Abstract
Acute myeloid leukemia (AML) is a devastating disease with an incidence that progressively increases with advancing age. Currently, only ∼40% of younger and 10% of older adults are long-term survivors. If untreated, the overall prognosis of AML remains dismal. Initiation of therapy at diagnosis is usually urgent. Barriers to successful therapy for AML are the attendant toxicities directly related to chemotherapy or those associated with inevitable aplasia. Organ dysfunction often further complicates such toxicities and may even be prohibitive. There are few guidelines to manage such patients and the fear of crossing the medico-legal abyss may dominate. Such clinical scenarios provide particular challenges and require experience for optimal management. Herein, we discuss select examples of common pretreatment comorbidities, including cardiomyopathy, ischemic heart disease; chronic renal failure, with and without dialysis; hepatitis and cirrhosis; chronic pulmonary insufficiency; and cerebral vascular disease. These comorbidities usually render patients ineligible for clinical trials and enormous uncertainty regarding management reigns, often to the point of withholding definitive therapy. The scenarios described herein emphasize that with appropriate subspecialty support, many AML patients with comorbidities can undergo therapy with curative intent and achieve successful long-term outcome.
Introduction
Although the initial therapy of acute myeloid leukemia (AML) has been standardized and forms the backbone of clinical trials, many patients do not receive conventional therapy at diagnosis due to existing or potential comorbidities which cause much anxiety among patients and clinicians. Herein, we present 8 selective, but common, clinical vignettes describing, with subspecialty advice, a pragmatic approach to the management of patients with AML who present with varying degrees of organ dysfunction. Adhering whenever possible to standard therapy had been the guiding principle in suggesting the optimal antileukemic therapy.1 The primary focus is on induction and consolidation with only general reference to allogeneic transplantation.
Scenario 1: patient with cardiomyopathy
A 57-year old man was admitted with a 2-week history of generalized weakness and mild dyspnea. He was found to have intermediate-risk AML (normal karyotype, no nucleophosmin [NPM1], CCAAT/enhancer-binding protein [CEBPA], or Fms-related tyrosine kinase 3 [FLT3]–internal tandem duplication [ITD] mutations identified). His past medical history is remarkable for ischemic heart disease with a myocardial infarction 11 months prior to admission. Otherwise, he was in good health. On admission, his white blood cell count was 43 × 109/L, with 91% blasts, hemoglobin 7.8 g/dL with platelets 22 × 109/L. The bone marrow (BM) was diffusely infiltrated with blast cells. He was afebrile with a normal chest radiograph. The echocardiogram revealed mildly reduced left ventricular systolic function with an ejection fraction (LVEF) of 42%.
Questions
Can standard induction and consolidation therapy be given to this patient with cardiomyopathy and, if not, what are the alternatives?
Can such a patient undergo allogeneic transplantation?
What is the best way to monitor the cardiac disease?
Is there a role for cardioprotective agents?
Cardiac dysfunction in newly diagnosed patients with AML is not an uncommon problem encountered in practice, particularly among older patients. This is even more relevant in the current era when standard induction therapy is given to an ever increasing older population. As anthracyclines form the core of induction therapy in AML, any cardiac dysfunction may limit the optimal treatment that can be given.
Several issues need to be considered. First, the most common approach to monitor cardiac function is the evaluation of LVEF. Radionuclide angiography measuring multigated blood pool imaging (multigated acquisition scan) or echocardiography (preferably, 3-dimensional) are commonly used. For added accuracy in marginal cases, as in this patient, measurement of LVEF by magnetic resonance imaging is considered by many to be the gold standard.2 However, we do not incorporate it into routine clinical practice. In general, because of the potential for progressive toxicity in a patient with cardiomyopathy, a LVEF of 45% as the threshold for using anthracyclines in the treatment of AML has been suggested3,4 (Table 1). Although such a cutoff is necessarily arbitrary, it is the most commonly used value for eligibility to large cooperative oncology group clinical trials in AML. Given the need to optimize the therapy in AML and being fully cognizant of the risk/benefit considerations, it may be entirely reasonable to offer standard induction therapy with very close cardiac monitoring. The choice is very individualized and will take into account the patient’s age, performance status, and any comorbidities.
Select precautions and recommendation for AML therapy in patients with heart disease
| . | Specific supportive care considerations . | Induction therapy . | Postremission strategy . |
|---|---|---|---|
| LVEF <45%3,4 | 1. Minimize IV infusions | 1. HiDAC or 7+3 | 1. Repeated HiDAC |
| 2. Repeat LVEF evaluation prior to each chemotherapy cycle | 2. If anthracycline used, consider epirubicin or mitoxantrone | 2. RIC allo-SCT may be considered for high-risk AML | |
| Ischemic heart disease15,17 | 1. Aspirin + beta-blocker | 1. If possible, postpone induction for few days | Based on leukemia risk stratification and LVEF |
| 2. Maintain hemoglobin >8 g/dL | 2. Use HiDAC | ||
| 3. Avoid anthracycline | |||
| 4. Aspirin throughout induction | |||
| 1. PCI prior to induction if active ischemia despite maximal noninvasive therapy | If not performed prior to induction, PCI is indicated for pending coronary obstruction prior to chemotherapy | ||
| 2. Bare metal stent |
| . | Specific supportive care considerations . | Induction therapy . | Postremission strategy . |
|---|---|---|---|
| LVEF <45%3,4 | 1. Minimize IV infusions | 1. HiDAC or 7+3 | 1. Repeated HiDAC |
| 2. Repeat LVEF evaluation prior to each chemotherapy cycle | 2. If anthracycline used, consider epirubicin or mitoxantrone | 2. RIC allo-SCT may be considered for high-risk AML | |
| Ischemic heart disease15,17 | 1. Aspirin + beta-blocker | 1. If possible, postpone induction for few days | Based on leukemia risk stratification and LVEF |
| 2. Maintain hemoglobin >8 g/dL | 2. Use HiDAC | ||
| 3. Avoid anthracycline | |||
| 4. Aspirin throughout induction | |||
| 1. PCI prior to induction if active ischemia despite maximal noninvasive therapy | If not performed prior to induction, PCI is indicated for pending coronary obstruction prior to chemotherapy | ||
| 2. Bare metal stent |
allo-SCT, allogeneic stem cell transplantation.
There are also alternative therapeutic options. One approach would be to avoid anthracyclines and offer this patient induction with high-dose cytarabine at doses that are adjusted for his age. Although several dosing options can be considered, our own preference, based on published experience, is a dose of 1.5 g/m2 given over 1 hour twice daily for 6 days.5 Gemtuzumab ozogamicin has also been used as a substitute for daunorubicin,6 although this is currently unavailable in much of the world. An amsacrine-based regimen has also been proposed,7 but this is not commonly used nowadays.
Several methods have been reported to potentially reduce the risk of progressive toxicity from anthracyclines.8 These include infusional administration of anthracyclines, or the use of the anthracenedione, mitoxantrone.9-12 Liposomal encapsulation, in various formulations, has been suggested to reduce cardiotoxicity,13 but there are no compelling data to support such a recommendation. There has been considerable discussion about the use of dexrazoxane as a chelator that may prevent anthracycline damage by binding to free iron radicals. Given the theoretic risk of retarding the desired clinical benefit, outside of a clinical trial we do not recommend any of these strategies.14,15 Impairment of heart function may restrict standard supportive measures during induction such as antibiotics, transfusions, and electrolyte replacement that are associated with large fluid volumes.
If this patient achieves complete remission with high-dose cytarabine, we would recommend offering postremission therapy with 2 additional cycles of the same regimen.5 Irrespective of the agent used, close ongoing cardiac monitoring remains essential.
The possibility of allogeneic transplantation in such patients is more complex than induction or consolidation therapy, mainly because of the prolonged immune suppression with its attendant complications. However, if the genetic prognostic factors are such that the graft-versus-leukemia effect is considered an essential component for curative therapy, a reduced-intensity conditioning (RIC) allogeneic transplantation can be judiciously considered with very close cardiac monitoring.
Comment
Overall, the outlook for patients with cardiomyopathy, if clinically stable, need not preclude therapy for AML.
Scenario 2: patient with ACS
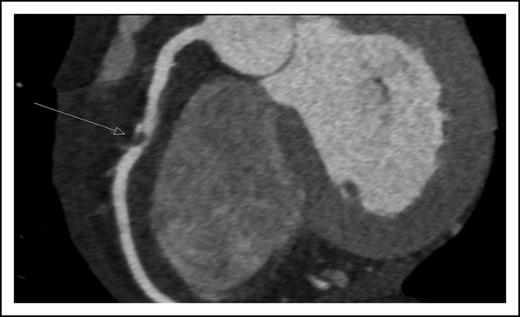
A 45-year-old man presented with new onset of chest pain and anemia. Cardiac computerized tomography revealed normal systolic function, but with significant narrowing of the right coronary artery (Figure 1). Acute coronary syndrome (ACS) was diagnosed. The white blood count was 10 × 109/L with 20% blasts. BM aspiration and biopsy were diagnostic for AML, molecularly characterized with normal cytogenetics and NPM1, as the only identified mutation.
