M., C.D., F.D.R., M.S., and T.P. contributed to the acquisition, analysis, or interpretation of data for the work; K.C.W., M.A.D., P.M., M.D., H.G., A.O., M.C., S.K., L.S., M.H.Z., J.S.-M., P.A., F.d.A., M.J.B., F.D.R., C.R., E.M.O., A.V., M.K., R.R., J.H., M.H., N.M., S.K., G.M., C.D., M.S., T.P., A.P., P.C., M.P., and A.M.C. contributed to revising the manuscript critically for important intellectual content and approved the final version of the manuscript; and P.A., M.J.B., F.D.R., C.R., E.M.O., A.V., R.R., J.H., M.H., S.K., G.M., C.D., N.M., M.S., T.P., A.P., P.C., M.P., and A.M.C. agreed to be accountable for all aspects of the work in ensuring that all questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict-of-interest disclosure: M.A.D. has received honoraria from Amgen, Celgene, Janssen, Novartis, Onyx, Ortho Biotech; A.P. has consulted for and received honoraria from Celgene, Janssen; M.C. has consulted for and received honoraria from Bristol-Myers Squibb, Celgene, Janssen, Millennium; M.D. has received honoraria from Amgen, Celgene, Janssen, Novartis, F.D.R. has received research funding and served on the speaker’s bureau for Celgene, Janssen; K.C.W. has consulted for and received honoraria and travel support from Amgen, Bristol-Myers Squibb, Celgene, Janssen, has received research funding from Celgene, Janssen, has consulted for and received honoraria from Onyx, received travel support from Novartis, Takeda, and has consulted for Noxxon; A.O. has participated in advisory boards and received honoraria from Celgene; M.J.B. has received personal fees from Janssen-Cilag and served on an advisory board for Celgene; H.G. has participated in advisory boards, speaker’s bureau, and received honoraria and research funding from Celgene; C.D. has received grants from Celgene and served on an advisory board for Celgene; M.K. reports personal fees from Amgen, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Takeda; P.A. has received personal fees and nonfinancial support during the conduct of a study from Celgene; R.R. has served on an advisory board for Novartis and Roche; J.S.-M. has consulted for Bristol-Myers Squibb, Celgene, Merck, Millennium, Novartis, Onyx; F.d.A. has received personal fees outside the submitted work from Amgen, Celgene, Janssen; S.K. has received honoraria from Celgene Germany; G.M. has served on an advisory board for Bristol-Myers Squibb, Celgene, Janssen, Novartis, Takeda; N.M., T.P., L.S., and M.H.Z. are employed by and have equity ownership with Celgene; M.S. and J.H. are employed by Celgene; P.M. has received honoraria from and consulted for Celgene, Takeda, Janssen, BMS; P.C., M.H., A.V., M.P., A.M.C., C.R., and E.M.O. declare no competing financial interests.
Correspondence: Meletios A. Dimopoulos, National and Kapodistrian University of Athens School of Medicine, Department of Clinical Therapeutics, Alexandra Hospital, 80 Vas. Sofias, 11528 Athens, Greece; e-mail: mdimop@med.uoa.gr.