Key Points
CD32a antibodies induce thrombocytopenia and hypersensitivity reactions in FCGR2A mice.
Effector-deficient CD32a antibodies prevent IgG-induced thrombosis and shock in FCGR2A mice.
Abstract
The CD32a immunoglobulin G (IgG) receptor (Fcγ receptor IIa) is a potential therapeutic target for diseases in which IgG immune complexes (ICs) mediate inflammation, such as heparin-induced thrombocytopenia, rheumatoid arthritis, and systemic lupus erythematosus. Monoclonal antibodies (mAbs) are a promising strategy for treating such diseases. However, IV.3, perhaps the best characterized CD32a-blocking mAb, was recently shown to induce anaphylaxis in immunocompromised “3KO” mice. This anaphylactic reaction required a human CD32a transgene because mice lack an equivalent of this gene. The finding that IV.3 induces anaphylaxis in CD32a-transgenic mice was surprising because IV.3 had long been thought to lack the intrinsic capacity to trigger cellular activation via CD32a. Such an anaphylactic reaction would also limit potential therapeutic applications of IV.3. In the present study, we examine the molecular mechanisms by which IV.3 induces anaphylaxis. We now report that IV.3 induces anaphylaxis in immunocompetent CD32a-transgenic “FCGR2A” mice, along with the novel finding that IV.3 and 2 other well-characterized CD32a-blocking mAbs, AT-10 and MDE-8, also induce severe thrombocytopenia in FCGR2A mice. Using recombinant variants of these same mAbs, we show that IgG “Fc” effector function is necessary for the induction of anaphylaxis and thrombocytopenia in FCGR2A mice. Variants of these mAbs lacking the capacity to activate mouse IgG receptors not only failed to induce anaphylaxis or thrombocytopenia, but also very potently protected FCGR2A mice from near lethal doses of IgG ICs. Our findings show that effector-deficient IV.3, AT-10, and MDE-8 are promising candidates for developing therapeutic mAbs to treat CD32a-mediated diseases.
Introduction
CD32a (Fcγ receptor IIa [FcγRIIa]) is one of several Fcγ receptor (FcγR)-type antibody receptors which bind the immunoglobulin G (IgG) “Fc” effector region as a ligand.1 IgG that is either immobilized on cell surfaces or clustered into immune complexes (ICs) can thus engage CD32a to induce inflammatory reactions in neutrophils, monocytes, platelets, and other immune cells, and this is thought to contribute to conditions as diverse as heparin-induced thrombocytopenia (HIT),2 rheumatoid arthritis,1 systemic lupus erythematosus (SLE),1 immune thrombocytopenia (ITP),3 and autoimmune hemolytic anemia.4 Animal model studies of these and other disorders suggest that therapeutic targeting of CD32a should have wide-ranging clinical utility.1,5
Several strategies have been pursued to suppress CD32a-mediated inflammation, including development of small-molecule inhibitors and recombinant soluble CD32a.1 However, preclinical development of CD32a-blocking monoclonal antibodies (mAbs) is conspicuously lacking compared with mAbs targeting other human FcγRs, such as CD16 (FcγRIII) and CD64 (FcγRI), which have been tested in clinical trials.1 The absence of CD32a mAb human clinical trials may be due, in part, to a surprising in vivo reaction in mice to the most widely used and highly characterized CD32a mAb, IV.3. Because mice lack a gene orthologous to human CD32a, few preclinical reports have described the use of CD32a mAbs in mice transgenic (Tg) for human CD32a (ie, CD32a-Tg mice). In one study, however, IV.3 induced anaphylaxis in CD32a-Tg triple-knockout “3KO” mice, which led the authors to conclude that targeting CD32a with divalent mAbs such as IV.3 should not be envisioned for human therapy.6 IV.3-induced anaphylaxis is surprising because IV.3 does not appear to activate CD32a ex vivo.7 However, the mechanisms by which IV.3 induces anaphylaxis have yet to be defined, and the potential contribution to anaphylaxis by the 3 FcγRs “knocked-out” in 3KO mice (CD16, CD32b, CD64) remains unexamined.
In this study, we addressed questions that are crucial for preclinical CD32a mAb development. First, to better define the mechanisms of IV.3-induced anaphylaxis, we investigated the extent to which immediate systemic reactions to CD32a mAbs require Fc function because the immunologic classification of hypersensitivity reactions such as anaphylaxis is typically organized around categories of Fc interactions with immunoglobulin receptors. Second, we examined systemic reactions to IV.3 in FCGR2A mice, which are CD32a-Tg, but also, unlike 3KO mice, possess all native FcγRs, which could potentially exacerbate or mitigate IV.3-induced anaphylaxis. Third, we aimed to determine whether anaphylactic reactions are unique to IV.3 or can also be observed with other CD32a-blocking mAbs.
Here, we report that 3 unique and well-characterized CD32a-blocking mAbs induce type II hypersensitivity reactions (T2HRs) in FCGR2A mice. We also report the novel finding of severe thrombocytopenic reactions, coincident with systemic anaphylaxis, in CD32a mAb-treated FCGR2A mice. We found that (1) effector-deficient variants of the same mAbs can be administered to CD32a-Tg mice without apparent systemic reactions and (2) mice so treated were protected from potentially lethal doses of CD32a-activating ICs. These findings establish previously unrecognized therapeutic potential for effector-deficient CD32a-blocking mAbs, which may prove beneficial for treating several inflammatory conditions, including but not limited to HIT, rheumatoid arthritis, SLE, ITP, and autoimmune hemolytic anemia.
Methods
Reagents and antibodies
M90 monoclonal IgG1 mAb (anti-human CD40L) was protein G purified from hybridoma (ATCC HB-12055)-conditioned media into azide-free phosphate-buffered saline (PBS), as described.8 The following were purchased from commercial sources: soluble human CD40L (Peprotech), mouse CD32 mAbs IV.3 (StemCell Technologies) and mouse AT-10 (Serotec), F(ab′)2-goat anti-human IgG (minimal cross-reactivity with mouse IgG; Jackson Immunoresearch Laboratories), fluorescein isothiocyanate–labeled anti-mouse CD41, CD62P, and control flow cytometry mAbs (BD Biosciences), and Alexa488-labeled goat-anti-human IgG (Invitrogen).
CD32a mAbs
Mouse IV.3 mAb (ATCC hybridoma #HB-217) was: produced in CL1000-bioreactor flasks using ultra-low-IgG fetal bovine serum (Invitrogen) in Hybri-Care medium (46-X; ATCC); protein G or A purified from conditioned media; dialyzed in PBS without azide, preservatives, detergents, or surfactants; and either refrigerated (4°C) for immediate use or frozen (−80°C) for later use. Frozen mAbs were thawed rapidly, clarified by centrifugation (>16 000g), refrigerated until used, and afterward discarded. IV.3 solutions were acceptably low in endotoxin levels (<1 U/mL, chromogenic limulus amebocyte lysate endotoxin assay; GenScript) and free of aggregates as assessed by dynamic light scattering (DLS; supplemental Figure 1, see supplemental Data available at the Blood Web site). IV.3 was deglycosylated with Endo S (NEB), which was then removed by chitin magnetic beads (NEB), followed by dialysis in PBS. For certain experiments, aglycosyl-IV.3 was labeled with AlexaFluor488. Antibody batches were verified for purity by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and concentration by IgG-calibrated Bradford assay. Prior to injection, all CD32a mAbs were validated functionally ex vivo for lack of apparent intrinsic CD32a-activating activity and for expected CD32a-blocking capacity (see supplemental Methods and supplemental Figures 1-9).
Recombinant IV.3 mAbs were made using modified versions of a published method.9 Briefly, complementary DNA synthesized from IV.3 hybridoma cell RNA was cloned into pFUSE antibody expression plasmids designed for generating mAbs having mouse variable light (VL) or heavy (VH) regions fused to human κ light or human IgG1 or human IgG2 heavy chain constant regions, respectively (InVivoGen). IgG1 and IgG2 plasmids were further modified by site-directed mutagenesis (Agilent Technologies) for point mutation (E269R and N297A) effector-deficient variants of chimeric mouse-human IV.3 (cIV.3). To ensure correct N-terminal variable regions, cIV.3 VH and VL sequences were derived from IV.3 hybridoma RNA using 5′ primers specific to the leader (L1 exon) sequences of the murine IGHV9-1 and IGKV2-137 germ line genes. cIV.3 VL and VH sequences were then codon optimized, regenerated synthetically (Eurofins), and ligated into pcDNA3.4 (Invitrogen) for higher mAb yield following transient transfection into Expi293F cells (Invitrogen). mAbs were purified from Expi293F-conditioned media as in the previous paragraph. Chimeric mouse-human AT-10 (cAT-10) and human MDE-8 mAbs were similarly prepared from codon-optimized variable region synthetic DNAs cloned into pcDNA3.4. This strategy allowed production of CD32a mAbs derived from clones IV.3,10 AT-10,11 and MDE-84 as IgG1 or IgG2 effector competent (native) or effector-deficient (E269R, N297A) variants. The phrase “CD32a mAbs” designates antibodies that bind CD32a as antigen irrespective of possible binding to CD32b. These CD32a mAbs localized specifically to platelets (supplemental Figure 2), lacked apparent intrinsic CD32a-activating activity ex vivo (supplemental Figure 3), and prevented platelet CD32a activation by immune complexes (supplemental Figure 4-6).
CD32a-Tg mice
Mice transgenic for human CD32a (“FCGR2A” mice3 ) were obtained from The Jackson Laboratory, bred on a C57BL6/J background, and housed at the University of Central Florida (UCF) Transgenic Animal Facility. All FCGR2A mice were genotyped (modified The Jackson Laboratory protocol) and used for experiments when 8- to 20 weeks old. All procedures were approved by the UCF Institutional Animal Care and Use Committee.
Counting of mouse platelets by flow cytometry
For counting platelets, blood (70 µL) was drawn from the retro-orbital plexus of isoflurane-anesthetized mice using heparin-coated capillary tubes and transferred to citrate anticoagulant (1:6 ratio). Platelets, identified by forward/side scatter and CD41 positivity, were counted by flow cytometry (FACSCanto II; BD Biosciences). Platelet counts before mAb injection indicated baselines (100%) per animal. Repeated blood draws may have caused small reductions in platelet counts for some animals.
CD32a mAb-induced systemic reactions
CD32a mAbs were IV injected (tail vein) into FCGR2A mice after measuring baseline central body temperature (CBT) by rectal thermometer and cytometric platelet counts. Hypothermia was identified by CBT reductions at 10, 20, or 30 minutes postinjection. Signs of apparent shock were assessed by observation of balance, mobility, and respiration, and were ranked as fatal, severe (complete immobility, apparent loss of consciousness), moderate (impaired mobility, irregular respiration), mild (lethargy, shallow respiration), or none, as described.8,12,13 Platelets were counted 30 minutes postinjection. Lungs were harvested en bloc for assessing thrombosis by hematoxylin-and-eosin (H&E) microscopy.8,12,13
STED microscopy
FCGR2A mouse platelet images were acquired on a Leica TCS SP8 gated STED microscope system using a 100×/1.40 oil objective, a white-light laser, LAS-AF v3.3x, and SVI Huygens Professional (for deconvolution) software. Agly-IV.3A488 was excited at 498 nm, emission was acquired from 506 to 579 nm, and for stimulated emission depletion, a 592 nm laser was used; phalloidinA555 excitation/emission settings were 554/559 to 631 nm.
Assessing IC-induced hypothermia, shock, thrombocytopenia, and thrombosis
Preformed ICs were made by mixing M90 (150 μg) with CD40L (50 μg) in PBS and incubating 5 minutes at room temperature. After measuring FCGR2A mouse baseline CBT and platelet counts, ICs were injected IV (200 μL bolus). Mice were then monitored (30 minutes) for signs of shock as described in “CD32a mAb-induced systemic reactions.” CBT was measured at 10, 20, and 30 minutes. Use of additional measures of anaphylaxis (eg, platelet-activating factor, histamine) were beyond the scope of this study. Thirty minutes post-IC injection, platelets were counted. Lungs were then harvested for H&E assessment of pulmonary thrombosis.
To assess potential protective effects of CD32a mAbs in IC-induced systemic reactions, FCGR2A mice were pretreated (IV) with effector-deficient CD32a mAbs (70-140 μg/mouse) 2 to 3 hours before IC injection. Other groups were pretreated (IV) with aspirin (15 mg/kg), bivalirudin (150 mg/kg), or eptifibatide (10 mg/kg).
Statistical analysis
Statistical analysis was conducted with SigmaPlot (version 12.5) or with SPSS (version 21.0). Except where otherwise noted, results are reported as mean ± standard deviation (SD), and differences between groups were analyzed by analysis of variance (ANOVA). P < .05 is considered statistically significant.
Results
IV.3 induces hypothermia and thrombocytopenia in FCGR2A mice
In unpublished pilot experiments, we observed apparent anaphylaxis in FCGR2A mice following IV injection of IV.3, acquired from a commercial source or prepared in-house, leading us to investigate mechanisms possibly involved. Following injection of various doses of IV.3 into FCGR2A mice, we observed acute systemic reactions, including dose-dependent hypothermia (Figure 1A), which indicated anaphylaxis, and thrombocytopenia (Figure 1B), which has not been previously described. Intraperitoneal injection of IV.3 (70 µg/mouse; n = 6) failed to induce anaphylaxis in FCGR2A mice yet severely depleted platelets (supplemental Figure 7). IV.3 (70 µg/mouse) injection into wild-type (WT) mice (n = 3) did not induce hypothermia or thrombocytopenia (not shown), indicating that our IV.3 preparation lacked the capacity to trigger observable acute systemic reactions via native mouse FcγRs (CD16, CD32b, CD64, and FcγRIV) independently of CD32a.
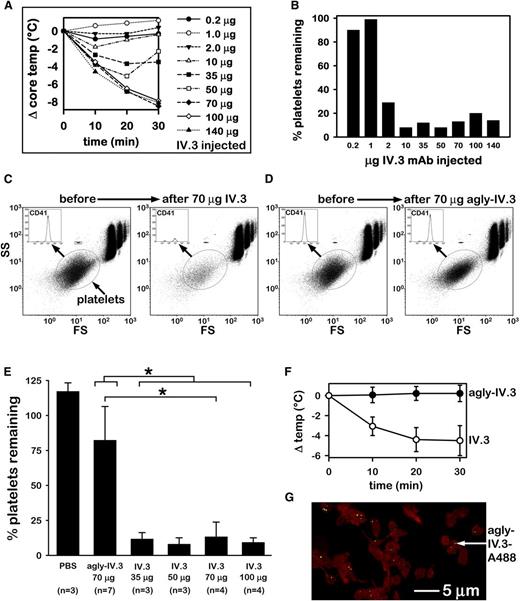
IV.3 induces Fc-mediated anaphylaxis and thrombocytopenia in FCGR2A mice. (A) Dose-dependent reduction in CBT induced by IV injection of mouse IV.3 in FCGR2A mice. (B) Platelet counts of mice after IV injection of IV.3. Animals represented are those of panel A (n = 1 per dose). (C) Representative flow cytometric analysis of CD41+ platelets (oval gate) in FCGR2A mouse whole blood before and 30 minutes after injection of 70 µg of IV.3. Cells in the upper right area are red blood cells (RBCs) and white blood cells (WBCs). Fluorescent beads (1.9 μm, Life Technologies; small boxed population above platelet oval) were used as a size reference and also to ensure consistent volume sampling between specimens. (D) Cytometric platelet count (as in panel C) of representative FCGR2A mouse before and 30 minutes after agly-IV.3 injection (70 μg). (E) Comparison of cytometric platelet counts (mean + SD) in whole blood from PBS, agly-IV.3, and IV.3-injected FCGR2A mice. *Significant difference (P < .05). A Kruskal-Wallis 1-way ANOVA on ranks identified a statistically significant difference (P = .003) in median values among all treatment groups. A t test showed that 70 µg of IV.3 differed significantly from 70 µg of agly-IV.3 (P < .001). A Mann-Whitney rank sum test showed that IV.3 (all mice injected with 35, 50, 70, or 100 µg of IV.3) differed significantly (P < .001) from 70 µg of agly-IV.3. (F) Comparison of body temperature change of mice injected with agly-IV.3 (70 µg/mouse; n = 7) or IV.3 (35 µg each; n = 4). Data in this panel (agly-IV.3 and IV.3) include mice from panel E. The 2 groups (agly-IV.3 vs IV.3) are statistically different based on a repeated measures test (F = 59.1; degrees of freedom = 1,9; P < .001). (G) High-resolution projection image of STED deconvolved microscopy sections (z stack; see supplemental Video) of FCGR2A mouse washed platelets (red) collected 24 hours after injection of 70 µg of agly-IV.3-A488 (green). Microscopic analysis shows agly-IV.3-A488 signal from objects ∼80-200nm in size and located in close physical proximity to platelet actin (labeled with phalloidin-Alexa 555). FS, forward scatter; SS, side scatter.
IV.3 induces Fc-mediated anaphylaxis and thrombocytopenia in FCGR2A mice. (A) Dose-dependent reduction in CBT induced by IV injection of mouse IV.3 in FCGR2A mice. (B) Platelet counts of mice after IV injection of IV.3. Animals represented are those of panel A (n = 1 per dose). (C) Representative flow cytometric analysis of CD41+ platelets (oval gate) in FCGR2A mouse whole blood before and 30 minutes after injection of 70 µg of IV.3. Cells in the upper right area are red blood cells (RBCs) and white blood cells (WBCs). Fluorescent beads (1.9 μm, Life Technologies; small boxed population above platelet oval) were used as a size reference and also to ensure consistent volume sampling between specimens. (D) Cytometric platelet count (as in panel C) of representative FCGR2A mouse before and 30 minutes after agly-IV.3 injection (70 μg). (E) Comparison of cytometric platelet counts (mean + SD) in whole blood from PBS, agly-IV.3, and IV.3-injected FCGR2A mice. *Significant difference (P < .05). A Kruskal-Wallis 1-way ANOVA on ranks identified a statistically significant difference (P = .003) in median values among all treatment groups. A t test showed that 70 µg of IV.3 differed significantly from 70 µg of agly-IV.3 (P < .001). A Mann-Whitney rank sum test showed that IV.3 (all mice injected with 35, 50, 70, or 100 µg of IV.3) differed significantly (P < .001) from 70 µg of agly-IV.3. (F) Comparison of body temperature change of mice injected with agly-IV.3 (70 µg/mouse; n = 7) or IV.3 (35 µg each; n = 4). Data in this panel (agly-IV.3 and IV.3) include mice from panel E. The 2 groups (agly-IV.3 vs IV.3) are statistically different based on a repeated measures test (F = 59.1; degrees of freedom = 1,9; P < .001). (G) High-resolution projection image of STED deconvolved microscopy sections (z stack; see supplemental Video) of FCGR2A mouse washed platelets (red) collected 24 hours after injection of 70 µg of agly-IV.3-A488 (green). Microscopic analysis shows agly-IV.3-A488 signal from objects ∼80-200nm in size and located in close physical proximity to platelet actin (labeled with phalloidin-Alexa 555). FS, forward scatter; SS, side scatter.
IV.3 reportedly lacks the intrinsic capacity to trigger signal transduction from CD32a ex vivo, except when heavily clustered, for example, by goat anti-mouse IgG. However, no published study has established whether IV.3 immediately activates CD32a in vivo. Because CD32a is the only IgG receptor on platelets in humans14 and in FCGR2A mice, we reasoned that if IV.3 directly immediately activated CD32a in vivo, divalent IV.3-F(ab′)2 fragments should induce thrombocytopenia in FCGR2A mice, as did whole IV.3. However, we were unable to produce IV.3-F(ab′)2 fragments by pepsin digestion, which others have also reported.15,16
We therefore deglycosylated IV.3 to generate agly-IV.3. Deglycosylation of asparagine at position 297 of the IgG Fc region dramatically reduces the capacity of IgG to activate FcγRs or complement C1q.17,18 Aglycosylated mAbs also exhibit long terminal half-life (t1/2) in rodents.19 Agly-IV.3 did not induce but rather fully blocked CD32a-mediated aggregation of washed human platelets, similarly as did undigested IV.3 (supplemental Figure 5). However, unlike IV.3 (Figure 1C), IV injected agly-IV.3 failed to induce thrombocytopenia in FCGR2A mice (Figure 1D). Whereas IV.3 doses ≥35 µg/mouse induced severe thrombocytopenia, 70 µg of agly-IV.3 did not (Figure 1E). Similarly, 35 µg of IV.3 induced hypothermia (ΔTmax = −4.5 ± 1.5°C) in FCGR2A mice, whereas 70 µg of agly-IV.3 did not (ΔTmax = +0.2 ± 0.8°C; Figure 1F, includes mice in Figure 1E). AlexaFluor488-labeled agly-IV.3 was detected on FCGR2A mouse platelets 24 hours after injection, as assessed by super-resolution STED microscopy (Figure 1G; supplemental Video). Confocal imaging of AlexaFluor488-positive platelets collected 30 minutes and 24 hours after IV injection of agly-IV.3A488 showed that platelets remained in the circulation and had acquired and retained fluorescent agly-IV.3 (supplemental Figure 9).
Together, the data in Figure 1 demonstrate that IV.3 induces anaphylaxis and thrombocytopenia in FCGR2A mice. Hypothermia following injection of such IgG antibodies into mice indicates passive systemic anaphylaxis.6 Our studies with agly-IV.3 show that anaphylaxis and thrombocytopenia in FCGR2A mice were mediated by the IV.3 effector region but also required CD32a because WT mice were unaffected by IV.3. These data indicate that IV.3 injected into FCGR2A mice did not immediately activate CD32a, but did specifically bind CD32a, thereby opsonizing platelets (and other cells that display CD32a), thus inducing systemic reactions. These data do not, however, indicate whether these systemic reactions are particular to IV.3 or may also be exhibited by other CD32a mAbs.
AT-10 and MDE-8 induce Fc-mediated hypothermia and thrombocytopenia in FCGR2A mice
We selected 2 other well-characterized clones for comparison with IV.3, with the rationale that if systemic reactions to IV.3 were indeed Fc mediated, other CD32a mAbs should be capable of inducing similar reactions. As originally described in 1991, AT-10 mAb binds CD32a with affinity (KD of 2 nM) comparable to that of IV.3 (KD of 5 nM).11,15 AT-10 has been extensively described in the relevant literature. MDE-8 mAb also binds human CD32a (KD of 9 nM) but comprises fully human amino acid sequences with well-defined CD32a-binding activity.4 Both AT-10 and MDE-8 mAbs were originally characterized for potential therapeutic use in vivo; however, neither has been reported to induce systemic reactions in FCGR2A mice. Importantly, AT-10 and MDE-8, like IV.3, potently prevent ligand binding to CD32a (eg, IgG-Fc-to-CD32a),4,11 yet lack the intrinsic capacity to induce CD32a-mediated platelet activation (supplemental Figures 3-5).
Because agly-IV.3 (Fc effector-deficient) mAb failed to induce systemic reactions in FCGR2A mice, the choice of Fc subclass (eg, IgG1 vs IgG2) as well as the manner in which comparable mAbs are rendered effector-deficient, represents a critical determinant of experimental design. In order to control the nature and extent of effector deficiency, we selected human IgG1 κ and human IgG2 κ as default formats for recombinant derivatives of IV.3, AT-10, and MDE-8. Effector competent (native) human IgG1 binds murine CD16, CD32b, CD64, and FcγRIV with relatively similar intermediate strengths, whereas native human IgG2 (like mouse IgG1) binds only CD16 and CD32b murine FcγRs.20 We selected 2 point mutations to render our recombinant mAbs effector-deficient: (1) mutation of glutamic acid to arginine at position 269 (E269R) of the IgG1 heavy chain ablates interactions with FcγRs while leaving interaction with complement C1q intact; (2) mutation of asparagine to alanine at position 297 (N297A) ablates both FcγR and C1q binding. In all, our recombinant mAbs for testing in FCGR2A mice included chimeric IV.3 (cIV.3), chimeric AT-10 (cAT-10), and human MDE-8. Each was produced in native and effector-deficient formats as IgG1, IgG1 E269R, IgG2, and IgG2 N297A intact whole IgG mAbs having human constant heavy and light chain sequences.
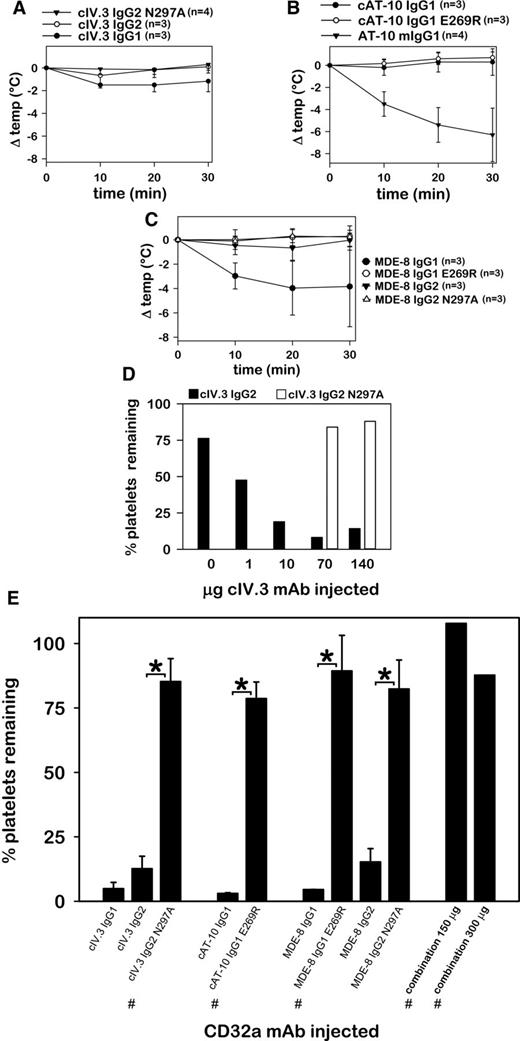
Hypothermia was induced in FCGR2A mice by cIV.3 IgG1 (ΔTmax = −1.0 ± 0.7°C; mean ± SD), by mouse AT-10 IgG1 (ΔTmax = −6.3 ± 2.4°C), and by MDE-8 IgG1 (ΔTmax = −4.0 ± 2.2°C), as shown in Figure 2A-C. Although cIV.3 IgG2 did not induce hypothermia (Figure 2A), this mAb caused a dose-dependent clearance of circulating platelets that was not observed with high doses of its effector-deficient variant, cIV.3 IgG2 N297A (Figure 2D). All CD32a mAbs in native format induced severe thrombocytopenia not observed in effector-deficient variants (Figure 2E). High dose injections of combined cAT-10 IgG1 E269R, cIV.3 IgG2 N297A, and MDE-8 IgG1 E269R (50 µg each mAb or 100 µg each mAb) failed to induce hypothermia or thrombocytopenia (Figure 2E). Pretreatment of FCGR2A mice with either MDE-8 IgG2 N297A (100 μg) or cAT-10 IgG1 E269R (140 μg) prevented thrombocytopenia and hypothermia induced by subsequent injection of 70 μg of IV.3 mouse IgG2b mAb (not shown). Together, the data in Figure 2 show that CD32a mAbs derived from 3 clone sources induce Fc-mediated systemic reactions in FCGR2A mice. Such reactions were not observed with effector-deficient variant mAbs.
IV.3, AT-10, and MDE-8 mAbs induce Fc-mediated hypothermia and thrombocytopenia. (A) Core body temperature changes in FCGR2A mice after IV injection of recombinant effector-competent (native) or effector-deficient chimeric IV.3 mAb. (B) CBT changes of mice injected with recombinant chimeric AT-10 in IgG1 (native) or IgG1 E269R (effector-deficient), or with mouse AT-10 (included for comparison). (C) Core body temperature changes following injection of native or effector-deficient MDE-8 mAbs. (D) Whole-blood platelet counts from FCGR2A mice following injection of different quantities (1 animal per dose) of native cIV.3 IgG2 or effector-deficient cIV.3 IgG2 N297A. (E) Bar chart showing the percentage of platelets remaining in circulation following IV injection of native and effector-deficient CD32a antibodies. The 3 antibody types marked with the # symbol were combined (50 µg each or 100 µg each) for high-dose combination injections, as designated. Unless otherwise noted (# symbol and panel D), each animal was injected with 70 to 140 µg of mAb. It should be noted that, due to insufficient yield, effector-deficient recombinant IV.3 (IgG1 E269R), as well as chimeric AT-10 (cAT-10) IgG2 and cAT-10 IgG2 N297A mAbs could not be extensively evaluated in FCGR2A mice.
IV.3, AT-10, and MDE-8 mAbs induce Fc-mediated hypothermia and thrombocytopenia. (A) Core body temperature changes in FCGR2A mice after IV injection of recombinant effector-competent (native) or effector-deficient chimeric IV.3 mAb. (B) CBT changes of mice injected with recombinant chimeric AT-10 in IgG1 (native) or IgG1 E269R (effector-deficient), or with mouse AT-10 (included for comparison). (C) Core body temperature changes following injection of native or effector-deficient MDE-8 mAbs. (D) Whole-blood platelet counts from FCGR2A mice following injection of different quantities (1 animal per dose) of native cIV.3 IgG2 or effector-deficient cIV.3 IgG2 N297A. (E) Bar chart showing the percentage of platelets remaining in circulation following IV injection of native and effector-deficient CD32a antibodies. The 3 antibody types marked with the # symbol were combined (50 µg each or 100 µg each) for high-dose combination injections, as designated. Unless otherwise noted (# symbol and panel D), each animal was injected with 70 to 140 µg of mAb. It should be noted that, due to insufficient yield, effector-deficient recombinant IV.3 (IgG1 E269R), as well as chimeric AT-10 (cAT-10) IgG2 and cAT-10 IgG2 N297A mAbs could not be extensively evaluated in FCGR2A mice.
Effector-deficient CD32a mAbs inhibit IgG IC-induced hypothermia and shock
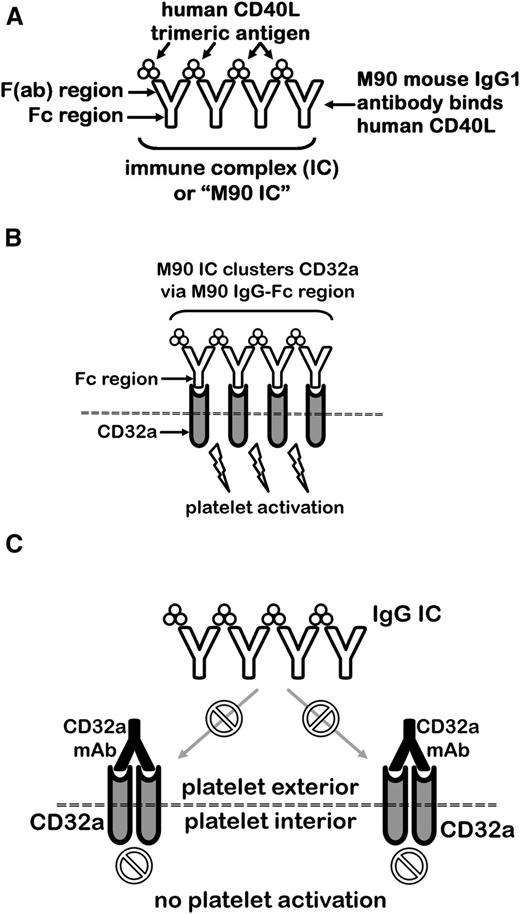
Having determined that acute systemic reactions induced by IV.3, AT-10, and MDE-8 could be prevented by ablating effector function, we sought to examine the capacity of our effector-deficient mAbs to inhibit CD32a activation by ICs in vivo. We previously demonstrated that the anti-CD40L mAb, M90, induces thrombocytopenia, thrombosis, hypothermia, and shock in FCGR2A mice, but not in littermate WT mice lacking CD32a.8,13 As illustrated in Figure 3A, M90 forms ICs with soluble human CD40L (M90 ICs), which are capable of triggering CD32a-dependent platelet activation. M90 IC-induced platelet activation (Figure 3B) is blocked by IV.3 (Figure 3B-C). IV.3-Fab-dependent binding to CD32a is >1000 times stronger than mouse IgG2b-Fc interactions with CD32a.10 Furthermore, human soluble CD40L does not efficiently bind mouse CD40, thereby limiting the possibility of unbound CD40L cytokine activity in FCGR2A mice.21 The M90 IC/FCGR2A mouse model is thus an excellent system for testing possible protective effects of CD32a mAbs in vivo.
Concept diagram for testing of recombinant CD32a mAbs in FCGR2A mice for protection against IC-mediated shock, thrombocytopenia, and thrombosis. (A) The CD40L antibody, M90, forms ICs with its trimeric ligand, CD40L. (B) The Fc regions of M90 ICs cluster platelet CD32a, causing robust activation. (C) CD32a-blocking mAbs such as IV.3, AT-10, and MDE-8 can prevent an IC from engaging CD32a.
Concept diagram for testing of recombinant CD32a mAbs in FCGR2A mice for protection against IC-mediated shock, thrombocytopenia, and thrombosis. (A) The CD40L antibody, M90, forms ICs with its trimeric ligand, CD40L. (B) The Fc regions of M90 ICs cluster platelet CD32a, causing robust activation. (C) CD32a-blocking mAbs such as IV.3, AT-10, and MDE-8 can prevent an IC from engaging CD32a.
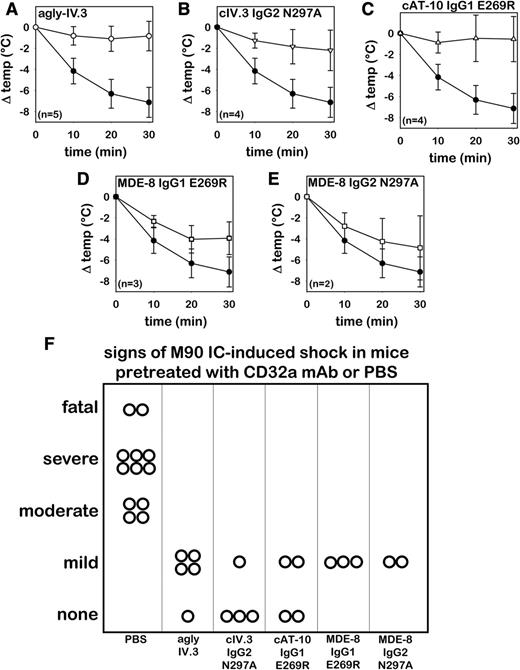
Effector-deficient CD32a mAbs, including agly-IV.3, inhibited hypothermia (Figure 4A-E) and signs of shock (Figure 4F) induced by M90 ICs. IV injection of Endo S (200 U) failed to protect against M90-IC challenge (n = 3 mice; data not shown), thus excluding the possibility that any residual Endo S might account for agly-IV.3 protection. Although MDE-8 derivatives prevented signs of shock comparably as did IV.3 and AT-10 derivatives, they provided only partial protection from M90 IC-induced hypothermia (Figure 4D-E). It is important to note that the hypothermia shown in these panels was induced by M90 ICs, not by the effector-deficient CD32a mAbs with which the mice were pretreated. M90 ICs induced moderate to severe shock and were fatal in 2 control FCGR2A mice.
Agly-IV.3 and recombinant effector-deficient CD32a mAbs inhibit M90 IC-induced shock. Core body temperature change in FCGR2A mice following IV injection of M90 ICs. Three hours prior to IC challenge, mice were pretreated with PBS (n = 12, solid circles) or with 1 of the following CD32a antibodies (open symbols): (A) Agly-IV.3 (n = 5); (B) cIV.3 IgG2 N297A (n = 4); (C) cAT-10 IgG1-E269R (n = 4); (D) MDE-8 IgG1-E269R (n = 3); and (E) MDE-8 IgG2-N297A (n = 2). (F) Signs of shock in control or CD32a-pretreated FCGR2A mice following M90 IC injection (same animals as panels A-E; each symbol represents 1 mouse).
Agly-IV.3 and recombinant effector-deficient CD32a mAbs inhibit M90 IC-induced shock. Core body temperature change in FCGR2A mice following IV injection of M90 ICs. Three hours prior to IC challenge, mice were pretreated with PBS (n = 12, solid circles) or with 1 of the following CD32a antibodies (open symbols): (A) Agly-IV.3 (n = 5); (B) cIV.3 IgG2 N297A (n = 4); (C) cAT-10 IgG1-E269R (n = 4); (D) MDE-8 IgG1-E269R (n = 3); and (E) MDE-8 IgG2-N297A (n = 2). (F) Signs of shock in control or CD32a-pretreated FCGR2A mice following M90 IC injection (same animals as panels A-E; each symbol represents 1 mouse).
Effector-deficient CD32a mAbs inhibit IgG immune complex-induced thrombocytopenia and thrombosis
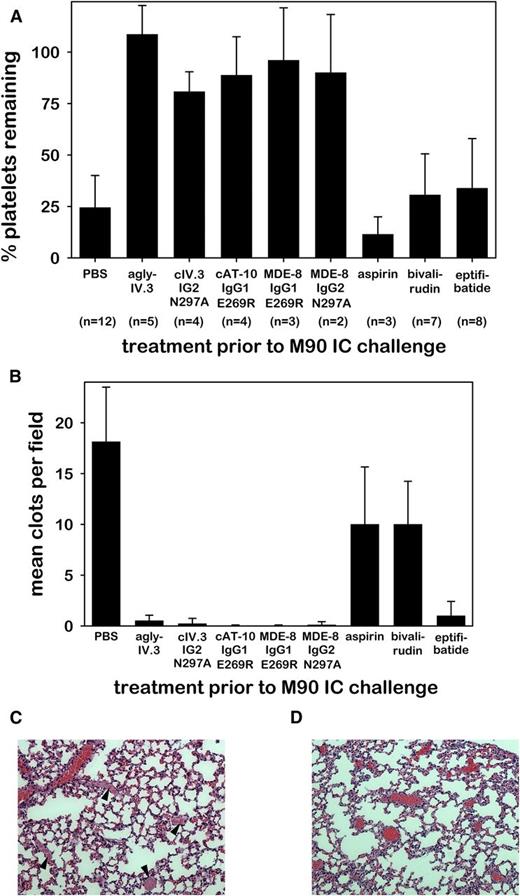
We recently reported that antiplatelet therapy with clopidogrel failed to prevent IC-induced thrombotic shock in FCGR2A mice.13 In contrast, we now show that effector-deficient CD32a mAbs very effectively protected FCGR2A mice from M90 IC-induced thrombocytopenia (Figure 5A) and thrombosis (Figure 5B), whereas aspirin and bivalirudin did not. The platelet GPIIb/IIIa inhibitor, eptifibatide, did inhibit IC-induced thrombosis, but had little effect on IC-induced thrombocytopenia (Figure 5B). Pervasive occlusive pulmonary thrombi were abundant in control mice (Figure 5C) but absent in mice pretreated with effector-deficient CD32a mAb (Figure 5D). These data also indicate that effector-deficient CD32a mAbs specifically bound CD32a in vivo.
Agly-IV.3 and recombinant effector-deficient CD32a mAbs inhibit M90 IC-induced thrombocytopenia and thrombosis. (A) Bar charts showing the percentage of platelets remaining in circulation of FCGR2A mice following the injection of M90 ICs. Three hours prior to IC challenge mice were pretreated with PBS or CD32a mAbs (as in Figure 4A-E) or with aspirin, bivalirudin, or eptifibatide. (B) Mean number of clots in the lung vasculature of animals pretreated as in panel A, obtained by counting intravascular clots in 5 fields per 3-μm section (mean + SD). The data in panels A and B represent the same animals. An all pairwise multiple comparison using the same Holm-Sidak 1-way ANOVA procedure for each data set (A-B) showed a difference between treatment groups (P < .001), and that each effector-deficient mAb (columns 2-6) differed significantly (P < .001) from PBS (column 1). (C) Representative H&E lung section (×200) of control mouse (from PBS group shown in panel B). Arrowheads identify occlusive intravascular thrombi. (D) Representative H&E lung section (×200) from cAT-10 IgG1 E269R pretreated FCGR2A mouse (from panel B) followed by anti-CD40L IC injection showing blood vessels containing erythrocytes (red) but lacking thrombi.
Agly-IV.3 and recombinant effector-deficient CD32a mAbs inhibit M90 IC-induced thrombocytopenia and thrombosis. (A) Bar charts showing the percentage of platelets remaining in circulation of FCGR2A mice following the injection of M90 ICs. Three hours prior to IC challenge mice were pretreated with PBS or CD32a mAbs (as in Figure 4A-E) or with aspirin, bivalirudin, or eptifibatide. (B) Mean number of clots in the lung vasculature of animals pretreated as in panel A, obtained by counting intravascular clots in 5 fields per 3-μm section (mean + SD). The data in panels A and B represent the same animals. An all pairwise multiple comparison using the same Holm-Sidak 1-way ANOVA procedure for each data set (A-B) showed a difference between treatment groups (P < .001), and that each effector-deficient mAb (columns 2-6) differed significantly (P < .001) from PBS (column 1). (C) Representative H&E lung section (×200) of control mouse (from PBS group shown in panel B). Arrowheads identify occlusive intravascular thrombi. (D) Representative H&E lung section (×200) from cAT-10 IgG1 E269R pretreated FCGR2A mouse (from panel B) followed by anti-CD40L IC injection showing blood vessels containing erythrocytes (red) but lacking thrombi.
Discussion
In this report, we show in FCGR2A mice that IV.3, AT-10, and MDE-8 mAbs induce hypothermia and thrombocytopenia not observed with effector-deficient variants of the same mAbs. Additionally, we show that these effector-deficient CD32a mAbs potently inhibit IC-induced shock and thrombosis. These findings must be considered in the context of the mouse model used. Several previously described models are relevant. Clynes and Ravetch showed that native mouse-activating FcγRs were pivotal to T2HRs induced by an IgG mAb that opsonized mouse platelets, leading to thrombocytopenia that was partially inhibited with the CD16/CD32b-blocking mAb, 2.4G2.22 Nieswandt and colleagues later showed that other platelet mAbs induced thrombocytopenia, with hypothermia inhibited by 2.4G2 mAb (where tested).23,24 These experimental ITP studies were conducted in mice lacking CD32a.
McKenzie and colleagues have shown that the addition of a human CD32a transgene, which places an IgG receptor on mouse platelets, increases the severity of reactions to platelet-opsonizing mAbs.3,25,26 In these FCGR2A mice, shock was exacerbated, rather than mitigated, by 2.4G2 mAb. Thrombosis, also, was observed in FCGR2A mice lacking native mouse FcγRs following treatment with a mAb that activated platelets via CD32a.25 FCGR2A mice, transgenic also for PF4, were later used to demonstrate thrombosis mechanistically similar to that of HIT in humans.27 We have since shown that M90 + CD40L ICs induce thrombocytopenia, hypothermia, shock, and thrombosis in FCGR2A mice.8,13 The relevance of these CD32a mouse models to human conditions is apparent, not only in the greater risk of thrombosis in HIT compared with ITP patients, but also in light of early CD40L mAb clinical trials, which were halted due to fatal thromboembolism in lupus patients.28 CD32a likely mediated such thrombosis.29
Hypothermia resulting from injection of a CD32a-activating mAb in FCGR2A mice was first reported by Taylor and colleagues in 2000.25 In 2005, a report by van Royen-Kerkhof et al showed that MDE-8 prevented hemolytic anemia in CD32a-Tg mice lacking functional CD16, CD64, and FcγRIV, but did not address the possibility of anaphylaxis had such FcγRs been present.4 More recently, IV.3 was surprisingly found to induce anaphylaxis in CD32a-Tg 3KO mice, which lack CD16, CD32b, and CD64 FcγRs.6 These researchers (with others) later reported that F(ab′)2 fragments of AT-10 and IV.3 potently inhibited CD32a-mediated arthritis in experimental mice.30 However, the in vivo use of whole IgG CD32a-blocking mAbs and their effects on platelets in the context of native FcγRs has not previously been described. Whole IgG mAbs are generally superior to F(ab′)2 fragments as therapeutics because they are less susceptible to degradation and have greater bioavailability. Also, whole CD32a-blocking mAbs may provide improved therapeutic specificity and reduced dosing compared with recombinant soluble CD32a.
In this report, we extend our previous work and that of others by identifying an in vivo mechanism of hypersensitivity reaction to CD32a-blocking whole IgG mAbs in CD32a-Tg mice also having the full repertoire of native mouse FcγRs. We describe novel data showing that IV.3, AT-10, and MDE-8 mAb derivatives can induce thrombocytopenia in FCGR2A mice. These reactions more closely resemble experimental ITP, which is classically a T2HR, than HIT, which is more strongly associated with thrombosis. Given that effector-deficient CD32a mAbs retain CD32a-blocking function in vivo, native CD32a mAbs likely bind CD32a in vivo, thereby opsonizing platelets while largely removing immediate function of the CD32a transgene product in FCGR2A mice (ie, functionally converting them to WT equivalents). Thromboembolism was not apparent in H&E-stained FCGR2A mouse lung sections from IV.3-injected mice (supplemental Figure 8). However, because IV.3 induces anaphylaxis in FCGR2A mice, the question of whether this and other CD32a mAbs might directly trigger CD32a-mediated cellular activation immediately after IV injection should be addressed with caution.
On the one hand, the observed clearance from the circulation of IV.3-opsonized platelets may have led to IV.3-Fc interactions with native mouse FcγRs and possibly subsequent CD32a-triggered degranulation in situ. On the other hand, effector-deficient CD32a mAbs failed to induce thrombosis or shock in FCGR2A mice. Because these mAbs do not induce anaphylaxis, our data suggest IV.3, AT-10, and MDE-8 do not directly activate platelets in vivo, as this would likely have caused thrombosis. Indeed, evidence of pulmonary thrombosis was not observed in effector-deficient CD32a mAb-treated mice injected with potentially lethal doses of M90 ICs. Thus, our findings demonstrate potent in vivo CD32a blocking by these mAbs.
Our study did not address the emerging biology of induced-inhibitory functions of CD32a,1,30 in which CD32a engagement may induce a bias toward inhibitory (eg, inhibitory immunoreceptor tyrosine-based activation motif [ITAMi]) rather than activating (eg, ITAM) cellular reactions. Future studies addressing whether AT-10, IV.3, or MDE-8 may induce CD32a–ITAMi function in platelets are warranted. In this study, we did not assess the capacity of our CD32a mAbs to suppress ongoing (chronic) IgG-mediated inflammation, which is clinically relevant and merits future investigation. We also did not address the question of how IV.3 induced release of anaphylactogens (platelet-activating factor, leukotrienes B4 and C4) variously from human monocytes and neutrophils.6 Because these cells display FcγRs other than CD32a, such reactions could potentially be Fc-mediated via non-CD32a FcγRs, but further studies with effector-deficient CD32a mAbs are needed on this matter. Similarly, our findings do not explain IV.3-induced passive cutaneous anaphylaxis because the mice used to demonstrate passive cutaneous anaphylaxis possessed mast cells having CD32a as the sole FcγR.6
Clearly, however, the presence of CD32a on platelets, the immunobiology of which was recently reviewed,31 fundamentally alters the biology of pathologic reactions to IgG antibodies. Nowhere is this more apparent than in the context of therapeutic mAbs, many of which are associated with hypersensitivity reactions (eg, infusion reaction, cytokine storm, anaphylaxis). Our data indicate that, when native FcγRs are present, anaphylaxis induced in FCGR2A mice by IV.3, AT-10, and MDE-8 is mechanistically Fc mediated. Effector-deficient variants of these mAbs may thus prove useful for therapy of conditions where CD32a participates in mediating disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Charles Hemphill and Jan Schroeder at Leica Microsystems for performing confocal and STED microscopy, Qun Huo at the Center for NanoScience Technology, University of Central Florida, for performing dynamic light scattering studies. The authors also thank Petronio Martins for technical assistance in antibody purification.
Authorship
Contribution: T.M. and A.A. designed the study, performed experiments, and wrote the paper; L.R.-C. designed and performed experiments and analyzed data; M.D. performed experiments, analyzed data, and wrote the paper; M.B. performed experiments and managed mouse colonies; H.D. performed histological analysis, performed experiments, and performed antibody purification; M.R.-A. helped with antibody production and experiments; and J.L.F. analyzed data and wrote the paper.
Conflict-of-interest disclosure: A patent application has been filed on subject matter described in this publication. The authors declare no competing financial interests.
Correspondence: Todd Meyer, Center for Thrombosis Research, Florida Hospital, 2566 Lee Rd, Winter Park, FL 32789; e-mail: todd.meyer@flhosp.org.