Key Points
Platelet-derived Dkk1 is the major Wnt antagonist that suppresses Wnt/β-catenin signaling during acute lung inflammation.
Intratracheal administration of Wnt3a or neutralization of Dkk1 inhibited neutrophil influx into the lungs.
Abstract
Neutrophil infiltration represents the early acute inflammatory response in acute lung injury. The recruitment of neutrophils from the peripheral blood across the endothelial-epithelial barrier into the alveolar airspace is highly regulated by the adhesion molecules on alveolar epithelial cells (AECs). Wnt/β-catenin signaling is involved in the progression of inflammatory lung diseases including asthma, emphysema, and pulmonary fibrosis. However, the function of Wnt/β-catenin signaling in acute lung inflammation is unknown. Here, we identified platelet-derived Dickkopf-1 (Dkk1) as the major Wnt antagonist contributing to the suppression of Wnt/β-catenin signaling in AECs during acute lung inflammation. Intratracheal administration of Wnt3a or an antibody capable of neutralizing Dkk1 inhibited neutrophil influx into the alveolar airspace of injured lungs. Activation of Wnt/β-catenin signaling in AECs attenuated intercellular adhesion molecule 1 (ICAM-1)/vascular cell adhesion molecule 1 (VCAM-1)-mediated adhesion of both macrophages and neutrophils to AECs. Our results suggest a role for Wnt/β-catenin signaling in modulating the inflammatory response, and a functional communication between platelets and AECs during acute lung inflammation. Targeting Wnt/β-catenin signaling and the communication between platelets and AECs therefore represents potential therapeutic strategies to limit the damage of acute pulmonary inflammation.
Introduction
Acute lung injury (ALI) and its more severe form, the acute respiratory distress syndrome (ARDS), are life-threatening diseases with a variety of causes, including sepsis, trauma, and viral or bacterial pneumonia.1 ALI/ARDS is characterized by the acute onset of hypoxemia, the damage of the capillary-alveolar interface, initiation of a proinflammatory cytokine and chemokine storm, and infiltration of inflammatory cells. ALI/ARDS is clearly recognized as a syndrome of acute inflammation with a mortality rate of up to 40% in human patients. Activation and transmigration of neutrophils through the endothelium, interstitium, and epithelium is the central event in the development of ALI/ARDS.2
Platelets are the central mediators of hemostasis of the circulatory system and also contribute to acute pulmonary inflammation by regulating the inflammatory response of endothelial cells and the activation of leukocytes.3-6 Acute pulmonary inflammation is associated with the activation of aggressive platelets in the blood and sequestration in pulmonary vascular beds. Platelet production following the onset of thrombocytopenia is increased up to 20-fold under the conditions of peripheral demand and inflammation.7 Platelets also aggregate with neutrophils or monocytes via cell surface P-selectin and provide supportive juxtacrine and paracrine signals.7-9 By interacting with endothelial cells, activated platelets cause increases in vascular permeability and promote the release of proinflammatory cytokines (interleukin-6 [IL-6]) or chemokines (IL-8) from endothelial cells.8,10 Platelet components are found in the bronchoalveolar (BAL) fluid from ARDS patients.11 However, it remains unclear whether platelets directly affect the functions of the alveolar epithelium during the development of ALI.
Wnt/β-catenin signaling is essential in maintaining proper embryonic lung development and regeneration of the lung epithelium after injury in adults.12-15 However, only limited evidence is available for a role of Wnt/β-catenin signaling in acute pulmonary inflammation.16-19 Wnt/β-catenin signaling inhibits the endothelial and epithelial inflammatory responses by suppressing proinflammatory cytokines (tumor necrosis factor α [TNFα] and IL-6),19,20 chemokines (IL-8 and CC chemokine ligand 2),21 adhesion molecules (vascular cell adhesion molecule 1 [VCAM-1] and intercellular adhesion molecule 1 [ICAM-1]),22 and other inflammatory regulators (nitric oxide synthase type 2, C8orf4 [TC1], and cyclooxygenase type 2)23 through interacting with nuclear factor-κB (NF-κB) signaling and Toll-like receptor–mediated signaling.21,23,24 In inflammatory lung diseases, suppression of Wnt/β-catenin signaling is observed in COPD25 and asthma patients,20,26 contributing to their dysfunctional epithelial repair. Inhibition of Wnt/β-catenin signaling is also reported in mechanical ventilation (MV)-insulted and Streptococcus pneumoniae–infected lungs.27,28 Elevation of the Wnt antagonist secreted frizzled-related protein 1 (sFRP1) has recently been discovered to be substantially responsible for the inhibition of Wnt/β-catenin signaling in experimental emphysema.29 Thus, it is important to explore the emerging roles of Wnt/β-catenin signaling in the pathogenesis of ALI/ARDS.
In the current study, we identified platelet-derived Dickkopf-1 (Dkk1) as a major Wnt antagonist capable of the suppression of Wnt/β-catenin signaling in alveolar epithelial cells (AECs). Administration of Wnt3a or neutralization of Dkk1 significantly reduced ICAM-1/VCAM-1–mediated neutrophil trafficking during acute lung inflammation. By targeting their promoters, Wnt/β-catenin signaling negatively regulated ICAM-1 and VCAM-1 expression in AECs. Our results indicate a novel function of Wnt/β-catenin signaling in AECs and a distinctive communication between platelets and the alveolar epithelium.
Materials and methods
Animals
Male C57BL/6N mice (6-8 weeks of age) and male Sprague-Dawley rats (180-200 g) were purchased from The Jackson Laboratory and Charles River, respectively. All animal experimental procedures were reviewed and approved by the institutional animal care committee of Oklahoma State University.
Cell isolation and culture
The alveolar epithelial type I cells (AEC I) cell line E10 was a generous gift from Dr M. Williams (Boston University) and was cultured in CMRL-1066 medium containing 10% heat-inactivated fetal bovine serum and 2.5 mM L-Glutamax. Primary alveolar epithelial type II cells (AEC II, >90%) were isolated from mice as described previously.30 AEC II were seeded in 12-well culture plates at 1 × 106 cells per well in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. AEC II were cultured for 4 to 5 days to allow them to transdifferentiate into AEC I–like cells. AECs (33% AEC I and 66% AEC II) were isolated from mice as described previously.31 Rat AEC II cells were isolated as previously described.32
Murine 2-hit acute pulmonary inflammation model
A 2-hit acute lung inflammation model with a moderate dose of lipopolysaccharide (LPS) followed by a high tidal volume MV was adopted as described before.33 Mice were divided into 6 groups (n = 8 per group): (1) spontaneous breathing nonventilated control (Blank), (2) LPS in phosphate-buffered saline (PBS) with MV (LM), (3) LPS in control-conditioned medium with MV (LM + Con_CM), (4) LPS in Wnt3a-conditioned medium with MV (LM + Wnt3a_CM), (5) LPS and mouse immunoglobulin G2b (IgG2b) in PBS with MV (LM + control IgG), and (6) LPS and anti-Dkk1 antibody in PBS with MV (LM + Anti-Dkk1). The mice were intratracheally instilled with LPS (Escherichia coli serotype 0111:B4, Sigma-Aldrich; 0.5 µg/g bodyweight, 1.5 µL/g) or 10× conditioned medium (1 µL/g). Sixty minutes later, the mice were ventilated on a small animal ventilator with the following settings: respiratory rate = 125 breaths per minute; inspiratory and expiratory ratio = 1:2; tidal volume = 12 mL/kg body weight; positive end expiratory pressure = 3 cm of H2O and an inspired oxygen fraction of 0.21 for 150 minutes.
Murine models of bacterial and influenza viral pneumonia
Mice were intranasally inoculated with Escherichia coli (strain 19138 from ATCC, 700 000 colony-forming units per mouse) or H1N1 influenza virus A/Puerto Rico/8/1934 (250 plaque-forming units (pfu) per mouse; ATCC). The animals were sacrificed on days 0 to 7 postinfection and the lung tissue and blood were collected.
Rat model of hyperoxia-induced ALI
Rats were exposed to >95% oxygen in a sealed Plexiglas chamber for 48, 60, or 72 hours as previously described.34
ELISA
Dkk1, soluble VCAM-1 (sVCAM-1), IL-6, and TNFα in BAL or plasma were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturers’ instructions (Quantikine mouse sVCAM-1 and Dkk1 immunoassay [R&D Systems] and mouse IL-6, TNFα ELISA Ready-SET-Go [eBioscience]).
Platelet isolation and stimulation
Platelets were isolated from mice as described previously with some modifications.8 The blood was collected into 3.8% sodium citrate, pH 7.4 (2:1, volume) by cardiac puncture, and centrifuged at 250g for 10 minutes to obtain the platelet-rich plasma (PRP). For platelet stimulation, PRP was treated with 100 μM thrombin receptor activating peptide (SFLLRN; Sigma-Aldrich) for the indicated times and then centrifuged at 657g for 7 minutes. The supernatant was collected as plasma and the platelet pellet was also collected. For platelet releasate collection, the PRP was centrifuged at 657g for 7 minutes to pellet the platelets. The platelets were then resuspended in Tyrode-HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer and stimulated with 0.1 U/mL thrombin (Sigma-Aldrich) for 90 minutes. The platelet releasate was collected by centrifugation at 2500g for 10 minutes at 4°C.
Cell-based Dkk1-binding assay
The binding of Dkk1 to cultured mouse AECs was assayed using an ELISA protocol modified from a previous report.35 Freshly isolated mouse AEC II cells were cultured on a fibronectin-coated 96-well plate to 100% confluence. Recombinant mouse Dkk1 (R&D Systems) was added into wells at different doses for 12 hours. The cells were fixed, washed with PBS containing 0.05% Tween 20, and incubated with anti-mouse Dkk1 (1:200; Abcam) mouse monoclonal antibody for 2 hours at room temperature. After being washed again, the cells were incubated with horseradish peroxidase–conjugated goat anti-mouse IgG for 1 hour. The cells were washed again and a tetramethylbenzidine substrate solution was added into the wells and color developed in 30 minutes. The intensity of the color was then measured at 450 nm.
Dual-luciferase assay
To measure promoter activity, E10 cells were seeded in 96-well plastic plates. After reaching ∼85% confluence, the cells were transfected with 100 ng/well pGL3_phVCAM-1, pGL3_phICAM-1 (Switchgear Genomics) or pGL3 (Promega) along with 2 ng/well phRL-TK with or without P65_pcDNA3.0 (Addgene) for the indicated time using Lipofectamine 2000. To measure NF-κB signaling activity, E10 cells were transfected with 100 ng/well pNFκB_luc (Promega) plasmid along with 2 ng/well phRL-TK with or without P65_pcDNA3.0 (Addgene) for the indicated times. For the TOP/FOPFLASH assay, E10 cells were transfected with 100 ng/well TOPflash or FOPflash (Millipore) along with 2 ng/well phRL-TK for the indicated times. The dual-luciferase assay was performed using the Dual-Luciferase Reporter Assay System (Promega).
Flow cytometry
Fluorescence-activated cell sorter (FACS) analysis of freshly isolated platelets was performed to quantify the activity of platelets by detecting the surface expression of P-Selectin (CD62P) according to a previous report.36 Briefly, PRP was collected from mice, fixed in 1% paraformaldehyde for 10 minutes, and suspended in FACS buffer. Then, 1 × 108 cells were stained with phycoerythrin (PE)-CD62P (eBioscience) and fluorescein isothiocyanate (FITC)-CD41 (platelet marker; eBioscience) antibodies or compatible IgGs (mouse IgG1κ for CD62P and rat IgG1κ for CD41) for 30 minutes at room temperature. FACS analysis was performed to identify the percentage of PE-CD62P–positive cells within the FITC-CD41–positive populations.
Cell adhesion assay
E10 cells were plated into 12-well plates and incubated with rat anti-mouse ICAM-1 (10 µg/mL, clone YN1/1.7.4; R&D Systems) or rat anti-mouse VCAM-1 antibodies (10 µg/mL, M/K-2.7; R&D Systems). Freshly isolated rat alveolar macrophages (AMϕ) or neutrophils were added onto the E10 cell monolayer and incubated for 30 minutes. Nonadherent cells were removed and the remaining cells were fixed in 4% paraformaldehyde and visualized by Wright-Giemsa staining. The attached AMϕ and neutrophils were counted.
Statistical analysis
All data are given as the mean ± standard error of the mean (SEM). For analysis of significant differences, 1-way analysis of variance (ANOVA) with post hoc Tukey test for multiple comparisons, 2-tailed Wilcoxon ranked test for nonparametric comparisons, or Student t test for 2 sets of data were applied. Significance was assumed when the P values were <.05.
Additional methods are included in supplemental Figures 1-11 (see supplemental Data available at the Blood Web site).
Results
Wnt/β-catenin signaling is inhibited during acute pulmonary inflammation
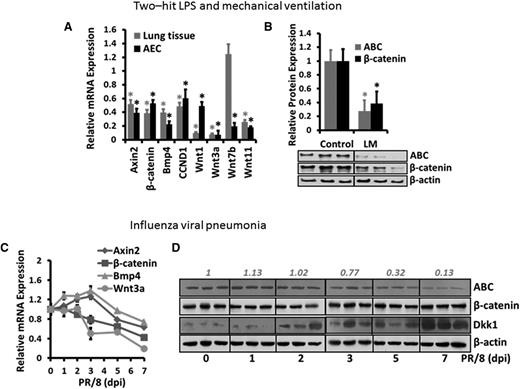
To determine whether Wnt/β-catenin signaling is modulated during acute lung inflammation, we quantified the messenger RNA (mRNA) expression of downstream genes of Wnt/β-catenin signaling and Wnt ligands in whole-lung homogenates and freshly isolated AECs from the lungs challenged with LM. mRNA expression of the downstream genes (Axin2, β-catenin, Bmp4, and CCND1) was significantly repressed in both the injured lungs and AECs (Figure 1A). The mRNA levels of canonical Wnt ligands (Wnt1, Wnt3a, Wnt7b, and Wnt11) were also remarkably reduced in LM-challenged lungs and AECs compared with the control, except that Wnt7b was slightly increased in injured lungs. The protein levels of the central signaling transducer β-catenin and the activated form of β-catenin (ABC) were both significantly decreased in LM-challenged lungs (Figure 1B). In another murine model of influenza viral pneumonia, mRNA expression of downstream genes (Axin2, β-catenin, and Bmp4) and a canonical Wnt ligand (Wnt3a) were also dramatically inhibited (Figure 1C). ABC was reduced by influenza virus infection (Figure 1D). However, the mRNA expression of canonical Wnt ligands (Wnt1, Wnt7b, and Wnt11) (supplemental Figure 1) and protein expression of β-catenin (Figure 1D) were not decreased in influenza viral pneumonia. Similarly, the downregulation of Wnt target genes, Wnt ligands, and β-catenin protein was also observed in hyperoxia-induced ALI (supplemental Figure 2).
Wnt/β-catenin signaling is inhibited during acute pulmonary inflammation. (A-B) Two-hit LPS and MV model: Mice were treated with LPS and MV (LM) or nonventilated (Control). The mRNA levels (A) of Wnt signaling components in the whole-lung tissues and freshly isolated AECs of LM-treated animals were analyzed by real-time PCR and plotted as a ratio against the control animals, with 18S rRNA as a reference gene. The protein levels (B) of ABC and total β-catenin in the whole-lung tissues of the control and LM-treated animals were determined by western blotting, with β-actin as an internal control. The density of the bands was quantified by ImageJ and normalized to β-actin. The results were expressed as a ratio of the control mice. Data are expressed as the mean ± SEM (n = 5 animals) and statistical significance was determined by Student t test. *P < .05 vs control. (C-D) Influenza viral pneumonia model: Mice were intranasally inoculated with H1N1 influenza A/PR/8/34 virus (250 pfu) and housed for 1 to 7 days (n = 3 per group). The mRNA levels (C) of Wnt signaling components in the whole-lung tissues were measured by real-time PCR and normalized to control animals (without infection) using 18S rRNA as a reference gene. The protein levels (D) of ABC, total β-catenin, and Dkk1 in whole-lung tissue of infected animals were determined by western blotting using β-actin as an internal control. The density of the ABC bands was quantified by ImageJ, normalized to control mice, and labeled on the top. PCR, polymerase chain reaction; rRNA, ribosomal RNA.
Wnt/β-catenin signaling is inhibited during acute pulmonary inflammation. (A-B) Two-hit LPS and MV model: Mice were treated with LPS and MV (LM) or nonventilated (Control). The mRNA levels (A) of Wnt signaling components in the whole-lung tissues and freshly isolated AECs of LM-treated animals were analyzed by real-time PCR and plotted as a ratio against the control animals, with 18S rRNA as a reference gene. The protein levels (B) of ABC and total β-catenin in the whole-lung tissues of the control and LM-treated animals were determined by western blotting, with β-actin as an internal control. The density of the bands was quantified by ImageJ and normalized to β-actin. The results were expressed as a ratio of the control mice. Data are expressed as the mean ± SEM (n = 5 animals) and statistical significance was determined by Student t test. *P < .05 vs control. (C-D) Influenza viral pneumonia model: Mice were intranasally inoculated with H1N1 influenza A/PR/8/34 virus (250 pfu) and housed for 1 to 7 days (n = 3 per group). The mRNA levels (C) of Wnt signaling components in the whole-lung tissues were measured by real-time PCR and normalized to control animals (without infection) using 18S rRNA as a reference gene. The protein levels (D) of ABC, total β-catenin, and Dkk1 in whole-lung tissue of infected animals were determined by western blotting using β-actin as an internal control. The density of the ABC bands was quantified by ImageJ, normalized to control mice, and labeled on the top. PCR, polymerase chain reaction; rRNA, ribosomal RNA.
Dkk1 is deposited in the lungs during acute lung inflammation
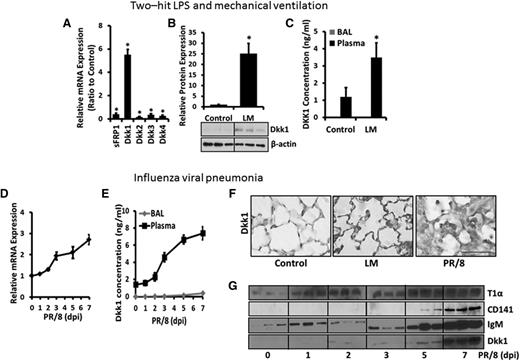
To identify the Wnt antagonist that is potentially responsible for this downregulation, we screened the mRNA expression of several Wnt antagonists (sFRP1 and Dkk1-4) in LM-challenged lungs and found that only Dkk1 was increased (Figure 2A). Dkk1 protein in the lung tissue was also upregulated by LM (Figure 2B). Furthermore, the mRNA (Figure 2D) and protein (Figure 1D) expression of Dkk1 were induced in influenza virus-infected lungs. Notably, Dkk1, which was reported to accumulate in the lungs of patients with idiopathic pulmonary fibrosis,37 was also significantly deposited in the alveoli of both the LM-challenged and the influenza virus-infected lungs (Figure 2F). However, Dkk1 mRNA in freshly isolated AECs (data not shown) and Dkk1 protein in the BAL of the LM-challenged and influenza virus-infected lungs (Figure 2C,E) were very low. However, Dkk1 in the plasma from both injured animals was remarkably increased compared with the control animals (Figure 2C,E). An increase in Dkk1 level in the BAL was observed during acute viral infection at day 7 compared with day 5 when endothelial cell damage and the disruption of the alveolar-vascular barrier occurred as evident by increases in CD141 and IgM (Figure 2G). Although both Dkk1 mRNA and protein were increased in the inflamed lungs, it is now recognized that platelets also contain functional mRNA.38 This finding indicated that the Dkk1 deposited in the alveoli likely originated from the circulating system although we cannot completely rule out the possibility that Dkk1 is generated from local lung cells.
Dkk1 is accumulated in the lung during acute pulmonary inflammation. (A-C) Two-hit LPS and MV model. (A) The mRNA expression of Wnt antagonists (sFRP1 and Dkk1-4) in the whole-lung tissues of LM-challenged and control mice was determined by real-time PCR and plotted as the fold change over control animals with 18S rRNA as a reference gene. (B) The protein levels of Dkk1 in the whole-lung tissues of control and LM-challenged animals were measured by western blotting using β-actin as a loading control. The density of the bands was quantified by ImageJ and normalized to β-actin. The result was expressed as a ratio to control animals. (C) The Dkk1 concentrations in the BAL and plasma of LM-challenged mice were measured by ELISA. (D-G) Influenza viral pneumonia model. (D) The mRNA level of Dkk1 in the whole-lung tissues of H1N1 influenza virus PR/8-infected mice for 0 to 7 days was analyzed by real-time PCR and normalized to the control mice with 18S rRNA as a reference gene. (E) The Dkk1 concentrations in the BAL and plasma of H1N1 influenza virus PR/8-infected mice were measured by ELISA. (F) Immunostaining of Dkk1 in the lungs of control, LM-challenged, and H1N1 influenza virus PR/8-infected (7 dpi) mice. Scale bar = 75 µm. (G) The protein levels of T1α (AEC I marker), CD141 (endothelial cell marker), IgM (epithelial-endothelial barrier damage marker), and Dkk1 in the BALs of H1N1 influenza virus PR/8-infected mice (day 0 to day 7) were determined by western blotting. Data are expressed as the mean ± SEM (n = 3-8 animals) and statistical significance is determined by the Student t test. *P < .05 vs control.
Dkk1 is accumulated in the lung during acute pulmonary inflammation. (A-C) Two-hit LPS and MV model. (A) The mRNA expression of Wnt antagonists (sFRP1 and Dkk1-4) in the whole-lung tissues of LM-challenged and control mice was determined by real-time PCR and plotted as the fold change over control animals with 18S rRNA as a reference gene. (B) The protein levels of Dkk1 in the whole-lung tissues of control and LM-challenged animals were measured by western blotting using β-actin as a loading control. The density of the bands was quantified by ImageJ and normalized to β-actin. The result was expressed as a ratio to control animals. (C) The Dkk1 concentrations in the BAL and plasma of LM-challenged mice were measured by ELISA. (D-G) Influenza viral pneumonia model. (D) The mRNA level of Dkk1 in the whole-lung tissues of H1N1 influenza virus PR/8-infected mice for 0 to 7 days was analyzed by real-time PCR and normalized to the control mice with 18S rRNA as a reference gene. (E) The Dkk1 concentrations in the BAL and plasma of H1N1 influenza virus PR/8-infected mice were measured by ELISA. (F) Immunostaining of Dkk1 in the lungs of control, LM-challenged, and H1N1 influenza virus PR/8-infected (7 dpi) mice. Scale bar = 75 µm. (G) The protein levels of T1α (AEC I marker), CD141 (endothelial cell marker), IgM (epithelial-endothelial barrier damage marker), and Dkk1 in the BALs of H1N1 influenza virus PR/8-infected mice (day 0 to day 7) were determined by western blotting. Data are expressed as the mean ± SEM (n = 3-8 animals) and statistical significance is determined by the Student t test. *P < .05 vs control.
Dkk1 is released from activated platelets and binds to AECs during acute pulmonary inflammation-associated thrombocytopenia
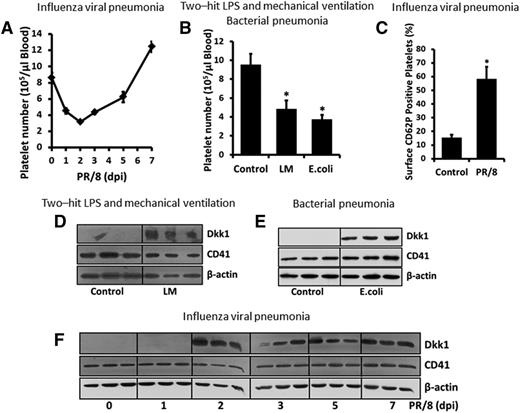
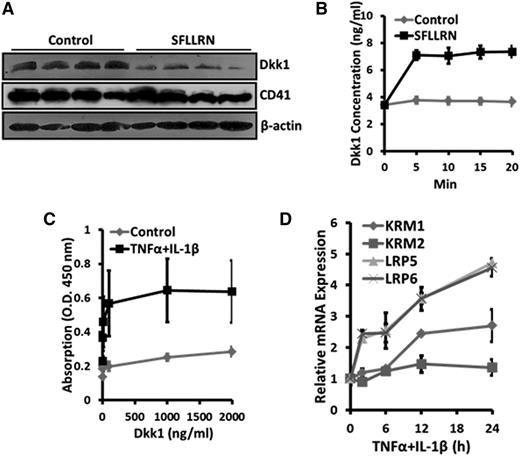
Platelets were recently identified as the major source of Dkk1 in the circulatory system.8 Thus, we first measured the number of circulating platelets in LM-challenged, live bacteria-infected, and influenza virus-infected animals. As we expected, there was a rapid and transient reduction in the number of circulating platelets, which represented the clinical condition of thrombocytopenia, in the animal model of influenza viral pneumonia (Figure 3A). In 2 other animal models of acute lung inflammation, the numbers of circulating platelets were also dramatically decreased (Figure 3B). Although the number of platelets was the lowest at 2 days postinfection, platelet activity was increased at the same time point, as shown by the increased surface expression of CD62P, the marker of platelet activation, during influenza virus infection (Figure 3C). Dkk1 protein levels were markedly elevated in the platelets isolated from 3 animal models of acute lung inflammation compared with control animals (Figure 3D-F). Dkk1 was very rapidly released from activated platelets upon stimulation of the thrombin receptor by SFLLRN (Figure 4A-B). To evaluate the potential paracrine regulation of Dkk1 from platelets on Wnt/β-catenin signaling in AECs, we measured the binding affinity of Dkk1 to AECs and found that the affinity was significantly increased upon TNFα/IL-1β stimulation (Figure 4C). TNFα/IL-1β treatment also upregulated the expression of the specific Dkk1 receptor Kermen-1 (KRM1) and the coreceptors for Wnt ligands (LRP5 and LRP6) (Figure 4D), suggesting that these receptors may contribute to the TNFα/IL-1β–induced increase of the binding of Dkk1 to AECs. The binding of Dkk1 to AECs repressed Wnt/β-catenin signaling (supplemental Figure 3A-B) as expected. Our results showed that Dkk1 released from platelets can be potentially used to manipulate Wnt/β-catenin signaling in AECs.
Dkk1 is induced in activated platelets during acute pulmonary inflammation-associated thrombocytopenia. (A-B) The circulating platelet count in H1N1 influenza virus PR/8-infected, LM-challenged, and live bacteria E coli–infected mice. (C) Platelets were isolated from H1N1 influenza virus PR/8-infected mice (2 dpi) and labeled with PE-CD62P and FITC-CD41 (1 μg/mL of each). Surface expression of CD62P (activation marker) was detected by FACS. (D-F) Protein levels of Dkk1 and CD41 (platelet marker) in circulating platelets in LM-challenged, live bacteria E coli–infected, and PR/8-infected mice were measured by western blot with β-actin as a loading control. Data shown are the mean ± SEM (n = 3-8 animals) and statistical significance is tested by the Student t test. *P < .01 vs control mice.
Dkk1 is induced in activated platelets during acute pulmonary inflammation-associated thrombocytopenia. (A-B) The circulating platelet count in H1N1 influenza virus PR/8-infected, LM-challenged, and live bacteria E coli–infected mice. (C) Platelets were isolated from H1N1 influenza virus PR/8-infected mice (2 dpi) and labeled with PE-CD62P and FITC-CD41 (1 μg/mL of each). Surface expression of CD62P (activation marker) was detected by FACS. (D-F) Protein levels of Dkk1 and CD41 (platelet marker) in circulating platelets in LM-challenged, live bacteria E coli–infected, and PR/8-infected mice were measured by western blot with β-actin as a loading control. Data shown are the mean ± SEM (n = 3-8 animals) and statistical significance is tested by the Student t test. *P < .01 vs control mice.
Dkk1 is released from activated platelets and binds to AECs during acute pulmonary inflammation. PRP, isolated from LM-challenged mice, was incubated with 100 μM SFLLRN for 20 minutes (A) or 5 to 20 minutes (B) and then centrifuged. The protein levels of Dkk1 and CD41 in the pellet were determined by western blotting (A) and the Dkk1 level in the supernatant was determined by ELISA (B). (C) Primary mouse AEC II was cultured for 4 days and then stimulated with TNFα and IL-1β (20 ng/mL of each) for 24 hours. Recombinant mouse Dkk1 was added to the cells at the indicated concentrations for 2 hours. Cell-based ELISA was performed to quantify the binding of Dkk1 to the stimulated AECs. (D) The mRNA levels of the Dkk1 receptors (KRM1, KRM2, LRP5, and LRP6) were analyzed by real-time PCR in the TNFα/IL-1β–stimulated AECs and normalized to control. Data are expressed as the mean ± SEM from 3 independent preparations.
Dkk1 is released from activated platelets and binds to AECs during acute pulmonary inflammation. PRP, isolated from LM-challenged mice, was incubated with 100 μM SFLLRN for 20 minutes (A) or 5 to 20 minutes (B) and then centrifuged. The protein levels of Dkk1 and CD41 in the pellet were determined by western blotting (A) and the Dkk1 level in the supernatant was determined by ELISA (B). (C) Primary mouse AEC II was cultured for 4 days and then stimulated with TNFα and IL-1β (20 ng/mL of each) for 24 hours. Recombinant mouse Dkk1 was added to the cells at the indicated concentrations for 2 hours. Cell-based ELISA was performed to quantify the binding of Dkk1 to the stimulated AECs. (D) The mRNA levels of the Dkk1 receptors (KRM1, KRM2, LRP5, and LRP6) were analyzed by real-time PCR in the TNFα/IL-1β–stimulated AECs and normalized to control. Data are expressed as the mean ± SEM from 3 independent preparations.
An antibody capable of neutralizing Dkk1 attenuates neutrophil infiltration during acute pulmonary inflammation
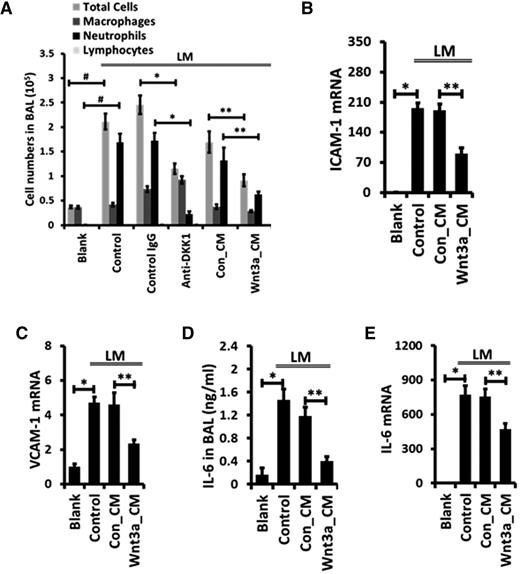
We investigated the effect of an antibody capable of neutralizing Dkk1 on the development of acute lung inflammation. Neutrophils were the major inflammatory cells recruited into the alveolar space in our 2-hit acute lung inflammation model, as revealed by differential immune cell count analysis of BAL (Figure 5A). Anti-Dkk1 antibody markedly inhibited neutrophil infiltration into the alveoli (Figure 5A). Whole-lung myeloperoxidase activity, a marker of neutrophils was also reduced by anti-Dkk1 antibody (supplemental Figure 4A). The results imply a potential role for Wnt/β-catenin signaling in regulating acute lung inflammation.
Intratracheal instillation of anti-Dkk1 antibody or Wnt3a attenuates neutrophil infiltration during LM-induced acute pulmonary inflammation. Mice were intratracheally instilled with LPS together with isotype-matched control IgG or function-blocking monoclonal antibody against Dkk1 (86 µg/mouse) and then mechanically ventilated (LM). Mice were also intratracheally coadministered with control (Con_CM) or Wnt3a-conditioned medium (Wnt3a_CM) along with LPS and ventilated as above. (A) Differential immune cell number in the BAL was counted. The mRNA levels of ICAM-1 (B), VCAM-1 (C), and IL-6 (E) in the whole-lung tissues were measured by real-time PCR and were normalized to blank control. (D) Concentrations of IL-6 in BAL were measured by ELISA. Data are expressed as the mean ± SEM (n = 5-8 animals). Statistical significance was determined by 1-way ANOVA analysis with post hoc Tukey test. *, **, and # represent P < .05.
Intratracheal instillation of anti-Dkk1 antibody or Wnt3a attenuates neutrophil infiltration during LM-induced acute pulmonary inflammation. Mice were intratracheally instilled with LPS together with isotype-matched control IgG or function-blocking monoclonal antibody against Dkk1 (86 µg/mouse) and then mechanically ventilated (LM). Mice were also intratracheally coadministered with control (Con_CM) or Wnt3a-conditioned medium (Wnt3a_CM) along with LPS and ventilated as above. (A) Differential immune cell number in the BAL was counted. The mRNA levels of ICAM-1 (B), VCAM-1 (C), and IL-6 (E) in the whole-lung tissues were measured by real-time PCR and were normalized to blank control. (D) Concentrations of IL-6 in BAL were measured by ELISA. Data are expressed as the mean ± SEM (n = 5-8 animals). Statistical significance was determined by 1-way ANOVA analysis with post hoc Tukey test. *, **, and # represent P < .05.
Administration of Wnt3a blocks ICAM-1/VCAM-1–mediated neutrophil infiltration during acute pulmonary inflammation
We activated Wnt/β-catenin signaling by administration of Wnt3a intratracheally and investigated its effect on the development of acute lung inflammation using 2-hit LPS and the MV mouse model. Compared with the control conditioned medium group (LM + Con_CM), Wnt3a (LM+Wnt3a_CM) significantly reduced the recruitment of neutrophils into the alveolar space, decreased the total cell number in the BAL, and attenuated myeloperoxidase activity in the whole-lung tissue (Figure 5A; supplemental Figure 4A).
The activation and transmigration of neutrophils are mediated by a series of adhesion molecules and chemotactic signaling.2 The mRNA expression of both ICAM-1 and VCAM-1 was significantly inhibited by Wnt3a in the LM-challenged lungs (Figure 5B-C). This impaired synthesis also led to a remarkable reduction in the sVCAM-1 concentration in the BAL of injured lungs (supplemental Figure 4B). However, mRNA levels of the major chemokines involved in neutrophil infiltration (KC, LIX, MCP-1, and MIP-2), the major cytokines (IL-1β, TNFα, and IL-10), and the growth factor, transforming growth factor β (TGFβ), were not changed by Wnt3a (supplemental Figure 4C).
Wnt/β-catenin signaling has been reported to limit the inflammatory functions of macrophages by reducing the release of TNFα and IL-6.19,20 Here, we observed that IL-6 in the BAL was markedly reduced after the administration of Wnt3a in LM-challenged lungs (Figure 5D), and this reduction was due to attenuated IL-6 mRNA synthesis by Wnt3a (Figure 5E). However, mRNA expression and the BAL level of TNFα showed no significant difference between Wnt3a-treated and control mice upon LM challenge (supplemental Figure 4C-D). Our results revealed a potentially novel mechanism that Wnt/β-catenin signaling utilizes to control neutrophil infiltration during acute inflammation.
Activation of Wnt/β-catenin signaling inhibits TNFα-induced ICAM-1/VCAM-1 expression
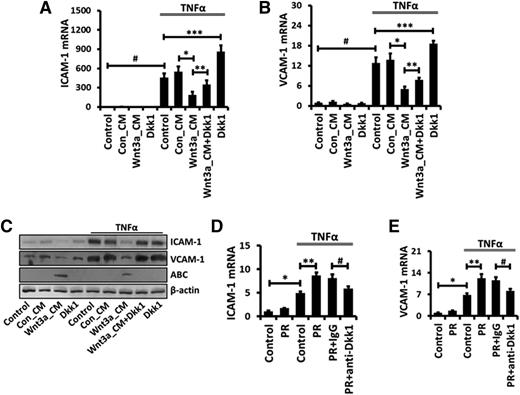
Both ICAM-1 and VCAM-1 are expressed in AECs and mediate the infiltration of immune cells during acute lung inflammation.35,39,40 To further elucidate the mechanism of Wnt/β-catenin signaling-mediated inhibition of ICAM-1 and VCAM-1 expression in injured lungs, we performed in vitro studies using E10 cells (a mouse AEC I line) and primary AEC I–like cells. TNFα stimulated the expression of ICAM-1 and VCAM-1 in a dose- and time-dependent manner in E10 cells (supplemental Figure 5A-D) and primary AEC I–like cells (supplemental Figure 5E-F). Wnt3a-conditional medium strongly reduced both ICAM-1 and VCAM-1 mRNA and protein expression stimulated by TNFα (Figure 6A-C). Recombinant Wnt3a protein had a similar effect (supplemental Figure 6). On the other hand, Dkk1 displayed the opposite effect on the induction of ICAM-1 and VCAM-1 expression. More importantly, platelet releasate augmented the TNFα-stimulated ICAM-1 and VCAM-1 expression in primary mouse AECs, and this augmentation was blocked by anti-Dkk1 antibody (Figure 6D-E). Our results further support a potential role for platelet-derived Dkk1 in controlling ICAM-1 and VCAM-1 expression during acute lung inflammation.
The platelet-derived Wnt antagonist Dkk1 is implicated in the stimulation of TNFα-mediated ICAM-1/VCAM-1 expression in AECs. E10 cells were incubated with 50% Con_CM or Wnt3a_CM together with 200 μM Dkk1 for 12 hours and then stimulated with 20 ng/mL TNFα for 2 hours (A-B) or 24 hours (C). Platelets were isolated from LM-challenged mice, resuspended in Tyrode-HEPES buffer, and treated with 0.1 U/mL thrombin for 90 minutes. Platelet releasate (PR) was collected and added to cultured primary mouse AECs together with isotype-matched control IgG or a function-blocking monoclonal antibody against Dkk1 (10 μg/mL) for 24 hours. After that, mouse AECs were stimulated with TNFα (20 ng/mL) for 2 hours (D-E). The mRNA levels of ICAM-1 (A,D) and VCAM-1 (B,E) were measured by real-time PCR using mouse 18S rRNA as a reference gene and normalized to control untreated cells. (C) Protein levels of ICAM-1, VCAM-1, and ABC were determined by western blotting with β-actin as an internal control. Data are expressed as the mean ± SEM from 3 independent preparations and statistical significance was tested with ANOVA analysis followed by post hoc Tukey test. *, **, ***, and # represent P < .05.
The platelet-derived Wnt antagonist Dkk1 is implicated in the stimulation of TNFα-mediated ICAM-1/VCAM-1 expression in AECs. E10 cells were incubated with 50% Con_CM or Wnt3a_CM together with 200 μM Dkk1 for 12 hours and then stimulated with 20 ng/mL TNFα for 2 hours (A-B) or 24 hours (C). Platelets were isolated from LM-challenged mice, resuspended in Tyrode-HEPES buffer, and treated with 0.1 U/mL thrombin for 90 minutes. Platelet releasate (PR) was collected and added to cultured primary mouse AECs together with isotype-matched control IgG or a function-blocking monoclonal antibody against Dkk1 (10 μg/mL) for 24 hours. After that, mouse AECs were stimulated with TNFα (20 ng/mL) for 2 hours (D-E). The mRNA levels of ICAM-1 (A,D) and VCAM-1 (B,E) were measured by real-time PCR using mouse 18S rRNA as a reference gene and normalized to control untreated cells. (C) Protein levels of ICAM-1, VCAM-1, and ABC were determined by western blotting with β-actin as an internal control. Data are expressed as the mean ± SEM from 3 independent preparations and statistical significance was tested with ANOVA analysis followed by post hoc Tukey test. *, **, ***, and # represent P < .05.
Because Wnt/β-catenin signaling normally regulates the promoter regions of its target genes,41 we examined the effect of Wnt3a on the promoter activities of ICAM-1 and VCAM-1. Wnt3a significantly repressed the TNFα-stimulated activity of the ICAM-1 and VCAM-1 promoters (supplemental Figure 7A-B). The promoters of ICAM-1 and VCAM-1 contain several NF-κB (P50/P65) binding sites, which are essential for their activation.42 We further explored whether Wnt3a can block NF-κB–mediated ICAM-1 and VCAM-1 expression. By overexpressing P65 in E10 cells, the promoter activities of both ICAM-1 and VCAM-1 were markedly induced, and this induction was inhibited by Wnt3a (supplemental Figure 6C-D). Our results suggest that both ICAM-1 and VCAM-1 are the target genes of Wnt/β-catenin signaling.
Functional crosstalk between NF-κB signaling and Wnt/β-catenin signaling has been reported to regulate the cell response to cytokines.21,23 We observed that Wnt3a inhibited NF-κB signaling activity in E10 cells stimulated by TNFα or P65 overexpression (supplemental Figure 8). However, we cannot detect the physical interaction between β-catenin and P50/P65, as previously reported21 (supplemental Figure 9). Wnt3a also did not affect the activation and nuclear translocation of P65 upon TNFα stimulation (supplemental Figure 10).
Activation of Wnt/β-catenin signaling attenuates ICAM-1/VCAM-1–mediated adhesion of alveolar macrophages and neutrophils to AECs
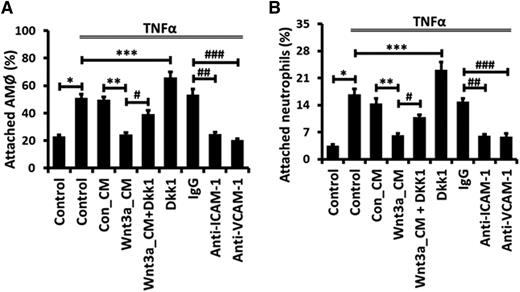
We further explored the functional significance of Wnt/β-catenin signaling-mediated inhibition of ICAM-1 and VCAM-1 expression. Both adhesion molecules are essential for neutrophil and macrophage adhesion35,40 and facilitate the transepithelial migration of monocytes upon influenza virus infection.39 We used an in vitro cell culture model to examine the adhesion of primary rat AMϕ or neutrophils to E10 cells. TNFα significantly increased the percentage of attached AMϕ or neutrophils to E10 cells (Figure 7). These increases were totally eliminated by immunoblockade with either anti-ICAM-1 or anti-VCAM-1 antibodies. Furthermore, Wnt3a dramatically reduced the adhesion of both types of immune cells to E10 cells. On the other hand, pretreatment with Dkk1 increased the attachment of AMϕ or neutrophils to E10 cells.
Wnt/β-catenin signaling reduces ICAM-1/VCAM-1–mediated adhesion of alveolar macrophages and neutrophils to AECs. E10 cells were incubated with 50% Con_CM or Wnt3a_CM together with 200 μM Dkk1 for 12 hours and then stimulated with 20 ng/mL TNFα for 24 hours, or E10 cells were incubated with control IgG or blocking mAbs against ICAM-1 or VCAM-1 for 30 minutes and then stimulated with 20 ng/mL TNFα for 24 hours. Next, adhesion of freshly isolated AMϕ or neutrophils to E10 cells was assayed. The values are presented as a percentage of attached AMϕ or neutrophils from 3 independent experiments and are tested for statistical significance by ANOVA analysis followed by the post hoc Tukey test. Data are expressed as the mean ± SEM. *, **, ***, #, ##, and ### represent P < .05.
Wnt/β-catenin signaling reduces ICAM-1/VCAM-1–mediated adhesion of alveolar macrophages and neutrophils to AECs. E10 cells were incubated with 50% Con_CM or Wnt3a_CM together with 200 μM Dkk1 for 12 hours and then stimulated with 20 ng/mL TNFα for 24 hours, or E10 cells were incubated with control IgG or blocking mAbs against ICAM-1 or VCAM-1 for 30 minutes and then stimulated with 20 ng/mL TNFα for 24 hours. Next, adhesion of freshly isolated AMϕ or neutrophils to E10 cells was assayed. The values are presented as a percentage of attached AMϕ or neutrophils from 3 independent experiments and are tested for statistical significance by ANOVA analysis followed by the post hoc Tukey test. Data are expressed as the mean ± SEM. *, **, ***, #, ##, and ### represent P < .05.
Discussion
In the 2-hit model of acute lung inflammation, repression of Wnt/β-catenin signaling in AECs was accompanied by the accumulation of the Wnt antagonist Dkk1 and the reduction of Wnt agonist synthesis. A similar phenomenon was also noticed in influenza virus pneumonia, bacterial pneumonia, and hyperoxic ALI. Our observations are consistent with the previous reports of suppression of Wnt/β-catenin signaling in inflammatory lung diseases.20,25-29 On the other hand, upregulation of Wnt/β-catenin signaling has been demonstrated in the resolution stage of ALI.43,44 Wnt/β-catenin signaling is activated in AEC II and is essential for alveolar epithelial repair following acute injury.13 Notably, transepithelial migration of neutrophils also activates Wnt/β-catenin signaling in AEC II and accelerates the process of repair.12 Activation of Wnt/β-catenin signaling in AEC is likely initialized by Wnt7b,45 which has been shown to activate Wnt/β-catenin signaling in airway ciliated epithelial cells following injury.14 All of these studies indicate a dynamic, complicated, and subtle response of Wnt/β-catenin signaling in inflammatory lung diseases.
sFRP1 is the only Wnt antagonist reported to be augmented in inflammatory lung diseases, including hyperoxic ALI and emphysema.29 Recent evidence has already pointed to an important role for Dkk1 in the development of inflammatory diseases such as atherosclerosis, arthritis, and pulmonary fibrosis by controlling fracture repair and pulmonary epithelial homeostasis.8,16,37 However, it is unclear whether the increased level of Dkk1 actively modulates these inflammatory diseases or is instead a consequence of the inflammatory process.46 Our current study suggests a profound role for elevated Dkk1 and the subsequent repression of Wnt/β-catenin signaling in the pathoprogression of acute lung inflammation by regulating ICAM-1/VCAM-1–mediated immune cell infiltration. As a potential therapeutic target, Dkk1 is also developed as a serum biomarker of inflammatory diseases and cancer patients.47,48 Neutralization therapy targeting Dkk1 has also shed light on patients with osteolytic disease.46,49 Here, we discovered the elevation of Dkk1 in both the BAL and the plasma in animal models of acute lung inflammation. If the same scenario can be confirmed in human patients, using Dkk1 neutralization therapy to limit acute pulmonary inflammation could reveal a safer method compared with that using Wnt3a to interfere with Wnt/β-catenin signaling activity in the lung because it avoids the aberrant activation of Wnt/β-catenin signaling, which could lead to the fibroproliferation of AECs43 and eventually pulmonary fibrosis or carcinoma.50,51
Acute lung inflammation is associated with increased platelet activation in the bloodstream and sequestration in the pulmonary vascular beds.3,7,52 The classic contribution of platelets to the progression of ALI/ARDS is their unique property of aggregation with neutrophils or monocytes, amplifying the inflammatory response, and disrupting the barrier function of endothelial cells.4,7,10 Blocking of the platelet-leukocyte interaction,53 platelet depletion,54,55 and antiplatelet therapy56,57 is protective in ALI/ARDS. Depletion of platelets reduces neutrophil recruitments into the lung in various ALI models including LPS-, acid-, transfusion- or sepsis-induced ALI.53-55 Thrombopoietin is a cytokine that regulates platelet production. The mice with the thrombopoietin receptor deficiency are protected from acid-induced ALI.54 As the major source of Dkk1 in the blood,8 platelets are likely the contributor to the Dkk1 in the alveolar space, considering the big difference in Dkk1 levels between the BAL and plasma. In addition to Dkk1, other platelet products such as regulated on activation normal T expressed and secreted (RANTES) and CD40 ligand are found in the alveolar space.37,58,59
Wnt3a reduces TNFα release in Mycobacterium-infected macrophages.19 Knockdown of β-catenin in macrophages increases LPS-induced IL-6 expression.20 Thus, macrophages may also contribute to the phenotypes we observed in our animal models. The interaction and relative contribution of macrophages and AECs to acute lung inflammation remain to be determined.
For the first time, we reported a specific and functional communication between platelets and AECs in inflammatory lung diseases (supplemental Figure 11). Manipulation of the function and life cycle of platelets using anticoagulants60,61 or platelet-stimulating agents62 could potentially be used to modulate Wnt/β-catenin signaling activity in patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Mary Williams (Boston University) for providing E10 cells with the permission of Dr Al Malkinson (University of Colorado). Hamster anti-mouse T1α antibody developed by Dr Andrew Farr (University of Washington) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development (NICHD) and maintained by the University of Iowa. The pcDNA3.0-based P65 overexpression plasmid was constructed by Dr Stephen Smale (University of California, Los Angeles) and obtained from Addgene (Cambridge, MA).
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute under award number R01 HL116876, the National Institute of General Medical Sciences under award number P20GM103648 (Molecular Biology Core), the Oklahoma Center for the Advancement of Science and Technology (HR14-060), and the Lundberg-Kienlen endowment fund. E.H. was supported by National Institutes of Health T35 OD011186-19 Short-Term Research Training for Veterinary Students.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: Y.G., A.M., E.H., C.Z., D.S., and T.W. performed experiments; Y.G. analyzed results and made the figures; and L.L. and Y.G. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lin Liu, Department of Physiological Sciences, Oklahoma State University, 264 McElroy Hall, Stillwater, OK 74078; e-mail: lin.liu@okstate.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal