Key Points
Ibrutinib allows for partial reconstitution of normal B cells and humoral immunity in patients with chronic lymphocytic leukemia.
Infection rate decreased with time on ibrutinib and was inversely correlated with improvements in serum IgA.
Abstract
Chronic lymphocytic leukemia (CLL) is characterized by immune dysregulation, often including hypogammaglobulinemia, which contributes to a high rate of infections and morbidity. Ibrutinib, a covalent inhibitor of Bruton tyrosine kinase (BTK), inhibits B-cell receptor signaling and is an effective, US Food and Drug Administration (FDA)-approved treatment of CLL. Inactivating germline mutations in BTK cause a severe B-cell defect and agammaglobulinemia. Therefore, we assessed the impact of ibrutinib on immunoglobulin levels, normal B cells, and infection rate in patients with CLL treated with single-agent ibrutinib on a phase 2 investigator-initiated trial. Consistent with previous reports, immunoglobulin G (IgG) levels remained stable during the first 6 months on treatment, but decreased thereafter. In contrast, there were a transient increase in IgM and a sustained increase in IgA (median increase 45% at 12 months, P < .0001). To distinguish the effects on clonal B cells from normal B cells, we measured serum free light chains (FLCs). In κ-clonal CLL cases, clonal (κ) FLCs were elevated at baseline and normalized by 6 months. Nonclonal (λ) FLCs, which were often depressed at baseline, increased, suggesting the recovery of normal B cells. Consistently, we observed normal B-cell precursors in the bone marrow and an increase in normal B-cell numbers in the peripheral blood. Patients with superior immune reconstitution, as defined by an increase in serum IgA of ≥50% from baseline to 12 months, had a significantly lower rate of infections (P = .03). These data indicate that ibrutinib allows for a clinically meaningful recovery of humoral immune function in patients with CLL. This trial was registered at www.clinicaltrials.gov as #NCT01500733.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by profound immune dysregulation resulting in significant infection-related morbidity and mortality.1 Since the 1950s,2,3 hypogammaglobulinemia has been reported in association with CLL and affects up to 85% of patients during the course of their disease.4 Deficiencies in immunoglobulin G (IgG) and its subclasses, IgA, and IgM may be present.5 Stage and duration of disease correlate with the severity of hypogammaglobulinemia.4 Although other immune defects, such as impairments of T-cell function, also contribute to infection risk, CLL patients with lower serum immunoglobulin levels appear to be particularly susceptible to severe and recurrent infections.6
Multiple mechanisms have been implicated in the development of hypogammaglobulinemia. In coculture experiments, CLL cells inhibit antibody production by bone marrow plasma cells via Fas/Fas ligand interaction.7 In addition, newly produced B cells in the peripheral blood are decreased in CLL patients compared with healthy controls,8 contributing to a smaller pool of antibody-producing cells. T and natural killer cells from CLL patients also downregulate antibody secretion by activated B cells from healthy donors in vitro.9-11 Moreover, an expanded population of CD30+ T cells commonly found in CLL inhibits isotype switching to IgG and IgA, even in nonclonal B cells.12
Disease-inherent immunodeficiency is further compounded by treatment. Standard anti-CD20–based chemoimmunotherapy for young, physically fit patients leads to significant neutropenia and infection13,14 and does not improve serum immunoglobulin levels, at least in the short-term.13 The addition of rituximab, although generally well tolerated, has been associated with fatal hepatitis B reactivation and progressive multifocal leukoencephalopathy.15 In an extended follow-up of 300 patients treated with chemoimmunotherapy, recurrent late cytopenias occurred in 28% and the risk of serious infections remained elevated during the first 2 years of remission.16 Thus, chemoimmunotherapy causes both short- and long-term impairments of the immune system.
B-cell receptor (BCR) signaling is critical in normal B lymphopoiesis and has been implicated in the pathogenesis of a number of different B-cell malignancies.17 In CLL, activation of BCR signaling, particularly within the microenvironment of secondary lymphoid tissues, drives survival and proliferation of tumor cells.18,19 Hence, there has been a growing interest in disrupting BCR signal transduction by targeting kinases downstream of the BCR.20-22 Ibrutinib, which covalently binds and irreversibly inhibits Bruton tyrosine kinase (BTK), has demonstrated clinical efficacy in CLL as well as other B-cell malignancies and is approved for second-line treatment of CLL and mantle cell lymphoma, and for front-line treatment of CLL with deletion 17p13.1 and Waldenström macroglobulinemia.17,23-27 Inactivating germline mutations in BTK underlie X-linked agammaglobulinemia, a primary immunodeficiency due to failure of mature B-cell development.28 However, the long-term immunologic consequences of pharmacologic BTK inhibition are unknown.
The initial phase 1b/2 trial of ibrutinib monotherapy for relapsed CLL reported grade ≥3 pneumonia in 12% of patients, including 3 deaths.23 Serious infections appeared to be related to disease rather than ibrutinib as the rate of grade ≥3 infections decreased following 6 months of treatment.23 Serum IgG was stable up to 12 months and an increase in IgA was observed.23 Similar findings were reported in previously untreated patients ≥65 years.29 A more recent randomized study of ibrutinib vs ofatumumab found that ibrutinib-treated patients developed more infections of any grade (70% vs 54%). However, treatment exposure was longer for the ibrutinib arm (median 8.6 vs 5.3 months) and the frequency of grade ≥3 infections was similar (24% vs 22%) between the 2 treatment groups.30
Given the important role of BTK in the development of normal B cells and the clinical relevance of hypogammaglobulinemia in CLL, we prospectively evaluated humoral immunity, normal B cells, and infection rate in CLL patients treated on our phase 2, investigator-initiated study with single-agent ibrutinib.24
Patients, material, and methods
Patients
Samples originated from a phase 2, investigator-initiated trial studying ibrutinib 420 mg once daily in patients with treatment naive and relapsed/refractory CLL ≥65 years of age or with TP53 aberration (NCT01500733).24,31 The study was approved by an institutional review board and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent.
The study enrolled 86 patients, of whom 2 were excluded from analysis due to enrollment deviation. Patient characteristics are summarized in supplemental Table 1 (see supplemental Data available at the Blood Web site). Analyses of laboratory parameters, including serum immunoglobulins, free light chains (FLCs), and flow cytometric analysis of B cells, were performed only in patients who received at least 12 months of ibrutinib and had paired data at the indicated time points. Patients receiving IV immunoglobulin replacement were excluded from analysis of immunoglobulin levels. Serum immunofixation electrophoresis (IFE) detected clonal gammopathy in 21 patients, including 1 with clonal IgA, 8 with clonal IgG, 10 with clonal IgM, and 2 with both clonal IgG and either clonal IgA or IgM. Samples with clonal immunoglobulins were analyzed separately and excluded from analysis of total immunoglobulins.
Laboratory analysis
Quantitation of serum immunoglobulins and FLCs was performed at the National Institutes of Health (NIH) Department of Laboratory Medicine. During the study period, the methodology used to measure serum immunoglobulins changed from a nephelometric to immunoturbidimetric assay (cobas 6000 analyzer; Roche). The normal reference ranges for each method are as follows: IgA, 0.91 to 4.99 g/L; IgG, 6.42 to 17.3 g/L; IgM, 0.34 to 3.42 g/L by nephelometry until February 7, 2014, and IgA, 0.7 to 4 g/L; IgG, 7 to 16 g/L; IgM, 0.4 to 2.3 g/L by immunoturbidimetry from February 8, 2014 onwards. To compare results over time, we normalized results across the different reference ranges.32
Polyreactive antibody titers in the sera were determined by a surrogate assay for polyreactivity using dinitrophenol (Invitrogen). Two-fold serially diluted serum was incubated on enzyme-linked immunosorbent assay plates previously coated with 5 µg/mL dinitrophenol. The bound antibody titers were determined by goat anti-human IgM, IgG, and IgA coupled with horseradish peroxidase (all from Southern Biotechnology Associates) and developed 2′,2′-azino-bis (3-ethylbenzthazoline-6-sulphonic acid). The polyreactive antibody titer was reported as the reciprocal of the serum dilution above-predetermined background activity.
Serum B-cell activating factor (BAFF) was measured according to the manufacturer’s specifications using a bead-based multiplex screening assay from R&D Systems on a Luminex IS100 instrument (Luminex Corp). Data were analyzed using Bio-Plex Manager Pro 6.1 analysis software (Bio-Rad).
Analysis of normal B cells was performed in the Flow Cytometry Laboratory (Laboratory of Pathology, National Cancer Institute, NIH). Specimens were processed within 12 hours of collection as previously described.33 Cells were stained with the following antibodies: anti-CD3-V500, CD5–peridinin chlorophyll (PerCP), CD5–allophycocyanin (APC), CD5-V450, CD11c-V450, CD14-AH7, CD19-PC7, CD20-PerCP, CD20-AH7, CD22–phycoerythrin (PE), CD22-PerCP, CD38-V450 CD45-V500, CD79b-PE, CD81–fluorescein isothiocyanate (FITC), κ-m-FITC, κ-p-APC (polyclonal rabbit anti-human, F(ab′)2), λ-m-PE, and λ-p-FITC (polyclonal rabbit anti-human, F(ab′)2) (all from BD Biosciences). Specimens were analyzed on a FACSCantoTMII flow cytometer (BD Biosciences) using FCS Express (De Novo Software). CLL cells were identified as previously described.34 Normal mature B cells were identified by appropriate levels of CD19, CD20, CD22, CD45, CD38, CD43, and CD81 expression and polyclonal expression of surface light chain immunoglobulin (supplemental Figure 1). B-cell precursors were identified by appropriate levels of CD19, CD20, CD10, CD34, and CD45 expression. The mean number of B cells analyzed in each specimen was 15 877 (range, 4356-92 793) at baseline and 174 653 (range, 3449-778 659) during treatment. CD3 and CD14 were used to exclude T cells and monocytes. Absolute normal B-cell count in the peripheral blood was calculated using the absolute CD19+ cell count and the percentage of normal CD19+ cells.
B-cell subset immunophenotyping was performed on cryopreserved peripheral blood mononuclear cells with the following antibodies: anti-CD3-V510, CD27-V421 (BioLegend); CD19-PerCP-Cy5-5, CD27-PE-Cy7 (eBioscience); Λ-FITC, κ-PE, IgG-PE-Cy7, CD5-FITC, CD5-APC, CD10-APC, CD20-APC-H7, and CD27-PE (BD Biosciences). Specimens were analyzed on a FACSCanto II flow cytometer (BD Biosciences) using FlowJo software (Tree Star).
Statistical analysis
The Wilcoxon signed-rank test was used to compare paired longitudinal data. The Mann-Whitney U test and the Pearson χ2 test were used to compare continuous and categorical variables between different groups, respectively. The mean number of the infections over time was estimated nonparametrically by the Nelson-Aalen method.35 Differences in the rate of infection between subgroups were examined using the Cox regression model for recurrent events.36
All statistical analyses were performed with JMP, version 10.0.0 (SAS Institute Inc) and the R statistical software, version 3.1.2 (R Foundation for Statistical Computing). Figures were created with GraphPad Prism version 6.02 for Windows (GraphPad Software).
Results
Change in serum immunoglobulins on ibrutinib
In agreement with previous reports,23,29 IgG remained stable during the first 6 months of treatment (Figure 1A, Table 1). However, by 12 months, a slight decrease in IgG was apparent (n = 35; median reduction, 4%; P < .0006). This decrease became more profound at 24 months (n = 21; median reduction, 23% (interquartile range [IQR], 9%-37%; P < .0001). In 6 of 18 patients (33%), a previously normal baseline IgG decreased below normal at 24 months. In 5 patients with follow-up of ≥36 months, IgG at 24 and 36 months were similar (median 7.9 and 8.1 g/L, respectively), suggesting that there was no further decline.
Serum IgG, IgA, and IgM levels on ibrutinib compared with pretreatment baseline. Scatter plot of change in (A) IgG (n = 35 at 6 and 12 months; n = 21 at 24 months), (B) IgA (n = 43 at 6 and 12 months; n = 28 at 24 months), and (C) IgM (n = 34 at 6 and 12 months; n = 23 at 24 months), in treatment-naive (○) and relapsed or refractory (♦) patients. Only paired data are shown. Horizontal lines denote median values. Titers of polyreactive (D) IgG, (E) IgA, and (F) IgM (mean ± SEM) prior to treatment (PRE), and at 6 and 12 months (n = 21). Statistical significance is indicated by *P < .05, **P < .01, ***P < .001, or ****P < .0001.
Serum IgG, IgA, and IgM levels on ibrutinib compared with pretreatment baseline. Scatter plot of change in (A) IgG (n = 35 at 6 and 12 months; n = 21 at 24 months), (B) IgA (n = 43 at 6 and 12 months; n = 28 at 24 months), and (C) IgM (n = 34 at 6 and 12 months; n = 23 at 24 months), in treatment-naive (○) and relapsed or refractory (♦) patients. Only paired data are shown. Horizontal lines denote median values. Titers of polyreactive (D) IgG, (E) IgA, and (F) IgM (mean ± SEM) prior to treatment (PRE), and at 6 and 12 months (n = 21). Statistical significance is indicated by *P < .05, **P < .01, ***P < .001, or ****P < .0001.
Serum immunoglobulins on ibrutinib
| . | IgG . | IgA . | IgM . |
|---|---|---|---|
| Normal range, g/L | 7-16 | 0.91-5 | 0.4-2.3 |
| Patients | n = 35* | n = 43* | n = 34* |
| Median (IQR), g/L | |||
| Pretreatment | 7.07 (5.71-9.03) | 0.47 (0.36-1.23) | 0.31 (0.25-0.40) |
| 6 mo | 6.84 (5.40-8.98) | 0.74 (0.45-1.56) | 0.36 (0.30-0.46) |
| P = .10 | P < .0001 | P = .003 | |
| 12 mo | 6.62 (4.79-9.03) | 0.77 (0.50-1.63) | 0.34 (0.29-0.46) |
| P = .0006 | P < .0001 | P = .09 | |
| 24 mo | 5.87 (4.39-7.91) | 0.72 (0.52-1.71) | 0.26 (0.17-0.35) |
| P < .0001, n = 21† | P < .0001, n = 28† | P = .3, n = 23† |
| . | IgG . | IgA . | IgM . |
|---|---|---|---|
| Normal range, g/L | 7-16 | 0.91-5 | 0.4-2.3 |
| Patients | n = 35* | n = 43* | n = 34* |
| Median (IQR), g/L | |||
| Pretreatment | 7.07 (5.71-9.03) | 0.47 (0.36-1.23) | 0.31 (0.25-0.40) |
| 6 mo | 6.84 (5.40-8.98) | 0.74 (0.45-1.56) | 0.36 (0.30-0.46) |
| P = .10 | P < .0001 | P = .003 | |
| 12 mo | 6.62 (4.79-9.03) | 0.77 (0.50-1.63) | 0.34 (0.29-0.46) |
| P = .0006 | P < .0001 | P = .09 | |
| 24 mo | 5.87 (4.39-7.91) | 0.72 (0.52-1.71) | 0.26 (0.17-0.35) |
| P < .0001, n = 21† | P < .0001, n = 28† | P = .3, n = 23† |
All P values reflect paired comparisons to pretreatment samples.
Patients who received ≥12 months of ibrutinib. Only paired data are shown.
Patients who received ≥24 months of ibrutinib. Between 12 and 24 months, 3 patients discontinued treatment: 2 withdrew consent and 1 progressed with transformation. The remaining patients continued ibrutinib, but had not reached their 24-month follow-up at the time of analysis.
In contrast to IgG, we observed an improvement in IgA within 6 months of starting ibrutinib (Figure 1B, Table 1) consistent with data reported by others.23,29 Compared with baseline, IgA further increased at 12 (n = 43; median increase, 45% [IQR, 29%-102%]; P < .0001) and 24 months of follow-up (n = 28; median increase, 64% [IQR, 21%--100%]; P < .0001). In 7 of 28 patients (25%), IgA more than doubled at 24 months. Overall, IgA increased in 82% of patients and decreased in 18%. Fourteen patients had a normal IgA at baseline and only 2 experienced a decrease on treatment. In both patients, IgA levels remained within the normal reference range.
Clonal IgM was detected in the serum of 11 patients (24%) by IFE. The light chain of the clonal IgM was λ in 7 patients, κ in 3 patients, and concordant with the surface light-chain restriction on CLL cells in all cases. In 1 patient, the clonal light chain could not be determined. Therefore, clonal serum IgM is likely tumor-derived. In support of this hypothesis, ibrutinib effectively decreased serum IgM at 6 months in all cases with clonal IgM (median reduction, 27% [IQR, 9%-39%]; P = .006). No change was detected in clonal IgG (n = 10) or clonal IgA (n = 2) during treatment.
Conversely, we observed an early, but transient, improvement in serum IgM in 34 patients without detectable paraprotein (median increase, 15% [IQR, 3%-30%] at 6 months; P = .003; Figure 1C, Table 1). At 24 months, IgM was no longer significantly different from pretreatment levels and 2 patients experienced a >50% decrease in serum IgM from baseline. IgM was normal in 8 patients at baseline then decreased in 7 of 8 during treatment with ibrutinib. In 3 patients, IgM dropped below normal at subsequent follow-up.
Overall, treatment-naive patients had higher baseline IgA compared with patients with relapsed/refractory CLL (P = .02, supplemental Figure 2B). Baseline IgG and IgM (supplemental Figure 2A,C) and changes in IgG, IgM, and IgA over time on ibrutinib were similar regardless of prior treatment history (data not shown).
Some CLL clones, particularly those of the IGHV unmutated subtype, express BCR specificity similar to that of B cells producing polyreactive antibodies.37 Therefore, we evaluated the serum levels of polyreactive antibodies pretreatment and on ibrutinib and found that both polyreactive IgA and IgM increased over time (P = .01 and P = .03, respectively, at 12 months; Figure 1E-F). Because one would expect treatment to decrease polyreactive antibodies if they were derived from CLL cells, this increase may in fact represent the recovery of nonleukemic polyreactive B cells.
Normalization of serum FLCs on ibrutinib
The observed changes in serum immunoglobulins suggest that ibrutinib may affect clonal and nonclonal B cells in opposite ways. We sought to investigate this further by analyzing the serum levels of FLCs. Patients were divided into 2 groups based on κ or λ light-chain restriction of their CLL cells as determined by flow cytometry. Overall, there were 39 κ-clonal and 28 λ-clonal CLL cases with longitudinal samples available up to 12 months on treatment.
In patients with κ-clonal CLL, the serum κ:λ ratio and κ FLCs at baseline were abnormally elevated and, thus, likely derived from tumor cells. Eight patients (21%) had a detectable monoclonal κ FLC by IFE. At 6 months, we observed normalization of the κ:λ ratio, primarily driven by a significant decrease in κ FLCs (P < .0001; Figure 2A-B, Table 2). On IFE, the monoclonal κ FLC was no longer detectable in 4 of 8 patients (50%) following ibrutinib therapy. In contrast, the nonclonal (λ) FLCs increased while on treatment (P = .0002 and P = .0005 at 6 and 12 months, respectively; Figure 2C, Table 2), suggesting the recovery of normal B cells.
Serum FLCs prior to treatment and on ibrutinib. Box and whisker plots (10th-90th percentile) of (A) serum κ:λ ratio, (B) κ FLC, and (C) λ FLC in κ-clonal CLL (n = 39). Box-and-whisker plots (10th-90th percentile) of (D) serum κ:λ ratio, (E) κ FLC, and (F) λ FLC in λ-clonal CLL (n = 28). Only paired data are shown. Y-axis is in log2 scale. Dotted lines illustrate the normal range. Statistical significance is indicated by *P < .05, **P < .01, ***P < .001, ****P < .0001, or ns (P ≥ .05) compared with pretreatment baseline.
Serum FLCs prior to treatment and on ibrutinib. Box and whisker plots (10th-90th percentile) of (A) serum κ:λ ratio, (B) κ FLC, and (C) λ FLC in κ-clonal CLL (n = 39). Box-and-whisker plots (10th-90th percentile) of (D) serum κ:λ ratio, (E) κ FLC, and (F) λ FLC in λ-clonal CLL (n = 28). Only paired data are shown. Y-axis is in log2 scale. Dotted lines illustrate the normal range. Statistical significance is indicated by *P < .05, **P < .01, ***P < .001, ****P < .0001, or ns (P ≥ .05) compared with pretreatment baseline.
Serum FLC and κ:λ ratio on ibrutinib, grouped by κ and λ light-chain restriction
| . | κ clonal CLL . | λ clonal CLL . | ||||
|---|---|---|---|---|---|---|
| κ:λ ratio . | κ FLC, mg/L . | λ FLC, mg/L . | κ:λ ratio . | λ FLC, mg/L . | κ FLC, mg/L . | |
| Normal range | 0.4-1.8 | 5.7-22.2 | 6.6-23.2 | 0.4-1.8 | 5.7-22.2 | 6.6-23.2 |
| Patients | n = 39 | n = 28 | ||||
| Median (IQR) | ||||||
| Pretreatment | 8.8 (2.5-28.6) | 67.1 (23.4-167.0) | 9.5 (6.2-12.2) | 0.2 (0.04-0.4) | 43.6 (27.4-84.9) | 8.7 (1.7-14.8) |
| 6 mo | 1.3 (1.0-1.9) | 14.2 (9.8-28.1) | 11.1 (7.1-15.6) | 0.5 (0.1-0.9) | 15.2 (10.2-21.0) | 9.1 (1.7-15.3) |
| P < .0001 | P < .0001 | P = .0002 | P < .0001 | P < .0001 | P = .7 | |
| 12 mo | 1.2 (0.9-1.7) | 13.0 (10.6-23.6) | 10.9 (7.7-13.8) | 0.7 (0.2-0.9) | 13.8 (8.4-18.1) | 8.6 (2.1-13.1) |
| P < .0001 | P < .0001 | P = .0005 | P < .0001 | P < .0001 | P = .7 | |
| . | κ clonal CLL . | λ clonal CLL . | ||||
|---|---|---|---|---|---|---|
| κ:λ ratio . | κ FLC, mg/L . | λ FLC, mg/L . | κ:λ ratio . | λ FLC, mg/L . | κ FLC, mg/L . | |
| Normal range | 0.4-1.8 | 5.7-22.2 | 6.6-23.2 | 0.4-1.8 | 5.7-22.2 | 6.6-23.2 |
| Patients | n = 39 | n = 28 | ||||
| Median (IQR) | ||||||
| Pretreatment | 8.8 (2.5-28.6) | 67.1 (23.4-167.0) | 9.5 (6.2-12.2) | 0.2 (0.04-0.4) | 43.6 (27.4-84.9) | 8.7 (1.7-14.8) |
| 6 mo | 1.3 (1.0-1.9) | 14.2 (9.8-28.1) | 11.1 (7.1-15.6) | 0.5 (0.1-0.9) | 15.2 (10.2-21.0) | 9.1 (1.7-15.3) |
| P < .0001 | P < .0001 | P = .0002 | P < .0001 | P < .0001 | P = .7 | |
| 12 mo | 1.2 (0.9-1.7) | 13.0 (10.6-23.6) | 10.9 (7.7-13.8) | 0.7 (0.2-0.9) | 13.8 (8.4-18.1) | 8.6 (2.1-13.1) |
| P < .0001 | P < .0001 | P = .0005 | P < .0001 | P < .0001 | P = .7 | |
All P values reflect paired comparisons to pretreatment samples.
In λ-clonal CLL, λ FLCs were elevated in 24 of 28 patients (86%) prior to treatment, of whom 12 had a detectable monoclonal λ FLC by IFE. On ibrutinib, the κ:λ ratio and λ FLCs became normal at 6 and 12 months (P < .0001; Figure 2D-E, Table 2). Three patients no longer had a detectable monoclonal λ FLC on IFE. In contrast to the observed increase of λ FLCs in κ-clonal patients, the nonclonal (κ) FLCs in λ-clonal patients remained mostly stable and increased in only a few patients (Figure 2F, Table 2).
Normal B-cell recovery in peripheral blood and bone marrow
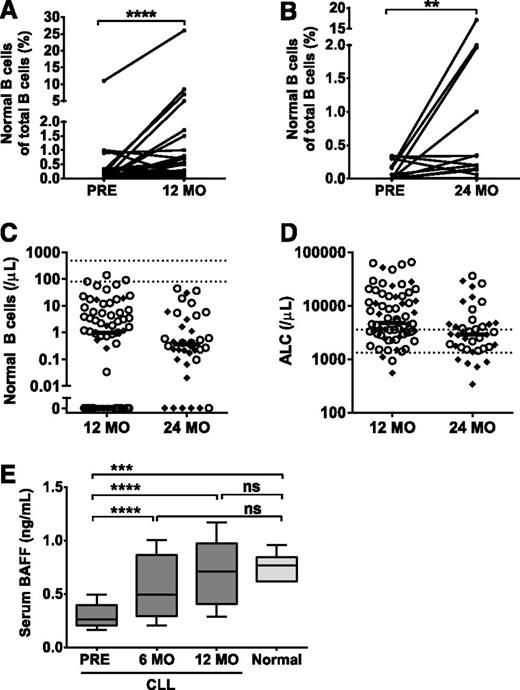
Given the observed increases in IgA and nonclonal FLCs, we hypothesized that normal B cells may be recovering on ibrutinib therapy. An increase in the percentage of normal B cells was noted at 12 and 24 months on ibrutinib compared with baseline (Figure 3A-B). However, the normal B-cell count remained abnormally low in the majority of patients (Figure 3C), even in those with effective control of their CLL who achieved a normal or near-normal absolute lymphocyte count (Figure 3D). In 3 patients, the normal B-cell count was within the reference range at 12 months. Although normal B cells in circulation were undetectable in 25 of 67 patients (37%) at 12 months, this was only the case in 6 of 38 patients (16%) at 24 months. Compared with those with relapsed/refractory disease, treatment-naive patients were more likely to have normal B cells at 12 months (29% vs 78%, P < .0001). At 24 months, this difference was no longer observed, suggesting that the effect of prior therapies may delay, but does not preclude, B-cell reconstitution on ibrutinib.
Detection of normal B cells and BAFF levels in the peripheral blood. (A) Percentage of normal B cells of total B cells at baseline (PRE) and 12 months (n = 45). (B) Percentage of normal B cells of total B cells at baseline (Pre) and 24 months (n = 16). (C) Absolute normal B-cell count and (D) ALC in treatment-naive (○) and relapsed/refractory (♦) patients at 12 (n = 67) and 24 months (n = 38) on log10 scale. Horizontal lines denote median values. Dotted lines illustrate the normal range. (E) Box-and-whisker plot (10th-90th percentile) of serum BAFF in ibrutinib-treated patients at baseline, 6, and 12 months (n = 21) and healthy controls (n = 6). Statistical significance is indicated by **P < .01, ***P < .001, ****P < .0001, or ns (P ≥ .05). ALC, absolute lymphocyte count.
Detection of normal B cells and BAFF levels in the peripheral blood. (A) Percentage of normal B cells of total B cells at baseline (PRE) and 12 months (n = 45). (B) Percentage of normal B cells of total B cells at baseline (Pre) and 24 months (n = 16). (C) Absolute normal B-cell count and (D) ALC in treatment-naive (○) and relapsed/refractory (♦) patients at 12 (n = 67) and 24 months (n = 38) on log10 scale. Horizontal lines denote median values. Dotted lines illustrate the normal range. (E) Box-and-whisker plot (10th-90th percentile) of serum BAFF in ibrutinib-treated patients at baseline, 6, and 12 months (n = 21) and healthy controls (n = 6). Statistical significance is indicated by **P < .01, ***P < .001, ****P < .0001, or ns (P ≥ .05). ALC, absolute lymphocyte count.
Next, we analyzed available bone marrow aspirates by flow cytometry to corroborate our observations in the peripheral blood. We found an upward trend in the percentage of normal B cells (Table 3). Due to the limited number of paired samples, there was insufficient power to detect a statistical difference. B-cell precursors were detected in the bone marrow of 14 of 31 patients (45%) at 12 months and 20 of 36 patients (56%) at 24 months. Bone marrow immunohistochemistry with CD5 and PAX5 visualized CD5−/PAX5+ B cells, consistent with normal B cells, in ibrutinib-treated patients (supplemental Figure 3).
Bone marrow cellularity and percentage of normal B cells and CLL cells on ibrutinib
| . | Cellularity, % . | CLL cells of total lymphocytes, % . | Normal B cells of total lymphocytes, % . |
|---|---|---|---|
| Pretreatment | |||
| Patients | n = 69 | n = 13 | n = 13 |
| Median (min-max) | 75 (30-100) | 95 (19-99) | 0.00* (0-1.49) |
| 12 mo | |||
| Patients | n = 55 | n = 30 | n = 29 |
| Median (min-max) | 45 (5-90) | 68 (17-97) | 0.33* (0-8.19) |
| 24 mo | |||
| Patients | n = 38 | n = 58 | n = 35 |
| Median (min-max) | 50 (15-80) | 58 (2-97) | 0.54* (0-17.93) |
| . | Cellularity, % . | CLL cells of total lymphocytes, % . | Normal B cells of total lymphocytes, % . |
|---|---|---|---|
| Pretreatment | |||
| Patients | n = 69 | n = 13 | n = 13 |
| Median (min-max) | 75 (30-100) | 95 (19-99) | 0.00* (0-1.49) |
| 12 mo | |||
| Patients | n = 55 | n = 30 | n = 29 |
| Median (min-max) | 45 (5-90) | 68 (17-97) | 0.33* (0-8.19) |
| 24 mo | |||
| Patients | n = 38 | n = 58 | n = 35 |
| Median (min-max) | 50 (15-80) | 58 (2-97) | 0.54* (0-17.93) |
P = .01 and .002 in an unpaired Wilcoxon analysis comparing normal B cells at pretreatment to 12 and 24 mo, respectively.
To understand the subset composition of the emerging B cells, we investigated the presence of immature/transitional (CD10+/CD27−), naive (CD10−/CD27−), and classic memory (CD27+) B cells in the peripheral blood of 4 patients. In 1 patient, the distribution of these immunophenotypic subsets resembled that of healthy donors (supplemental Figure 4A).38 However, the B-cell subsets were abnormally distributed in the remaining 3 patients (supplemental Figure 4B of representative case). Thus, although there is an overall increase in normal B cells in ibrutinib-treated patients, the recovery of specific B-cell subsets can differ from healthy individuals.
One reason for the low numbers of normal B cells in patients with CLL may be a competition for critical survival factors. In particular, BAFF is required for B-cell survival and maturation39 and is diminished in CLL patients.40,41 Serum BAFF levels are inversely correlated with the number of B cells expressing BAFF receptor, including CLL cells.40 In agreement with previous reports, pretreatment serum BAFF levels in our patients were substantially lower than in healthy controls.39 Following initiation of ibrutinib, serum BAFF increased to normal levels (Figure 3E).
Infection risk
Next, we sought to explore the contribution of changes in immunoglobulins and normal B cells on the risk of infection in our patients. At a median follow-up of 27.8 months, 69 of 84 patients (82%) developed a total of 177 infections. These included 25 grade 3-4 infections in 20 patients (24%) and 4 grade 5 events. Infections were more frequent during the first 6 months with an average rate of 16.3 infections per 100 patient-months compared with 6.9 thereafter (supplemental Figure 4A). Respiratory tract infections were the most common (65%), followed by gastrointestinal or genitourinary (16%) and skin (13%) infections. Patients with relapsed/refractory CLL had more infections of any grade than previously untreated patients (risk ratio, 1.58; P = .002; Figure 4A). Prior treatment was also associated with a higher rate of grade ≥3 infections (risk ratio, 2.23; P = .03; supplemental Figure 4B).
Cumulative mean number of infections. (A) Cumulative mean number of infections in TN, R/R, and all patients. (B) Cumulative mean number of infections in patients with ≥ (dotted line) or <1.5 FC (solid line) in IgA at 12 months compared with pretreatment baseline. FC, fold change; R/R, relapsed/refactory; TN, treatment-naive.
Cumulative mean number of infections. (A) Cumulative mean number of infections in TN, R/R, and all patients. (B) Cumulative mean number of infections in patients with ≥ (dotted line) or <1.5 FC (solid line) in IgA at 12 months compared with pretreatment baseline. FC, fold change; R/R, relapsed/refactory; TN, treatment-naive.
Using the improvement in IgA as a measure of immune reconstitution, we compared infection rates in patient groups divided by the median fold-change in IgA at 12 months compared with baseline. Patients with ≥50% increase in IgA had a lower rate of infection (risk ratio, 0.61; P = .03; Figure 4B) than patients with <50% increase in IgA. The difference remained significant (P = .03) after adjustment for prior treatment status. In contrast, baseline immunoglobulin levels and changes in IgG and IgM were not correlated with infection risk (supplemental Table 2).
Discussion
The essential role of BTK in B-cell maturation and immunoglobulin production is demonstrated by loss-of-function mutations that cause X-linked agammaglobulinemia.28 Given that ibrutinib leads to sustained inhibition of BTK, clinical trials investigating its use have monitored immunoglobulin levels in patients on treatment. A consistent but surprising observation has been the increase in IgA seen in these studies.23,29 Here, we confirmed this finding in a larger cohort of patients and extended the follow-up to 24 months. In addition, we found that the rate of infection was relatively reduced in patients with greater improvements in IgA. Using serum FLCs to distinguish clonal CLL cells from normal B cells, we demonstrated an increase in nonclonal λ FLCs in patients with κ-clonal CLL, suggesting reconstitution of B-cell immunity. Flow cytometry of peripheral blood and bone marrow identified an expanding nonclonal B-cell population, albeit at low levels, as treatment with ibrutinib continued. However, the recovery of specific B-cell subsets can be different than what is observed in healthy individuals. Whether this difference is related to CLL or treatment with ibrutinib is unknown.
Ibrutinib had a variable effect on the different immunoglobulin classes. IgA increased significantly and remained above baseline levels in most patients after 2 years of treatment. IgG, unaffected in the short-term, decreased with longer duration on drug. Compared with previous studies, our analysis, which incorporated IFE, accounted for the confounding effects of clonal immunoglobulins produced by CLL cells or contributed by an unrelated monoclonal gammopathy. By separating patients with and without clonal IgM, we were able to distinguish the divergent changes in serum IgM levels. In patients with clonal IgM, ibrutinib decreased IgM levels consistent with an antitumor effect, whereas IgM levels in patients without paraprotein transiently increased.
Multiple factors likely contribute to the improvement in specific immunoglobulin classes. The rapidity of increase in IgA suggests that preexisting antibody-producing cells may be secreting more immunoglobulins, whereas CLL cells, which impair immunoglobulin production,7,8 are removed by ibrutinib. Additionally, stimulatory signals provided by increasing BAFF levels may promote the emergence of normal B cells. In agreement with previous reports, we found that serum BAFF levels were suppressed in CLL patients at baseline40,41 and rapidly increased to normal levels during treatment with ibrutinib. BAFF binds to BAFF receptor, the cognate receptor on B cells, to activate the alternative nuclear factor κB pathway and drive cell survival and proliferation.42 BAFF has been shown to support CLL cell survival in vitro,43,44 raising the question of whether BAFF signaling could support the survival of remaining CLL cells. However, ibrutinib was previously shown to inhibit BAFF-R signaling45 and analyses of CLL cells from patients receiving ibrutinib demonstrated sustained inhibition of BCR and nuclear factor κB signaling and tumor proliferation.46
The risk of infection in CLL patients is associated with the severity of hypogammaglobulinemia and is somewhat mitigated by IV immunoglobulin replacement.1,6 We observed that among our patients, those with an increase in IgA of ≥50% above baseline had a lower rate of infections than those with an increase of <50%, even after adjustment for prior treatment status. Furthermore, prior treatment was associated with a lower baseline IgA, but not the change in IgA on ibrutinib. Secretory IgA coats mucosal surfaces where it binds and prevents epithelial adherence and translocation of pathogens.47 Because most patients with IgA deficiency are asymptomatic, the role of IgA in mucosal immunity is often considered redundant.47 However, when multiple facets of the immune system are disrupted, such as in CLL,1 IgA may have a greater role in protection against infections. Alternatively, the increase in IgA may only be an indicator of improved immune function. Whether IgA directly contributes to, or is a surrogate for, improved immunity against infections requires further investigation.
In addition to improvements in total IgA and IgM, we found a corresponding increase in the titers of polyreactive antibodies. The increase in polyreactive antibodies during treatment with ibrutinib may be analogous to the emergence of polyreactive B cells in individuals with X-linked agammaglobulinemia caused by inactivating mutations in BTK.48 Polyreactive antibodies belong to the natural antibody repertoire of the innate immune system as a first line of defense against encapsulated bacteria49,50 and possibly contributed to the lower rate of infection seen in our patients. Future work will address whether patients treated with ibrutinib have improved responses to vaccines that may further decrease infection-related morbidity.
Targeting BCR signaling represents a paradigm shift in the treatment of CLL and other B-cell malignancies. Ibrutinib, the first approved drug targeting this pathway, is generally well tolerated, but continued safety monitoring is required to assess the long-term safety of a potent inhibitor of key pathways in B-cell development and maturation. In our cohort of ibrutinib-treated patients with CLL, we observed improvements in IgA associated with a reduced rate of infections, but also a decline in IgG that was only apparent after prolonged treatment. At present, the latter does not appear to have an adverse impact. However, because ibrutinib may be given indefinitely, extended follow-up is needed to determine the immunologic consequences of prolonged BTK inhibition.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank our patients for participating in these studies, Pharmacyclics for providing ibrutinib, research support, and comments on the manuscript, and Leigh Samsel from the NHLBI Flow Cytometry core for performing BAFF measurements.
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Authorship
Contribution: A.W., G.A., and C.S. designed the study and wrote the manuscript; C.S., X.T., Y.S.L., and A.L. collected and analyzed data; S.E.M.H. assisted with analysis and manuscript revisions; M.Z.F., A.W., J.V., S.S., and G.E.M. implemented the clinical trials; S.G. and A.L.N. performed experiments on polyreactive antibodies; D.S., M.S.-S., C.Y., L.K., and S.M. performed flow cytometric analyses; I.M. performed immunohistochemistry; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: A.W. received research funding from Pharmacyclics, Inc. The remaining authors declare no competing financial interests.
Correspondence: Adrian Wiestner, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, 10 Center Dr, Building 10, CRC 3-5140, Bethesda, MD 20892-1202; e-mail: wiestnea@nhlbi.nih.gov.
References
Author notes
A.W. and G.A. are co-senior authors.