B-cell chronic lymphocytic leukemia (B-CLL) is defined by the accumulation of CD5+ B cells in the periphery and bone marrow. This disease is not characterized by highly proliferative cells but rather by the presence of leukemic cells with significant resistance to apoptosis and, therefore, prolonged survival. B-lymphocyte stimulator (BLyS) is a newly identified tumor necrosis factor (TNF) family member shown to be critical for maintenance of normal B-cell development and homeostasis and it shares significant homology with another TNF superfamily member, APRIL. The striking effects of BLyS on normal B-cell maintenance and survival raises the possibility that it may be involved in pathogenesis and maintenance of hematologic malignancies, including B-CLL. In this study, we investigated the status of APRIL and BLyS expression, as well as their receptors, in this disease. All B-CLL patient cells studied expressed one or more of 3 known receptors for BLyS; however, the pattern of expression was variable. In addition, we demonstrate for the first time that B-CLL cells from a subset of patients aberrantly express BLyS and APRIL mRNA, whereas these molecules were not detectable in normal B cells. Furthermore, we provide in vitro evidence that BLyS protects B-CLL cells from apoptosis and enhances cell survival. Because these molecules are key regulators of B-cell homeostasis and tumor progression, leukemic cell autocrine expression of BLyS and APRIL may be playing an important role in the pathogenesis of this disease.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is the most common leukemia in the Western world and is defined by the accumulation of CD5+ B cells with prolonged survival in the periphery and bone marrow.1,2 One of the current challenges to a better understanding of this disease is the extensive clinical and biologic heterogeneity found among patients with B-CLL. B-CLL cells are defined by a unique set of cell surface molecules: CD19+, CD5+, CD23+, and low levels of surface immunoglobulin (Ig). However, additional biologic features permit further subcharacterization of B-CLL, including Ig heavy-chain variable region somatic mutation status and CD38 expression levels.1,2 Although numerous advances have been made in our understanding of B-CLL, the molecular pathogenesis of this disease remains to be elucidated. However, it has been demonstrated that certain molecular alterations, including overexpression of antiapoptotic proteins and recurring chromosomal abnormalities, can be found regularly in B-CLL.1 2
In this regard, members of the tumor necrosis factor (TNF) superfamily have been shown to be key mediators in the formation and regulation of normal B-cell responses.3 B-lymphocyte stimulator, BLyS,4 also called B cell–activating factor (BAFF),5 TNF homologue that activates apoptosis, nuclear factor κB (NF-κB), and c-Jun NH2-terminal kinase (THANK),6 TNF and apoptosis ligand-related leukocyte-expressed ligand 1 (TALL-1),7 or zTNF48 is a newly identified TNF family member expressed by monocytes, macrophages, and dendritic cells.4,5,7 It has been shown to be critical for maintenance of normal B-cell development and homeostasis.9-11 Similar to other members of the TNF superfamily, BLyS is a type II membrane protein that can act in either a membrane-bound form or be proteolytically cleaved into a soluble cytokine.3 BLyS shares significant homology with a proliferation-inducing ligand (APRIL), which has been found to stimulate tumor cell growth12 as well as proliferation of primary lymphocytes.13 Of interest, APRIL is expressed by a variety of human cancers, including lymphoma.12 Initial studies on the effects of BLyS on B-cell physiology suggested that it costimulated B-cell proliferation and Ig secretion.4,5Subsequently, a role for BLyS in controlling cell survival was proposed because B cells isolated from BLyS transgenic mice have elevated Bcl-2 levels and prolonged survival compared to wild-type B cells.8,14 Moreover, it was found that BLyS increased the survival of resting or activated B cells and cooperated with CD40L to attenuate B-cell apoptosis.15 Transgenic overexpression of BLyS in mice results in elevated numbers of mature B cells in the spleen and periphery and development of autoimmunelike manifestations reminiscent of systemic lupus erythematosus.14,16 In addition, as BLyS transgenic mice age, they develop a secondary pathology, Sjögren syndrome, which is associated with intense B-cell activity and germinal center formation in the exocrine glands.17 Importantly, some of these latter biologic parameters, for example, elevated Bcl-2 levels, apoptosis resistance, and autoimmune disorders, are frequently observed in B-CLL. Additionally, patients with Sjogren syndrome are at high risk for development of B-cell malignancies, predominantly B-cell non-Hodgkin lymphomas (NHL).18 19
Three receptors, B-cell maturation antigen (BCMA),20transmembrane activator and CAML interactor (TACI),21 and BAFF-R22 have been identified as receptors for BLyS. BCMA and BAFF-R are exclusively expressed on B lymphocytes, whereas TACI can be found on B cells and activated T cells. TACI and BCMA can also bind to APRIL, whereas BAFF-R is specific for BLyS. A key role for BAFF-R in BLyS binding has been suggested by studies demonstrating that A/WySnJ mice, which carry a mutation in the BAFF-R, have a loss of follicular and marginal zone B cells in secondary lymphoid organs, a phenotype similar to BLyS-deficient mice.10,22-24 Thus, BAFF-R appears to be the primary receptor for BLyS responsible for B-cell development and survival. However, TACI-deficient mice were found to have an accumulation of splenic B cells and TACI−/− B cells have increased Ig production, suggesting an important role for this receptor in B-cell homeostasis.25 26 A role for BCMA in B-cell function remains to be elucidated.
Although there appears to be an essential role for BLyS in B-cell development and survival, the precise mechanism of BLyS action is less clearly understood. Treatment of A20 lymphoma cells with BLyS results in NF-κB and JNK activation.27 Overexpression of BCMA in 293 cells activates the transcription factors Rel/NF-κB, JNK, Elk-1, and p38 kinase28,29 and activation of TACI in Jurkat T cells results in transcriptional activation of AP-1, NF-κB, and nuclear factor of activated T cells (NF-AT).30Additionally, BLyS activates RelB and p50 in resting and CD40L-activated mouse splenic B cells.15 These findings suggest an important role for BLyS in activation of NF-κB.
The striking effects of BLyS on normal B-cell maintenance and survival raises the possibility that the BLyS-TACI/BCMA/BAFF-R receptor system may be involved in pathogenesis and maintenance of B-cell malignancies. Accordingly, we began an investigation of the status of APRIL and BLyS expression in B-CLL. Of great interest, we show for the first time that B-CLL cells from a subset of patients aberrantly express BLyS and APRIL. In addition, exogenous BLyS promoted B-CLL cell survival and soluble decoy receptors enhanced B-CLL apoptosis.
Materials and methods
Cells and reagents
Using previously described methods,31peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors or untreated B-CLL patients providing written informed consent. As shown in Table 1, B-CLL patients studied represented all Rai stages of disease. B-CLL and normal B lymphocytes were purified using anti-CD19 microbeads (Miltenyi, Auburn, CA). B-CLL purity was assessed by CD20 or CD5 expression and found to be uniformly more than 99%. Soluble Flag-BLyS, BCMA-Fc, and anti-BLyS (Buffy-1) were purchased from Alexis Biotechnology (San Diego, CA). Human IgG, Fc fragment, was purchased from Jackson Immunoresearch (West Grove, PA). Fluorescein isothiocyanate (FITC)–conjugated anti-Flag M2 was purchased from Sigma Chemical (St Louis, MO). Anti–poly-adenosine 5′-diphosphate-ribose polymerase (PARP) was purchased from Becton Dickinson (San Diego, CA). The anti–TACI-biotin antibody was generated by immunization of mice with recombinant TACI and biotinylated using NHS-biotin.30 Jurkat cells stably expressing BLyS were used as a positive control.
Clinical features of B-CLL patients
| Patient no. . | Sex . | Age, y . | Rai . | CD38, % . | VH mutation status . |
|---|---|---|---|---|---|
| 1 | F | 54 | 4 | 21 | Germ line |
| 2 | M | 69 | 4 | 93 | Mutated |
| 3 | F | 81 | 4 | 4 | Mutated |
| 4 | M | 69 | 3 | 9 | NA |
| 5 | F | 69 | 1 | 5 | NA |
| 6 | M | 75 | 0 | 12 | Mutated |
| 7 | M | 71 | 4 | 35 | Germ line |
| 8 | M | 46 | 4 | 0 | NA |
| 9 | M | 61 | 4 | 25 | Mutated |
| 10 | M | 87 | 0 | 0 | Mutated |
| 11 | F | 85 | 0 | 58 | Germ line |
| 12 | M | 81 | 2 | 81 | Mutated |
| 13 | M | 49 | 0 | 1 | Mutated |
| 14 | M | 73 | 4 | 2 | Mutated |
| 15 | M | 61 | 4 | 5 | Mutated |
| 16 | M | 43 | 2 | 2 | Mutated |
| 17 | M | 65 | 0 | 0 | NA |
| 18 | M | 50 | 0 | 2 | Mutated |
| 19 | F | 69 | 4 | 1 | Mutated |
| 20 | M | 78 | 4 | 87 | Germ line |
| 21 | M | 59 | 0 | 2 | Mutated |
| 22 | F | 79 | 4 | 86 | Germ line |
| 23 | F | 91 | 4 | 3 | Mutated |
| Patient no. . | Sex . | Age, y . | Rai . | CD38, % . | VH mutation status . |
|---|---|---|---|---|---|
| 1 | F | 54 | 4 | 21 | Germ line |
| 2 | M | 69 | 4 | 93 | Mutated |
| 3 | F | 81 | 4 | 4 | Mutated |
| 4 | M | 69 | 3 | 9 | NA |
| 5 | F | 69 | 1 | 5 | NA |
| 6 | M | 75 | 0 | 12 | Mutated |
| 7 | M | 71 | 4 | 35 | Germ line |
| 8 | M | 46 | 4 | 0 | NA |
| 9 | M | 61 | 4 | 25 | Mutated |
| 10 | M | 87 | 0 | 0 | Mutated |
| 11 | F | 85 | 0 | 58 | Germ line |
| 12 | M | 81 | 2 | 81 | Mutated |
| 13 | M | 49 | 0 | 1 | Mutated |
| 14 | M | 73 | 4 | 2 | Mutated |
| 15 | M | 61 | 4 | 5 | Mutated |
| 16 | M | 43 | 2 | 2 | Mutated |
| 17 | M | 65 | 0 | 0 | NA |
| 18 | M | 50 | 0 | 2 | Mutated |
| 19 | F | 69 | 4 | 1 | Mutated |
| 20 | M | 78 | 4 | 87 | Germ line |
| 21 | M | 59 | 0 | 2 | Mutated |
| 22 | F | 79 | 4 | 86 | Germ line |
| 23 | F | 91 | 4 | 3 | Mutated |
Mutated VH indicates a greater than 2% deviation from the most similar germ line sequence.
PCR analysis
For reverse transcription–polymerase chain reaction (RT-PCR), the TRIzol reagent (Invitrogen, Carlsbad, CA) was used to isolate total RNA from freshly isolated CD19+ normal B cells, CD19+ B-CLL cells, or monocyte-enriched PBMCs obtained from B and T lymphocyte–depleted peripheral blood. RNA was converted into cDNA using the First-Strand cDNA Synthesis Kit (Amersham Pharmacia, Little Chalfont, Buckinghamshire, England) according to the manufacturer's instructions. BLyS, APRIL, BAFF-R, BCMA, and β-actin cDNAs were detected by PCR amplification with HotStarTaq(Qiagen, Valencia, CA) in steps of 1 minute each at 94°C, 60°C and 72°C for 35 cycles, using primers previously described as being specific for BLyS5 (5′ GGA GAA GGC AAC TCC AGT CAG AAC and 3′ CAA TTC ATC CCC AAA GAC ATG GAC) and APRIL12 (5′ CCA GCC TCA TCT CCT TTC TTG C and 3′ TCA CAG TTT CAC AAA CCC CAG G). BAFF-R, BCMA, and β-actin primers were designed using the published cDNA nucleotide sequences for BAFF-R (5′ GGA AGA CCC AGG AAC CAC and 3′ AAG GCA AGC ACA CCA AA), BCMA (5′ TTA CTT GTC CTT CCA GGC TGT TCT and 3′ CAT GAA ACC AAG GAA GTT TCT ACC), or β-actin (5′ GGA TCC GAC TTC GAG CAA GAG ATG GCC AC and 3′ CAA TGC CAG GGT ACA TGG TG).
Real-time PCR was performed on RNA samples isolated from CD19+ B-CLL cells, HL60 myelomonocytic cells, or HT2260 and Raji B lymphoblastic cells. HeLa RNA was purchased from BD Pharmingen (Franklin Lakes, NJ). Quantitative PCR was carried out with the use of double fluorescently labeled probes (synthesized by Applied Biosystems, Foster City, CA) specific for cyclophilin-B or BLyS. The probes were labeled at the 5′ end with the fluorescent reporter dye VIC and at the 3′ end with tetramethylrhodamine (TAMARA) as the quencher. cDNA synthesis and quantitative PCR was performed in a single 50-μL reaction containing 25 μL OneStep RT-PCR Master Mix containing AmpliTaq Gold DNA Polymerase (Applied Biosystems), 1.25 μL 40 × MultiScribe reverse transcriptase (Applied Biosystems), 125 mM sequence specific hybridization probes for BLyS (VIC-CCA CCA GCT CCA GGA GAA GGC AAC TC-TAMRA) or cyclophilin-B mRNA (VIC-AGC ATC TAC GGT GAG CGC TTC CCC-TAMRA), 300 nM forward and reverse primers for BLyS (5′ CGC GGG ACT GAA AAT CTT TG and 3′ CAC GCT TAT TTC TGC TGT TCT GA) or cyclophilin-B (5′ GGA GAT GGC ACA GGA GGA AA and 3′ CGT AGT GCT TCA GTT TGA AGT TCT CA), and 1 to 5 μg RNA. cDNA synthesis and RT-PCR were performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems) with the following thermal cycler protocol. Step 1: 48°C for 30 minutes; step 2: 95°C for 10 minutes; step 3: 40 cycles of denaturation at 95°C for 15 seconds and annealing at 60°C for 1 minute.
Quantitative PCR analysis was completed using ABI PRISM 7000 SDS Software. Cτ values were collected for cyclophilin and BLyS during log phase of the cycle. BLyS levels were normalized to cyclophilin for each sample (ΔCτ = Cτ BLyS − Cτ cyclophilin) and compared with the values obtained for a known positive control (HL60) using the following formula 100/2ΔΔCτ where ΔΔCτ = Cτ unknown − ΔCτ HL60.
Caspase-3 activity assay
B-CLL cells were isolated, washed, and cultured (10 × 106 cells/mL) in phenol red-free RPMI supplemented with 0.5% bovine serum albumin (BSA) and 0.1 μg/mL Flag-BLyS, 10 μM chlorambucil, 10 μg/mL human IgG, Fc fragment, or 1 μg/mL BCMA-Fc at 37°C. After 18 hours of incubation, cells were washed and plated into a 96-well plate (5 × 106 cells/well in 25 μL phenol red-free RPMI in triplicate). Select wells were incubated with the caspase-3–specific inhibitor Ac-DEVD-CHO (50 nM final; Biomol, Plymouth Meeting, PA) for 30 minutes at 37°C prior to addition of 50 μL of the caspase-3–specific fluorogenic substrate Ac-DEVD-AMC (33 μM final; Biomol) in substrate buffer (100 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 10% sucrose, 0.1% CHAPS (3[3-chloroaminopropyl diethyl-ammonio[-1-propane sulfonate], 5 mM DTT [dithiothreitol], and 0.0001% NP40). Caspase-3 activity was measured by cleavage of the caspase-3–specific substrate and was assessed by fluorescence using a CytoFluor Multi-well Plate Reader (Applied Biosystems).
Western blot analysis
B-CLL cells, stimulated as described above, were directly lysed in 150 μL Laemmli sample buffer. Cell lysates (10 × 106 cells/lane) were boiled, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were transferred to an Immobilon P membrane (Millipore, Bedford, MA). Membranes were incubated with 1 μg/mL anti-PARP followed by horseradish peroxidase (HRP)–linked goat antimouse secondary antibody. Immunoreactive proteins were detected using enhanced chemiluminescence (Pierce, Rockford, IL).
Results
Expression of TACI, BCMA, and BAFF-R in CLL B cells
Initially, we examined TACI expression by B-CLL cells and normal B cells. Using fluorescence-activated cell sorting (FACS), we detected significant levels of TACI on 23 of 23 B-CLL samples and all normal B-cell samples tested (Figure 1A and data not shown). Additionally, binding of soluble BLyS, which binds TACI, BCMA, and BAFF-R, was detected on all B-CLL (23 of 23) as well as all normal B cells examined (Figure 1A and data not shown). Mature B cells have been reported to express both BCMA and BAFF-R, but because antibodies to either of these receptors are not yet available, we used RT-PCR to measure expression of both receptors. As shown in Figure 1B, 7 of 7 B-CLL samples expressed BAFF-R mRNA as did normal B cells (3 of 3 and data not shown). BCMA mRNA was expressed at low levels in normal B cells (3 of 3 and data not shown) and there was a variable pattern of expression in the B-CLL samples. Thus, similar to normal human B cells, B-CLL cells express BLyS receptors and have the ability to bind soluble BLyS.
Expression of BCMA, TACI, and BAFF-R in CLL B cells.
(A) Surface expression of TACI and soluble BLyS binding was determined by FACS. CD19+ B-CLL, CD19+ normal B cells, or BLyS-Jurkat cells were incubated with biotin-conjugated anti-TACI or Flag-BLyS (gray histograms) for 30 minutes on ice, washed, and incubated with the respective secondary antibodies, Red 670-streptavidin, or anti–Flag-FITC. Isotype and fluorochrome controls were done for each sample (open histograms). The cells were analyzed for immunofluorescence on a FACS Vantage (Becton Dickinson). Collected data were analyzed using CellQuest software. (B) Expression of BCMA and BAFF-R mRNA was analyzed by RT-PCR in CD19+B-CLL and CD19+ normal B cells.
Expression of BCMA, TACI, and BAFF-R in CLL B cells.
(A) Surface expression of TACI and soluble BLyS binding was determined by FACS. CD19+ B-CLL, CD19+ normal B cells, or BLyS-Jurkat cells were incubated with biotin-conjugated anti-TACI or Flag-BLyS (gray histograms) for 30 minutes on ice, washed, and incubated with the respective secondary antibodies, Red 670-streptavidin, or anti–Flag-FITC. Isotype and fluorochrome controls were done for each sample (open histograms). The cells were analyzed for immunofluorescence on a FACS Vantage (Becton Dickinson). Collected data were analyzed using CellQuest software. (B) Expression of BCMA and BAFF-R mRNA was analyzed by RT-PCR in CD19+B-CLL and CD19+ normal B cells.
BLyS protects CLL B cells from apoptosis
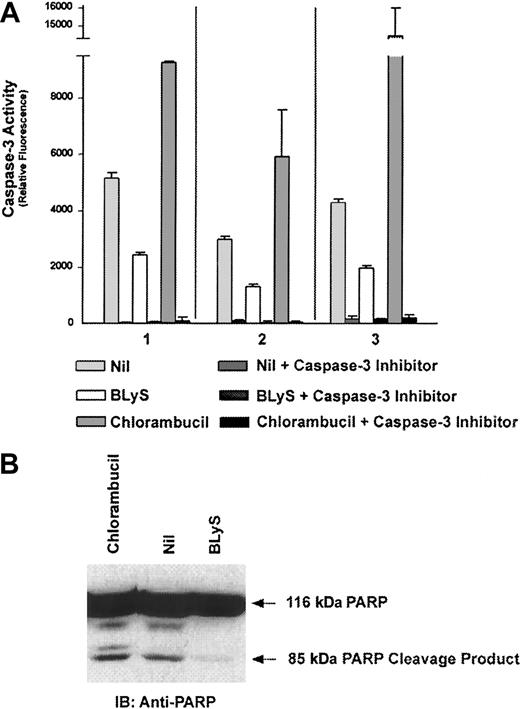
Initially, it was proposed that BLyS functioned to enhance B-cell proliferation following anti-IgM or CD40 stimulation as well as immunoglobulin secretion.4,5 However, recent literature now suggests that the predominant function of BLyS is maintenance and survival of the B-cell population and attenuation of B-cell apoptosis.15 Because B-CLL cells express the receptors for BLyS and they are characterized by high resistance to apoptosis, we next wanted to determine the influence of BLyS on CLL B cells. Using caspase-3 activation and PARP degradation as hallmark indicators of apoptosis,32 we examined the effects of BLyS on CLL B-cell survival. Three B-CLL patient samples treated with BLyS for 18 hours had an average of a 55% reduction in the level of caspase-3 activity compared with untreated controls (Figure2A). The caspase-3–specific inhibitor Ac-DEVD-CHO was used as a control to demonstrate the specificity of the assay. Cleavage of PARP was also assessed after treatment of B-CLL cells with BLyS. Similar to what was seen in the caspase-3 activity assays, there was a significant reduction in the level of PARP degradation in the presence of BLyS (Figure 2B). B-CLL cells were treated with 10 μM chlorambucil as a positive control for caspase-3 activation and PARP cleavage.33 These data clearly indicate that BLyS reduces the level of apoptosis and enhances survival of B-CLL B cells.
Attenuation of B-CLL apoptosis by BLyS.
B-CLL cells from 3 patients (nos. 1-3) were cultured in phenol red-free RPMI supplemented with 0.5% BSA and 0.1 μg/mL Flag-BLyS or 10 μM chlorambucil at 37°C for 18 hours. (A) Caspase-3 activity was determined using the fluorescent caspase-3–specific substrate Ac-DEVD-AMC as described in “Materials and methods.” Apoptosis, as measured by caspase-3 activity, is detected by cleavage of Ac-DEVD-AMC and subsequent fluorescence. The caspase-3–specific inhibitor was included in all conditions to demonstrate specificity. (B) PARP degradation was assessed by Western blot B-CLL cells, stimulated as described above, were directly lysed, separated by SDS-PAGE, and proteins were transferred to an Immobilon P membrane. Membranes were incubated with 1 μg/mL anti-PARP followed by HRP-linked goat antimouse secondary antibody.
Attenuation of B-CLL apoptosis by BLyS.
B-CLL cells from 3 patients (nos. 1-3) were cultured in phenol red-free RPMI supplemented with 0.5% BSA and 0.1 μg/mL Flag-BLyS or 10 μM chlorambucil at 37°C for 18 hours. (A) Caspase-3 activity was determined using the fluorescent caspase-3–specific substrate Ac-DEVD-AMC as described in “Materials and methods.” Apoptosis, as measured by caspase-3 activity, is detected by cleavage of Ac-DEVD-AMC and subsequent fluorescence. The caspase-3–specific inhibitor was included in all conditions to demonstrate specificity. (B) PARP degradation was assessed by Western blot B-CLL cells, stimulated as described above, were directly lysed, separated by SDS-PAGE, and proteins were transferred to an Immobilon P membrane. Membranes were incubated with 1 μg/mL anti-PARP followed by HRP-linked goat antimouse secondary antibody.
Expression of BLyS by CLL B cells
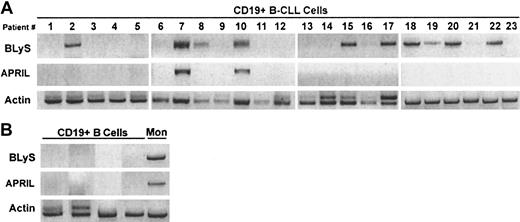
Because of evidence in the literature demonstrating tumor cell autocrine expression of APRIL,12 we next wished to determine whether B-CLL cells could express BLyS or APRIL in an autocrine manner. To examine this possibility, we used RT-PCR to detect BLyS and APRIL mRNA in highly purified CD19+ peripheral blood B cells from B-CLL patients. Of great interest, BLyS mRNA was detected in 11 of 23 patients and APRIL mRNA was detected in 2 of 23 patients (Figure 3A). By contrast, normal CD19+ peripheral blood B cells failed to express either molecule (Figure 3B). Monocyte-enriched PBMCs were used as a positive control for BLyS and APRIL expression (Figure 3B).
Expression of BLyS and APRIL mRNA in CLL B cells.
Expression of BLyS and APRIL mRNA was analyzed by RT-PCR in 23 CD19+ B-CLL (A) or in CD19+ normal B cells and monocyte-enriched PBMCs (B).
Expression of BLyS and APRIL mRNA in CLL B cells.
Expression of BLyS and APRIL mRNA was analyzed by RT-PCR in 23 CD19+ B-CLL (A) or in CD19+ normal B cells and monocyte-enriched PBMCs (B).