Coronary computerized tomography demonstrating atherosclerotic occlusion of the right coronary artery.
Coronary computerized tomography demonstrating atherosclerotic occlusion of the right coronary artery.
Questions
Is the treatment of AML feasible in the setting of ACS?
What is the optimal antiplatelet therapy?
Should coronary intervention precede induction?
The combination of ACS and AML presents an unusually complicated predicament. For this patient, immediate medical therapy should include the combination of aspirin, beta-blockers,16,17 allopurinol, hydration, correction of electrolyte imbalances, and maintenance of hemoglobin level of 8 g/dL.18 Hydroxyurea is indicated for a rapidly increasing blast count. If ischemic symptoms subside, we would monitor the patient without initiating induction therapy for 5 to 7 days. If the patient remains stable and without symptoms, induction of remission is warranted.19 Although the patient presents with a normal cardiac function, active coronary disease increases the risk for anthracycline toxicity.15 This risk needs to be balanced against the importance of using anthracyclines for remission induction. Therefore, either 3+7 with standard anthracycline doses or high-dose cytarabine is a reasonable option for induction.20
Aspirin is the drug of choice for ACS21 and reduces progression to myocardial infarction or death in 30 days from 13.4% to 4.2%.22 Therefore, although there are no established guidelines in this setting, we recommend aspirin throughout induction while attempting to maintain, arbitrarily, the platelet count above 30 × 109/L. The addition of anticoagulation, or other antiplatelet agents, may further modestly reduce the death risk; however, such practice increases the risk of bleeding and should be avoided.
For this patient, if signs or symptoms of ischemia persist, percutaneous coronary intervention (PCI) should be discussed. Intractable ischemia must be resolved prior to initiating induction therapy. The risks of progression to myocardial infarction, life-threatening arrhythmia, and sudden death are increased with stress, electrolyte imbalance, and hypoxia, all common during induction. Therefore, it is suggested that if symptoms of ischemia persist despite optimal medical therapy, revascularization should precede induction therapy. Following PCI, dual antiplatelet therapy is indicated to minimize the risk of stent thrombosis. In most patients with AML, induction should not be significantly delayed. Therefore, implantation of a drug-eluting stent that requires longer duration of dual antiplatelet therapy should be avoided if possible. Because the risk of stent thrombosis peaks during the first 14 days after PCI, and with bare-metal stent decreases thereafter, clopidogrel discontinuation should be considered 14 days after PCI to coincide with the development of induction-related severe thrombocytopenia.
Comment
Overall, the presence of ACS should not discourage aggressive therapy for AML.
Scenario 3: patient with newly diagnosed AML
A 55-year-old woman presents with newly diagnosed AML. Biallelic mutation in CEBPA is detected with a normal karyotype. She has a history of diabetes mellitus and diabetic nephropathy with chronic renal failure and a creatinine of 3.0 mg/dL, but does not require dialysis.
Questions
What precautions are necessary?
What is the best approach to induction and postremission therapy?
Are there required chemotherapy dose adjustments?
The treatment of a patient with newly diagnosed AML and chronic renal failure presents several challenges. A joint effort between the hematologist and nephrologist is required. The earliest issue which needs attention is the potential for tumor lysis syndrome (TLS). An elevated pretreatment serum creatinine >1.4 mg/dL is an independent risk factor for the development of both laboratory and clinical TLS.23 In cases of severe TLS, leukapheresis may be considered, even at leukocyte levels lower than the standard threshold for leukapheresis.24 In a patient with impaired renal function, intensive fluid administration needs to be carefully monitored to avoid fluid overload or volume depletion. We use loop diuretics to increase urine flow rate. The administration of sodium bicarbonate is no longer generally recommended.25 Moreover, in patients with severe renal failure it is contraindicated because of concern for metabolic alkalosis that may develop due to the retention of sodium bicarbonate. Allopurinol can be administered in doses adjusted for the degree of renal function.26 Rasburicase may be used if needed.
The best approach to induction and postremission therapy in a patient with chronic renal failure is unknown. Because cytarabine is generally metabolized by liver cytidine deaminase, dose reduction is not required when standard doses (<400 mg/m2 per day) are administered.27 However, when higher doses are given (2-3 g/m2) to patients with renal dysfunction, neurotoxicity has been observed.28 In a study of 110 patients with AML given high-dose cytarabine, among patients with estimated creatinine clearance <60 mL per minute, 76% were complicated by neurotoxicity compared with 8% of those with creatinine clearance >60 mL per minute.28 The recommended dose adjustments are as follows: if the creatinine clearance is 46 to 60 mL per minute, administer 60% of the dose; if the creatinine clearance is 31 to 45 mL per minute, administer 50% of the total dose; and if the creatinine clearance is <30 mL per minute, consider an alternative agent29 (Table 2). However, despite such an elegant algorithm, given the concerns of neurotoxicity (admittedly often reversible28 ) and hepatotoxicity, we do not administer high-dose cytarabine to patients with renal impairment. We maintain this practice even among patients with core-binding factor AML for whom it has been suggested that high-dose cytarabine may be particularly effective in prolonging survival.30
Select precautions and recommendation for AML therapy in patients with renal or hepatic dysfunction
| . | Specific supportive care considerations . | Induction therapy . | Postremission strategy . |
|---|---|---|---|
| Renal failure27,29,32 | 1. Caution with fluid balance | 1. “3+7” with regular cytarabine dose | 1. Mitoxantrone + etoposide preferred |
| 2. Loop diuretics | 2. Reduce daunorubicin by 50% if creatinine >3 mg/dL | 2. Dose-adjusted HiDAC not recommended | |
| 3. Avoid sodium bicarbonate | |||
| 4. Leukapheresis for TLS prevention | |||
| 5. Adjust allopurinol dose | |||
| Dialysis27 | Use dialysis to balance electrolytes and prevent TLS | “3+7” with reduced anthracycline dose: depending on anticipated prognosis from the renal disease | Repeat induction regimen |
| Hepatic cirrhosis65,66 | Careful monitoring for coagulopathy | Dose reduction based on bilirubin blood level | |
| If bilirubin >5 mg/dL, maximum cytarabine dose is 50 mg/m2 |
| . | Specific supportive care considerations . | Induction therapy . | Postremission strategy . |
|---|---|---|---|
| Renal failure27,29,32 | 1. Caution with fluid balance | 1. “3+7” with regular cytarabine dose | 1. Mitoxantrone + etoposide preferred |
| 2. Loop diuretics | 2. Reduce daunorubicin by 50% if creatinine >3 mg/dL | 2. Dose-adjusted HiDAC not recommended | |
| 3. Avoid sodium bicarbonate | |||
| 4. Leukapheresis for TLS prevention | |||
| 5. Adjust allopurinol dose | |||
| Dialysis27 | Use dialysis to balance electrolytes and prevent TLS | “3+7” with reduced anthracycline dose: depending on anticipated prognosis from the renal disease | Repeat induction regimen |
| Hepatic cirrhosis65,66 | Careful monitoring for coagulopathy | Dose reduction based on bilirubin blood level | |
| If bilirubin >5 mg/dL, maximum cytarabine dose is 50 mg/m2 |
“3+7”, 3 days of daunorubicin and 7 days of cytarabine.
The anthracyclines are primarily eliminated by renal and hepatic aldoketo reductase and biliary excretion. Less than 20% is eliminated in the urine.27 Of all anthracyclines, pharmacokinetic data are most robust for doxorubicin.31 Yet, there are no consistent recommendations regarding the need for dose reduction.32-34 Among anthracyclines, daunorubicin has the greatest fractional renal clearance, and we follow the common recommendation to reduce the dose by 50% for a creatinine >3 mg/dL.32
With dose reduction for anthracyclines and standard-dose cytarabine (100 mg/m2 per day for 7 days by continuous induction), such conventional induction chemotherapy can be administered even to patients with very low creatinine clearance. As consolidation, one could give the identical induction regimen, but we would use the combination of mitoxantrone plus etoposide. The dose of mitoxantrone does not require adjustment.32,35,36 Etoposide is primarily eliminated by the liver and kidney, but pharmacokinetics are apparently the same in patients with end-stage renal disease as in normal individuals.37 Therefore, dose modifications are regarded as empiric. We adjust the dose and administer 75% of the total dose if the creatinine clearance is 10 to 50 mL per minute and give 50% of the dose if the creatinine clearance is <10 mL per minute.29
Scenario 4: patient with renal dysfunction
What if a patient on chronic dialysis presents with AML?
Questions
Is AML therapy feasible in patients on chronic dialysis?
Should the goals of AML therapy be altered because of kidney disease?
What chemotherapy regimens can be safely administered?
Considerations related to impaired renal function, as outlined above, also apply to patients on chronic dialysis (Table 2). Ironically, hemodialysis is an effective treatment of intractable fluid overload, hyperkalemia, hyperuricemia, hyperphosphatemia, or hypocalcemia38 and its frequency can be adjusted to prevent life-threatening electrolyte abnormalities.
Awareness and rapid response to early signs of infections are of particular importance, because life-threatening infections are common among chronic dialysis patients even without leukemia.39 Furthermore, among chronic dialysis patients, asymptomatic carriers of resistant bacteria are prevalent.40-42
A curative approach to AML may be futile among patients in whom the predicted survival due to their kidney disease is short even prior to the development of leukemia.43,44 Realistic expectations and the desired goals of therapy must be discussed between the patient, family, nephrologist, and hematologist. For a newly diagnosed AML patient who has a favorable prognosis based on the dialysis comorbidity scale,43,44 AML therapy with curative intent is reasonable.
The induction regimen and chemotherapy doses are similar to those described above for patients with severely impaired renal function. Because anthracyclines are not eliminated by hemodialysis, no dose augmentation is required.31
As postremission therapy,45 high-dose cytarabine should be avoided due to potential neurotoxicity.46 We would recommend repeating the induction regimen for 1 to 2 cycles or, alternatively, administer mitoxantrone and etoposide in reduced doses. Allogeneic transplantation in patients on chronic dialysis has been reported.47,48 Ordinarily, we do not consider it except in rare cases of a young highly motivated individual who has a very poor-prognosis genotype.
Comment
Renal dysfunction presents a significant barrier to treatment of AML. Meticulous attention to metabolic abnormalities and chemotherapy dose adjustment are critical.
Scenario 5: patient with hepatitis
A 26-year-old woman presents with core-binding factor AML with the t(8;21)(q22;q22) translocation. Her past medical history is unremarkable. She now resides in the United Kingdom, has traveled extensively as part of a diplomat’s family, and was born in China where she spent the first 6 years of her life. In her metabolic profile, the liver and renal function tests are normal. The patient is hepatitis B surface antigen (HBsAg)-positive and antibody to hepatitis B core antigen (anti-HBc)-positive. Antibodies to hepatitis B surface antigen (anti-HBS) are negative.
Questions
Do doses of induction and consolidation need to be attenuated?
Would such a serologic profile preclude an option of allogeneic transplantation?
Is there an optimal antiviral agent and recommended duration for prophylaxis?
Are the guidelines similar if such a patient presents in frank reactivation of hepatitis with markedly abnormal liver enzymes?
What is the best way to monitor response to antiviral therapy?
Should every AML patient, even if they have not lived in high-risk areas, be screened for hepatitis B virus?
What if the patient is a carrier of hepatitis C?
This patient clearly is a carrier of hepatitis B virus and is at significant risk for hepatitis B virus (HBV) reactivation (HBVr). In general, HBsAg and anti-HBc are positive in such patients. Anti-HBc is the most sensitive test for previous exposure to hepatitis B and may be positive in the absence of detectable HBsAg. In rare cases, both the surface antigen and antibodies to the core antigen may be negative, whereupon prior exposure can be detected by sensitive polymerase chain reactions (PCRs) for DNA load. However, this is not part of routine practice in most parts of the world.
Prophylaxis is essential for patients receiving severe immunosuppressive therapy. AML induction and consolidation therapy certainly fall into this category.49 The efficacy of such prophylaxis has been demonstrated in several controlled trials.50,51 With prophylaxis, induction and consolidation therapy can be given at standard doses, and if indicated, allogeneic transplantation can be undertaken. The presence of antibody to HBsAg may offer some protection, but this is probably insufficient to discard prophylaxis.52
Whether patients who are at low risk for HBVr should be screened is controversial. However, given the high risk of morbidity and mortality (∼25%),53 and in light of the marked efficacy of prophylaxis, we routinely screen all newly diagnosed AML patients for HBsAg and anti-HBc.54,55
Lamivudine remains the most commonly used agent as prophylaxis, which should ordinarily be continued for a total of 6 to 12 months. The ideal monitoring of response uses DNA viral load. However, lamivudine is associated with a high rate of developing drug resistance over time (>20% at 1 year). Therefore, in those parts of the world where cost is not the primary determining factor, therapy with next-generation nucleotide analogs should be given to AML patients. Entecavir is an alternative to lamivudine, and is particularly suitable for naive patients with no history of resistance to therapy or prior reactivation.56 In the latter case, the preferred agent is tenofovir disoproxil fumarate.
In the presence of HBVr, with high HBV DNA levels and frank hepatitis, prompt use of antiviral therapy does not in itself permit the use of standard chemotherapy for AML. Management of such cases is complex, leads to significant delays and, often, discontinuation of therapy. Considerable experience is needed in navigating the risks of progressive hepatic failure with ongoing immunosuppression.57-59
In patients with cancer, reactivation of hepatitis C virus (HCV) is far less common and associated with significantly lower rates of severe hepatitis than HBV reactivation.60 Current guidelines by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America do not recommend prophylaxis for HCV reactivation, certainly not in asymptomatic carriers or in those with noncirrhotic hepatitis.61 Among patients with cirrhosis, the risk of life-threatening reactivation is greater.62
Nevertheless, this is clearly an area of flux in the era of novel therapies for HCV. There has been a suggestion that patients receiving potent immunosuppressive targeted therapies should be closely monitored for potential development of hepatic flare,63 but this is controversial in a new era of clinical investigations, and there are no special guidelines for patients with AML.
Comment
Although not proven, AML and its therapy are sufficiently immunosuppressive to recommend prophylaxis for all carriers of hepatitis B. Prophylaxis is not recommended for carriers of hepatitis C.
Scenario 6: patient with cirrhosis
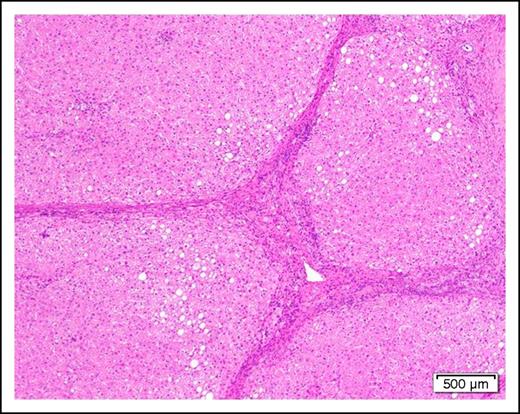
A 58-year-old man with cirrhosis (Figure 2) of the liver attributable to chronic alcohol abuse is found to have AML with the mixed-lineage leukemia translocation t(4;11)(q21;q23). He has evidence of portal hypertension as manifested by mild ascites and edema of the lower extremities. The serum albumin is mildly low at 3.0 g/dL and the prothrombin time and partial thromboplastin time are slightly prolonged.
Photomicrograph of a cirrhotic liver illustrating well developed fibrous septa separating irregular regenerative nodules. The hepatocytes show mild steatosis (hematoxylin and eosin stain, original magnification ×40).
Photomicrograph of a cirrhotic liver illustrating well developed fibrous septa separating irregular regenerative nodules. The hepatocytes show mild steatosis (hematoxylin and eosin stain, original magnification ×40).
Questions
Can patients with cirrhosis receive treatment of AML?
What is the preferred regimen?
Are there dose modifications of chemotherapeutic agents?
The treatment of a patient with cirrhosis of the liver and newly diagnosed AML is complicated. Underlying cirrhosis of the liver presents a number of potential issues. These include third spacing of fluid, hepatic dysfunction with perturbed drug metabolism, portal hypertension and variceal bleeding, malnutrition and coagulopathy due to impaired synthetic function as well as thrombocytopenia related to splenic sequestration. As a result, many patients may not be candidates for intensive chemotherapy. The Child-Pugh score is an important tool for determining the prognosis of patients with cirrhosis.64 Coagulation abnormalities need to be very closely followed, with aggressive replacement for any evidence of bleeding. In general, we would maintain a platelet count >20 × 109/L throughout induction. The therapeutic strategy depends on drug metabolism and dose modification in the setting of hepatic dysfunction.
Because anthracyclines are metabolized primarily by the liver, dose modifications based on the total bilirubin and hepatic transaminases are required.65,66 For example, we give 75% of the dose of daunorubicin if the total bilirubin is 1.5 to 3 mg/dL and/or the aspartate transaminase (AST) is 60 to 180, 50% dose if the total bilirubin is 3.1 to 5 mg/dL and/or the AST is >180; we do not administer daunorubicin at all if the total bilirubin is >5 mg/dL.65,66 For cytarabine, because the drug is partially detoxified in the liver, we adjust the dose and administer 50% of the total dose for any elevation in the AST or alanine transaminase67 or total bilirubin >2 mg/dL.68 Regarding high-dose cytarabine (HiDAC) for consolidation, theoretically the same criteria for dose modification apply. However, given the numerous potential complications in a patient with cirrhosis of the liver and the risk of cerebellar toxicity, we rarely administer HiDAC in this clinical condition.69,70
Decitabine is eliminated by cytidine deaminase which is found intracellularly in the liver. Most clinical trials involving decitabine and azacitidine excluded patients with significant liver disease. Azacitidine has the potential for hepatotoxicity in patients with liver disease.71,72 Therefore, we do not administer hypomethylating agents to patients with significant preexisting hepatic impairment.
In practice, a patient with cirrhosis and a bilirubin level >5 mg/dL is precluded from optimal induction and consolidation therapy. Low-dose cytarabine may be used; however, our practice is to offer a 7- to 10-day course of cytarabine at a dose not exceeding 50 mg/m2 per day, given that with standard-dose cytarabine 35% of patients can achieve complete remission (CR).
Allogeneic transplantation is obviously hazardous and we would not do it for anyone with uncompensated cirrhosis. However, in a patient with Child I cirrhosis, who is in CR, we would consider RIC transplantation.
The one theoretic exception where underlying cirrhosis may not preclude treatment of AML may be patients with acute promyelocytic leukemia.73 In fact, in preclinical models, all-trans retinoic acid has been proposed as a treatment of cirrhosis of the liver because this agent decreases liver fibrosis by reducing transforming growth factor β 1, interleukin-6, and type I collagen.74,75
Comment
Cirrhosis of the liver presents a serious dilemma; dose reduction is often required and curative options are usually precluded if associated with severe hyperbilirubinemia.
Scenario 7: patient with COPD
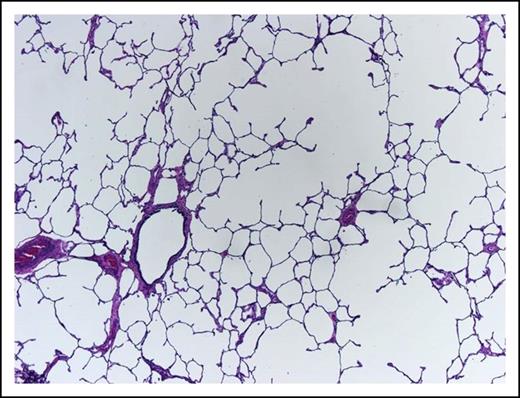
A 62-year-old woman, heavy smoker, known to suffer from pulmonary emphysema (Figure 3) and secondary pulmonary hypertension, presents with pancytopenia and 30% blasts on BM examination. At rest, she is comfortable with oxygen saturation of 90% but even ordinary physical activity causes undue dyspnea. In retrospect, her hemoglobin was falling slowly during the last year and severe dysplastic changes are evident in the BM. Unfavorable-risk AML is diagnosed after trisomy 8 and deletion 7 are reported by rapid fluorescence in situ hybridization evaluation.
This lung shows centrilobular emphysema with dropout of alveolar walls surrounding the bronchiole (hematoxylin and eosin stain, original magnification ×4).
This lung shows centrilobular emphysema with dropout of alveolar walls surrounding the bronchiole (hematoxylin and eosin stain, original magnification ×4).
Questions
What is the best first-line therapy in this woman?
What can be achieved beyond remission?
Are there special recommendations for such patients?
AML patients with chronic obstructive lung disease (COPD) are usually excluded from clinical trials. Therefore, data addressing outcome of such patients are scanty. In a large retrospective study, following intensive induction therapy, grade 3-4 respiratory complications requiring support were present in 16% of AML patients and 8% developed respiratory failure.76,77 In addition, pulmonary hypertension raises the mortality from sepsis.78 At the age 62 years, with COPD, patients with AML that evolved from myelodysplastic syndrome need to overcome significant obstacles inherent in intensive chemotherapy.
The Sorror comorbidity index assigns 2 or 3 points for moderate or severe pulmonary abnormalities such as in this patient.79 A Sorror index of 3 is the cutoff for poorer allogeneic hematopoietic cell transplantation (allo-HCT) outcome even if RIC is carried out80,81 that similarly applies to intensive induction therapy.82,83 Given an induction mortality of close to 85% for patients with a Sorror score of 3 or more, the risk of using optimal induction therapy is probably excessive. Hypomethylating therapy may be an alternative to intensive induction as it may be efficacious for “low proliferative” leukemia as well as in frail patients.84-87 Yet, although hypomethylating agents are better tolerated by patients with comorbidities, the CR rate is only 18% to 24%. A similar 2- to 3-year survival can be expected in patients over 70 years of age following either standard induction or hypomethylating agents.87 In contrast, in patients 60 to 70 years old, longer survival has been reported if intensive induction is used (25% vs 5% to 8%). For patients with COPD with a slowly proliferating leukemia or with an adverse karyotype, a reasonable strategy is to use hypomethylating therapy for 3 to 4 months followed by RIC allo-HCT.88-91
For this patient, we would initiate comprehensive pulmonary function evaluation as results of pulmonary function tests are predictive for long-term outcome of allo-HCT.92,93 If the patient has a comorbidity score of 3 or greater, and this is consistent with the bedside clinical assessment, we would initiate induction therapy with hypomethylating agents. We would not ordinarily proceed with allogeneic transplantation in this setting. If, on the other hand, the comorbidity score is <3 and the patient is clinically judged as able to tolerate intensive myelosuppression, we would initiate standard induction, recognizing the potential for pulmonary and infectious complications. Intensive support throughout induction is essential as every effort must be made to avoid mechanical ventilation.
As a heavy smoker, this patient is at a particularly high risk for invasive pulmonary infection.94 Maintaining a high index of suspicion and initiating antifungal prophylaxis are indicated.
Comment
The management of AML patients with COPD is fraught with hazards. In the absence of unequivocal guidelines, the approach to therapy requires experience, careful judgment, and subspecialty support.
Scenario 8: patient with ICH
A 57-year-old woman is diagnosed with acute myelomonocytic leukemia 6 weeks following an intracranial hemorrhage (ICH) involving her right basal ganglia. At the time of the ICH, the blood counts were normal. Her medical history is remarkable only for untreated hypertension. Following successful rehabilitation, she currently can manage her daily life activities but requires walker assistance for mobilization. Currently, her white blood count is 45 × 109/L with 90% blasts. No metaphases are available for analysis, but Flt3-ITD mutation is detected by PCR. The hemoglobin is 11.2 g/dL and the platelet count is 90 × 109/L. Blood coagulation test results are normal. No new neurological abnormalities are present.
Questions
What is the risk of rebleeding in the central nervous system (CNS)?
And how does this impact on the therapy for AML?
In a patient with a prior ICH, blood pressure control reduces recurrent CNS bleeding risk by 50%.95 In addition, urgent antileukemia therapy with the aim to control the blast count is indicated because hyperleukocytosis is associated with increasing bleeding risk.96-98 A platelet count threshold of 50 × 109/L has been suggested, although there are no convincing data to support this.99 The risk of rebleeding decreases with time from the primary bleeding event. In this patient, given that she is 6 weeks after the acute event, we would, arbitrarily, maintain the platelet count above 30 × 109/L during standard induction therapy. Such a recommendation may be analogous to guidelines for a similar clinical scenario regarding the use of anticoagulation following ICH.100 Induction with attenuated anthracycline doses should be avoided as the severity of thrombocytopenia is identical for daunorubicin (45-90 mg/m2) doses in both younger and older patients with AML.101,102
In older patients, in whom the risk of rebleeding is significantly high and allogeneic transplantation is not a practical option, we consider less myeloablative therapy, such as hydroxyurea, low-dose cytarabine, or hypomethylating agents.100,103
This patient has a high risk for CNS involvement based on the myelomonocytic lineage morphology and leukocytosis104-106 but there is no firm evidence to suggest a connection between ICH and CNS involvement of leukemia.107 Although ICH preceded the diagnosis of leukemia by 6 weeks and the patient has poorly controlled hypertension as a risk factor for ICH, we would evaluate for the presence of CNS leukemia with a lumbar puncture and imaging.
Comment
Patients with ICH can receive optimal induction therapy, provided the platelets are kept at relatively safe levels.
Conclusion
In conclusion, the presentation of patients with preexisting comorbidities is common and the therapy is challenging. Although certainly not all-encompassing, select common scenarios were presented emphasizing practical approaches for patients who nevertheless require therapy. The catastrophic prognosis for untreated AML often permits and encourages the adoption of pragmatic risk taking. With appropriate subspecialty support, many patients with AML who present with organ dysfunction can be treated successfully.
Acknowledgments
The authors acknowledge the following physicians for their subspecialty advice: Mark Klutstein, Yoav Luria, Arthur Kerner, Daniel Kurnik, Sergio Girlat, William Travis, Richard Steingart, Jinru Shia, and Carlos Flombaum. The authors also thank Hillard Lazarus and Aaron Rapoport for review of the manuscript and helpful suggestions. Finally, the authors thank Sonia Kamenetsky for assistance in the preparation of this manuscript.
Authorship
Contribution: Y.O., M.S.T., and J.M.R. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yishai Ofran, Department of Hematology and Bone Marrow Transplantation, Rambam Health Care Campus, 8, Ha’Aliya St, Haifa 31096, Israel; e-mail: y_ofran@rambam.health.gov.il.