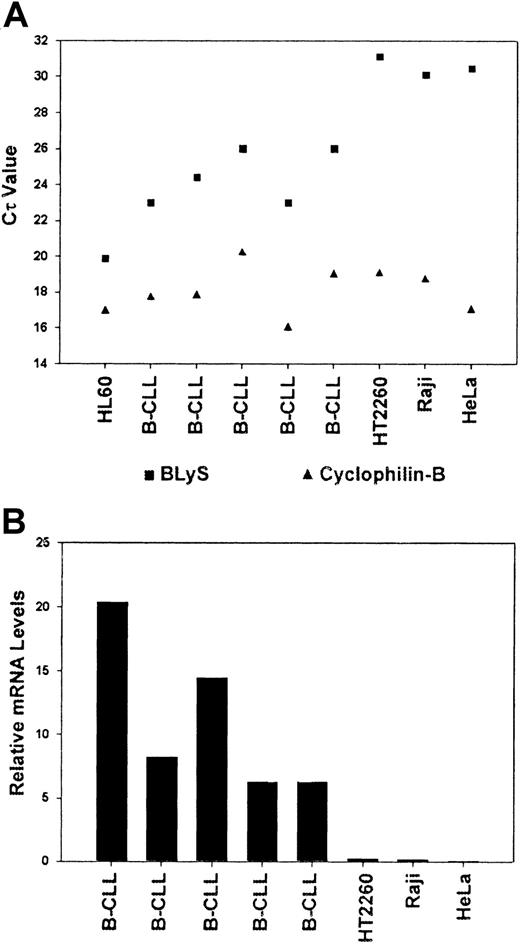
To further confirm the RT-PCR data, we next analyzed B-CLL cells for expression of BLyS by quantitative real-time PCR (Figure4A). BLyS and cyclophilin-B were amplified from 5 B-CLL patient samples, HL60 cells, which are known to express BLyS,34 the Raji and HT2260 B-cell lymphoma lines, and HeLa cells. The mRNA expression levels of cyclophilin-B and BLyS are shown in Figure 4A. For analysis of BLyS expression, each sample was normalized to cyclophilin-B and compared with the levels of BLyS expression in HL60 cells (see “Materials and methods”). Relative to HL60 cells, CLL B cells expressed lower levels (80%-95% less) of BLyS mRNA (Figure 4B). However, B-CLL cells clearly expressed BLyS, whereas the 2 B-cell lymphoma lines and HeLa cells were found to be negative.
Quantitative expression of BLyS mRNA in CLL-B cells.
Real-time PCR was performed on RNA samples isolated from CD19+ B-CLL cells, HL60 myelomonocytic cells, HT2260 and Raji B lymphoblastic cells, or HeLa cells. Quantitative PCR analysis was completed using ABI PRISM 7000 SDS software as described in “Materials and methods.” Cτ values were collected for cyclophilin and BLyS during log phase of the cycle and are shown in panel A. (B) BLyS levels were normalized to cyclophilin for each sample. HL60 cells were assigned a relative expression value of 100.
Quantitative expression of BLyS mRNA in CLL-B cells.
Real-time PCR was performed on RNA samples isolated from CD19+ B-CLL cells, HL60 myelomonocytic cells, HT2260 and Raji B lymphoblastic cells, or HeLa cells. Quantitative PCR analysis was completed using ABI PRISM 7000 SDS software as described in “Materials and methods.” Cτ values were collected for cyclophilin and BLyS during log phase of the cycle and are shown in panel A. (B) BLyS levels were normalized to cyclophilin for each sample. HL60 cells were assigned a relative expression value of 100.
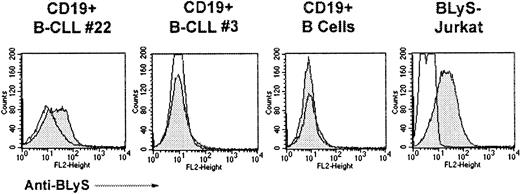
In addition to our PCR approach, we also examined B-CLL cells for cell surface expression of BLyS (Figure 5). B-CLL cells that were shown to express BLyS mRNA (Figure 3A) were also found to express low levels of cell surface BLyS, whereas B-CLL cells that were negative by RT-PCR, were found to be negative for BLyS protein by FACS. As expected, normal B cells were deficient for BLyS expression. Jurkat T cells stably expressing BLyS served as a positive control. Work is currently underway to look for APRIL protein in B-CLL cells by Western analysis, because it appears that APRIL may be directly secreted without intermediate expression on the cell surface.35 Taken together, these results suggest that B CLL cells from a subset of patients aberrantly express BLyS and APRIL, whereas these molecules were undetectable in normal B cells.
Cell surface expression of BLyS on CLL-B cells.
Surface expression of BLyS was determined by FACS. CD19+B-CLL, CD19+ normal B cells, or BLyS-Jurkat cells were incubated with anti-BLyS (gray histograms) and a secondary antibody, anti–rat-PE. Isotype and fluorochrome controls were done for each sample (open histograms).
Cell surface expression of BLyS on CLL-B cells.
Surface expression of BLyS was determined by FACS. CD19+B-CLL, CD19+ normal B cells, or BLyS-Jurkat cells were incubated with anti-BLyS (gray histograms) and a secondary antibody, anti–rat-PE. Isotype and fluorochrome controls were done for each sample (open histograms).
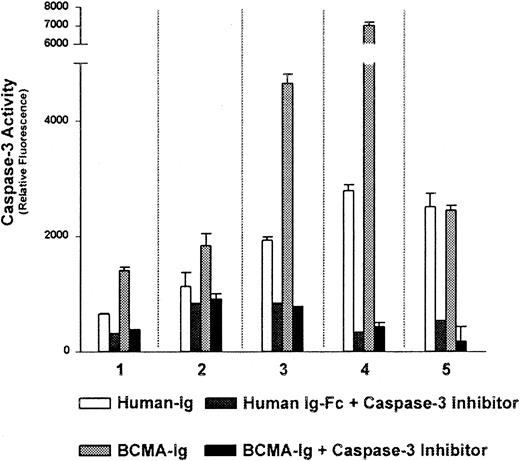
The data shown in Figure 2 demonstrated the ability of exogenous BLyS to protect B-CLL cells from undergoing apoptosis. We next wished to determine whether we could demonstrate a role for autocrine BLyS in the maintenance and survival of CLL B-cells. To accomplish this, we used a soluble decoy receptor approach. Thus, we examined the effects of BCMA-Fc, which binds with high affinity to soluble BLyS and APRIL,8,11 36 on B-CLL cell survival. Using caspase-3 activation as a measurement of apoptosis we found that incubation of CLL B cells, all of which expressed message for BLyS, with BCMA-Fc resulted in enhanced caspase-3 activity in 4 of 5 patient samples. Incubation of B-CLL cells with BCMA-Fc resulted in an average of a 2.1-fold increase in caspase-3 activity compared with the negative human IgG-Fc control (Figure 6). These results suggest that treatment of CLL B cells with a blocking reagent that binds soluble BLyS or APRIL may enhance apoptosis and decrease cell survival.
Autocrine produced BLyS protects B-CLL cells from apoptosis.
B-CLL cells from 5 patients (nos. 1-5) were cultured in phenol red-free RPMI supplemented with 0.5% BSA and 1 μg/mL BCMA-Fc or 10 μg/mL human Ig-Fc at 37°C for 18 hours. Caspase-3 activity was determined using the fluorescent caspase-3–specific substrate Ac-DEVD-AMC as described in “Materials and methods.” Apoptosis, as measured by caspase-3 activity, is detected by cleavage of Ac-DEVD-AMC and subsequent fluorescence. The caspase-3–specific inhibitor was included in all conditions to demonstrate specificity.
Autocrine produced BLyS protects B-CLL cells from apoptosis.
B-CLL cells from 5 patients (nos. 1-5) were cultured in phenol red-free RPMI supplemented with 0.5% BSA and 1 μg/mL BCMA-Fc or 10 μg/mL human Ig-Fc at 37°C for 18 hours. Caspase-3 activity was determined using the fluorescent caspase-3–specific substrate Ac-DEVD-AMC as described in “Materials and methods.” Apoptosis, as measured by caspase-3 activity, is detected by cleavage of Ac-DEVD-AMC and subsequent fluorescence. The caspase-3–specific inhibitor was included in all conditions to demonstrate specificity.
Discussion
The initial aim of our studies was to examine the role of BLyS and its receptors in B-CLL. Our results demonstrated that all B-CLL patients tested express TACI and BAFF-R, whereas only a subset express BCMA. Additionally, not only do B-CLL cells express the receptor for BLyS, they also have the ability to bind soluble BLyS. Finally, we also present evidence for the first time that B-CLL cells may also express BLyS and APRIL.
The expression of TACI and BAFF-R by B-CLL cells was expected, because normal mature B cells express these receptors. However, the variable expression of BCMA by CLL B cells is intriguing and the significance of these finding is unclear at this time. However, it will be of utmost importance to more thoroughly examine the expression levels of TACI, BCMA, and BAFF-R in B-CLL in light of data suggesting that expression levels of BAFF-R highly influence the lifespan of B cells.37 A more definitive analysis of BCMA and BAFF-R protein expression by B-CLL cells is currently underway, however, this has been challenging because of a lack of reagents. Altered expression of BLyS receptors and ligands for these receptors may therefore contribute to the progressive accumulation of malignant B cells characteristic of B-CLL.
Recent data support the notion that BLyS is essential to B-cell development and survival; however, the precise mechanism of BLyS action is less clearly understood. The ability of BLyS to bind 3 distinct receptors, BAFF-R, TACI, and BCMA, further complicates the picture. BAFF-R– and BLyS-deficient mice have similar phenotypes suggesting that BAFF-R is the predominant receptor for BLyS. However, BLyS clearly binds to and signals through TACI and BCMA. We provide evidence in this report that similar to normal B cells, B-CLL cells have enhanced cell survival in response to soluble BLyS. The ability of BLyS to down-regulate caspase-3 activity and PARP cleavage in B-CLL cells suggests a possible role for this protein in the maintenance and survival of malignant B-CLL cells.
Currently, the molecular mechanisms underlying the pathogenesis of B-CLL are poorly defined. However, there is a growing literature suggesting that CLL B cells have elevated levels of Bcl-238as well as constitutive activation of NF-κB.39-41Because Bcl-2 and NF-κB play a central role in regulating cell survival, changes in their activity and expression levels likely play a critical role in the progression of this disease. Of interest, treatment of normal B cells, as well as B lymphoma lines, with BLyS results in activation of NF-κB,15,27 and B cells from BLyS transgenic mice have elevated levels of Bcl-2.14 16
Accordingly, we investigated the possibility that deregulated expression of BLyS may contribute to the reduced levels of apoptosis found in B-CLL cells. In this report, we show for the first time that B-CLL cells from a subset of patients aberrantly express BLyS and APRIL, whereas these molecules were undetectable in normal B cells. The inability of normal B cells to produce BLyS is consistent with previous reports in the literature that have failed to detect BLyS expression in normal resting or activated B cells.4 34 Although there was no apparent association between Rai stage, CD38 expression, or mutation status and BLyS or APRIL expression (Table 1), this will need to be rigorously tested using larger numbers of B-CLL patient samples. Quantitative analysis of BLyS expression revealed that BLyS mRNA was found at low levels compared with HL60 cells and that it was virtually undetectable in B-cell lymphoma lines. Our conclusion that some B-CLL cells may express autocrine BLyS is further supported by our ability to detect low cell-surface expression of BLyS on CLL-B cells from some patients and the ability of a BCMA-Fc decoy receptor to increase apoptosis. Of interest, only 4 of 5 patient B-CLL samples cultured with BCMA-Fc displayed enhanced apoptosis. Although the significance of this observation remains unclear at this time, this result is consistent with the heterogeneity of this disease and may reflect heterogeneity in receptor expression levels or balance in expression between BAFF-R, TACI, and BCMA, or the inability or inefficiency of the decoy receptor to displace autocrine BLyS from cell-surface receptors.
The mechanism by which some B-CLL cells acquire the ability to express BLyS and APRIL is currently unknown. However, evidence suggests that the locus for BLyS on chromosome 13q32-343 is amplified in human B-cell malignancies.42 Additionally, the ability of tumor cells to produce autocrine factors that support their growth is a common theme in many tumorigenesis models. Of interest, APRIL, another TNF superfamily member with homology to BLyS, is expressed by and enhances the in vivo growth rate of tumor cells.12Although we have no evidence at this time that CLL-B cells express BLyS in vivo, we believe that it is unlikely that ex vivo manipulation of the CLL B cells would influence BLyS expression specifically in CLL B cells. Although CD19 microbead purification of CLL B cells may influence protein expression, because we purified normal and CLL B cells using identical methods, and because we did not find BLyS in either normal B cells or in a subset of B-CLL samples, we believe it is unlikely that our method of purification artificially induced BLyS expression. In addition, BLyS mRNA was expressed in patient samples not treated with CD19 microbeads that were more than 95% B-CLL cells (results not shown). Taken together, we believe our data strongly suggest that CLL B cells have the ability to synthesize BLyS.
As noted previously, TACI is expressed on activated T cells.30 An additional biologic aspect relevant to our findings is the recent report that BAFF (BLyS) was able to induce T-cell activation and division under appropriate culture conditions.43 In addition, Wang and colleagues44 showed that TACI-ligand interactions were required for T-cell activation and collagen-induced arthritis in mice. Of interest, B-CLL patients not only have a clonal expansion of B cells at presentation, but also an expansion of polyclonal T cells.45 Thus, this raises the possibility that T-cell expansion may occur in part as a consequence of elevated BAFF/BLyS in the microenvironment. In this scenario of elevated BAFF/BLyS, however, it is difficult to explain the diminished normal B-cell compartment typically seen in B-CLL patients. One possible explanation for this observation is that the levels of BAFF-R, TACI, or BCMA expression on B-CLL cells may differ from normal B cells thereby providing a competitive advantage, that is, increased survival, to the leukemic population.
Autocrine expression of BLyS by B-CLL cells has exciting implications and suggests the possibility that agents that inhibit BLyS binding to B-CLL cells may induce apoptosis. We have shown for the first time that B-CLL cells from a subset of patients aberrantly express BLyS and APRIL. Additionally, all B-CLL patients tested expressed TACI and BAFF-R, whereas only a minor subset expressed BCMA. Furthermore, we provide evidence that BLyS protects B-CLL cells from apoptosis and enhances cell survival, whereas the BCMA-Fc decoy receptor interrupted this autocrine loop resulting in enhanced cell death. We believe that these results are the first to show this unique autocrine pathway related to apoptosis in B-CLL. Importantly, our results also suggest that therapeutic agents that inhibit BLyS binding to B-CLL cells may have clinical efficacy.
We thank Ms Renee Tschumper and Nancy Bone for their help in determining B-CLL immunoglobulin mutation status and levels of CD38 expression.
Prepublished online as Blood First Edition Paper, June 14, 2002; DOI 10.1182/blood-2002-02-0558.
Supported by National Institutes of Health grant CA62228 (D.F.J.). A.J.N. was supported by a postdoctoral trainee award from the National Institutes of Health, grant CA09441.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Diane F. Jelinek, Department of Immunology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail:jelinek.diane@mayo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal