Key Points
IL-6 is dysregulated after experimental allogeneic SCT and promotes alloantigen-dependent Th17 expansion within the lung.
IL-6 is dysregulated in patients with IPS after clinical allogeneic SCT.
Abstract
Idiopathic pneumonia syndrome (IPS) is a relatively common, frequently fatal clinical entity, characterized by noninfectious acute lung inflammation following allogeneic stem cell transplantation (SCT), the mechanisms of which are unclear. In this study, we demonstrate that immune suppression with cyclosporin after SCT limits T-helper cell (Th) 1 differentiation and interferon-γ secretion by donor T cells, which is critical for inhibiting interleukin (IL)-6 generation from lung parenchyma during an alloimmune response. Thereafter, local IL-6 secretion induces donor alloantigen-specific Th17 cells to preferentially expand within the lung, and blockade of IL-17A or transplantation of grafts lacking the IL-17 receptor prevents disease. Studies using IL-6−/− recipients or IL-6 blockade demonstrate that IL-6 is the critical driver of donor Th17 differentiation within the lung. Importantly, IL-6 is also dysregulated in patients undergoing clinical SCT and is present at very high levels in the plasma of patients with IPS compared with SCT recipients without complications. Furthermore, at the time of diagnosis, plasma IL-6 levels were higher in a subset of IPS patients who were nonresponsive to steroids and anti-tumor necrosis factor therapy. In sum, pulmonary-derived IL-6 promotes IPS via the induction of Th17 differentiation, and strategies that target these cytokines represent logical therapeutic approaches for IPS.
Introduction
Allogeneic stem cell transplantation (alloSCT) is a curative treatment of most hematologic malignancies; however, the success of this treatment is limited due to major complications, principally graft-versus-host disease (GVHD). Acute GVHD affects the skin, liver, and gastrointestinal (GI) tract, is mediated by donor T cells within the transplanted graft, and is the main cause of mortality in these patients.1 Idiopathic pneumonia syndrome (IPS) is characterized by acute, noninfectious, lung inflammation that typically occurs within the first 100 days of SCT, is resistant to therapy, and is usually fatal.2,3 Whether IPS truly represents GVHD has been debated because of the lack of apoptosis in lung tissue that is the pathognomonic feature of GVHD in other target organs.4 We and others5,6 have demonstrated that interferon (IFN)-γ regulates the development and severity of IPS following SCT and that this requires signaling through nonhematopoietic cells. However, the mechanism and most importantly, the relevance to clinical IPS remain to be fully elucidated.
In this study, we demonstrate that interleukin (IL)-6 derived from lung parenchyma is critical to the development of donor T-helper (Th) 17 cell differentiation within the lung and this cytokine is negatively regulated by donor T-cell–derived IFN-γ. Furthermore, we demonstrate that the conditioning and immune suppression regimens used following clinical SCT generate an IFN-γ–deplete, IL-6–high environment conducive to severe pulmonary inflammation and confirm IL-17A as a logical therapeutic target.
Materials and methods
Mice
Female C57Bl/6 (referred to as B6.WT herein; H-2b), BALB/c.WT (H-2d), and B6D2F1 (H-2b/d) mice were purchased from the Animal Resources Centre (Perth, Western Australia, Australia). B6.IFN-γR−/− and BALB/c.IFN-γ−/− mice were purchased from the Jackson Laboratories (Bar Harbor, ME). BALB/c CD45.1 mice were obtained from the Peter MacCallum Cancer Centre (East Melbourne, Victoria, Australia). B6.IL-6−/− mice were kindly provided by S. Alexander (University of Sydney, New South Wales, Australia). BALB/c.IL-17RA−/− mice were obtained from Amgen Inc. (Seattle, WA). B6.IL-17-Cre and B6.Rosa-26-eYFP mice were kindly provided by B. Stockinger and crossed to generate B6.IL-17-eYFP fate map reporter mice.7 β-Actin-luciferase background TEa mice have been described (TEaluc+).8
alloSCT
Animal procedures were approved by the QIMR Berghofer Medical Research Institute’s Animal Ethics Committee. Recipient mice were transplanted and monitored daily as described previously.5,9,10 Briefly, total body irradiation (TBI) (137Cs source) was split into 2 doses and separated by 3 hours to minimize GI toxicity. Radiation doses were as follows: B6.WT, B6.IFN-γR−/−, B6.IL-6−/−, 1000cGy; B6D2F1 mice, 1100cGy unless otherwise stated. Recombinant human granulocyte colony-stimulating factor (G-CSF; Amgen Inc., Thousand Oaks, CA) was administered to donor mice subcutaneously (10 µg/dose per animal for 6 days).11 Mice were transplanted with either 25 × 106 T-cell replete or 20 × 106 T-cell deplete (TCD) G-CSF mobilized splenocytes. For bone marrow transplantation (BMT), mice were transplanted with 107 TCD BM and 1 × 106 splenic T cells. GVHD was assessed using established scoring systems12 and mice with clinical scores ≥6 were euthanized in accordance with institutional guidelines. Cyclosporin (CsA) (Novartis Pharma, Switzerland) was administered by intraperitoneal (IP) injection at the doses described.
Cytokine/cytokine receptor blockade
Rat anti-mouse IL-6R monoclonal antibody (mAb) (MR16-1, provided by Chugai Pharmaceutical Co, Japan) was administered IP at 500 μg/dose on day −1 and day +3 post-SCT as previously described.13 Rat anti-mouse IL-17A mAb (M210) was provided by Amgen Inc (Thousand Oaks, CA) and administered by IP injection at 100 μg/dose every alternate day starting at day 0. Rat anti-mouse IFN-γ mAb (XMG1.2) was produced in-house and administered at 500 ug/dose on day 0 and subsequently every 3 days thereafter. Rat IgG was purchased from Sigma-Aldrich (St. Louis, MO). IgG1 (Mac 49), IgG2a (Mac 4), and IgG2b (Mac 5) isotype control mAbs were produced in-house.
Patient samples
Ethics approval to undertake these studies was obtained from both the QIMR Berghofer Medical Research Institute and the Royal Brisbane and Womens’ Hospital Human Ethics Committees with written informed consent obtained from participating patients. Recipients were an unreported cohort of patients who received either a myeloablative conditioning regimen consisting of cyclophosphamide at 60 mg/kg per day for 2 days and 12 Gy of fractionated TBI given over 3 days, or a reduced intensity conditioning regimen consisting of fludarabine at 25 mg/m2 per day for 5 days (days −7 to −3) and melphalan at 120 mg/m2 on day −2. All patients received a T-cell replete G-CSF–mobilized peripheral blood stem-cell graft from HLA (A, B, C, DR, DQ)-matched or (1 or 2 Ag) mismatched related or unrelated donors. GVHD prophylaxis consisted of CsA and MTX on days +1 (15 mg/m2), 3, 6, and 11 (10 mg/m2). Peripheral blood was collected from patients prior to transplantation (day −7) and periodically posttransplant. Additional samples analyzed for plasma IL-6 levels were collected from patients with IPS who were enrolled on a separate clinical study conducted at the University of Michigan and the Dana-Farber Cancer Institute or on a multicenter study conducted jointly by the Children’s Oncology Group and the Pediatric Blood and Marrow Transplant Consortium. These trials were registered at www.clinicaltrials.gov as #NCT00141739 and #NCT00309907.
Statistical analysis
The Mann-Whitney U test was used for the statistical analysis of all animal and clinical data with the exception of the clinical cytokine profiles over time where the nonparametric Kruskal–Wallis multiple comparisons test was employed. P < .05 was considered statistically significant. Data are presented as mean ± SEM.
For further details, please see supplemental Methods, available on the Blood Web site.
Results
IL-6 is dysregulated after clinical SCT but IFN-γ is not
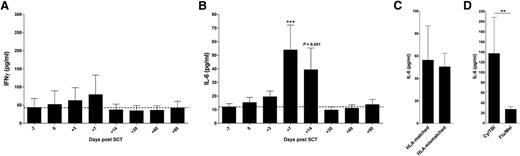
We and others have previously described the ability of IFN-γ to regulate lung injury after experimental alloSCT. In rodent models of alloSCT, which use conditioning with TBI alone, GVHD typically targets the GI tract within the context of a proinflammatory cytokine storm dominated by IFN-γ and tumor necrosis factor (TNF). Although IPS can be seen in rodent models early after alloSCT, it characteristically occurs 4 to 6 weeks after SCT at a time when IFN-γ is no longer detectable systemically.14-16 However, in the absence of IFN-γ, a cytokine known to regulate IPS, severe lung disease develops in the second week after SCT.5 To understand IFN-γ generation after clinical transplantation, we determined cytokine levels in a large unreported clinical cohort of SCT recipients (n = 50) receiving immune suppression with CsA and methotrexate (MTX) in Brisbane, Australia. The cumulative incidence of acute GVHD grades 2 to 4 by day 100 in these patients was 45% but IPS did not occur; thereby reflecting the low use of TBI-based conditioning in this cohort. Surprisingly, IFN-γ levels did not increase in the first 3 months in patients following SCT (Figure 1A), in contrast to murine BMT models without immune suppression. Other cytokines, including TNF, IL-4, and IL-17A were not elevated in the first 2 months post alloSCT in which IPS develops (data not shown). In contrast, IL-6 was increased, peaking a week after SCT (Figure 1B). The induction of IL-6 7 days after SCT was not associated with HLA-matching (Figure 1C), but was dependent on conditioning intensity (Figure 1D). The levels of IL-6 in patients at day 7 after SCT who subsequently developed acute GVHD grades 2 to 4 were 89 ± 46 pg/mL vs 26 ± 9 pg/mL in patients who developed no acute GVHD (P = .044), reflecting a relatively limited dynamic range.
IFN-γ secretion is suppressed whereas IL-6 is dysregulated after clinical SCT. Plasma (A) IFN-γ and (B) IL-6 levels were measured prior to SCT conditioning (day −7) and up to day 90 post-SCT while receiving calcineurin-based immune suppression (CsA or tacrolimus and MTX). IFN-γ levels after transplant (beyond day 0) are not significantly different to that of pre transplant (day −7). IL-6: ***P = .0007 vs day +7. (C) Plasma IL-6 levels in HLA-matched (n = 29) vs HLA-mismatched (n = 21) recipients at day +7 post-SCT. (D) Plasma IL-6 levels at day +7 post-SCT in recipients receiving either TBI (n = 12) vs reduced intensity conditioning (fludarabine/melphalan; n = 38). **P = .003.
IFN-γ secretion is suppressed whereas IL-6 is dysregulated after clinical SCT. Plasma (A) IFN-γ and (B) IL-6 levels were measured prior to SCT conditioning (day −7) and up to day 90 post-SCT while receiving calcineurin-based immune suppression (CsA or tacrolimus and MTX). IFN-γ levels after transplant (beyond day 0) are not significantly different to that of pre transplant (day −7). IL-6: ***P = .0007 vs day +7. (C) Plasma IL-6 levels in HLA-matched (n = 29) vs HLA-mismatched (n = 21) recipients at day +7 post-SCT. (D) Plasma IL-6 levels at day +7 post-SCT in recipients receiving either TBI (n = 12) vs reduced intensity conditioning (fludarabine/melphalan; n = 38). **P = .003.
CsA inhibits IFN-γ but not IL-6 dysregulation after experimental murine BMT
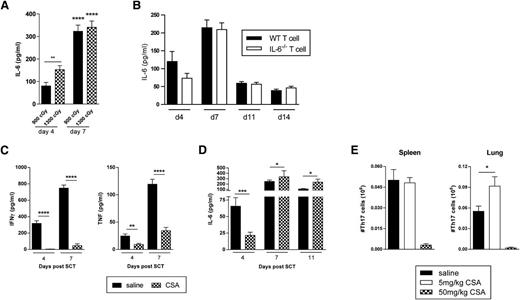
To understand the effect of the standard immune suppression used in clinical SCT on cytokine generation, we undertook murine allogeneic BMT experiments after TBI and in the presence or absence of GVHD prophylaxis with CsA. IL-6 dysregulation was exaggerated after high TBI doses early after transplant and these levels increased over time (Figure 2A). Furthermore, IL-6 dysregulation still occurred in the absence of any IL-6 secretion from donor T cells (Figure 2B), suggesting that standard immune suppression with T-cell targeted agents may have a limited effect on this cytokine. In the presence of CsA, the generation of systemic IFN-γ was prevented and TNF generation was attenuated (Figure 2C), consistent with the fact that these cytokines were derived from donor T cells.5,17 In contrast, effects on IL-6 levels were indeed limited and appeared to be enhanced in the second week of BMT by CsA administration (Figure 2D). Given that IL-6 promotes Th17 generation, we examined Th17 numbers in lung tissue following allogeneic BMT with or without immunosuppression. We performed experiments transplanting T cells from B6.IL-17eYFP fate map reporter mice into B6D2F1 recipients treated with CsA, and harvested spleen and lung tissue on day 7 after BMT. This demonstrated that Th17 differentiation, like Th1 differentiation, was inhibited early after BMT by CsA but was promoted at low doses, specifically within the lung (Figure 2E). Thus, CsA administration resulted in the IFN-γ deplete cytokine environment previously described as conducive to the development of IPS,5 while permitting high levels of IL-6 to be generated.
CsA effects on IFN-γ and IL-6 following experimental BMT. (A) IL-6 levels measured in sera of B6D2F1 recipients who received lethal irradiation doses of either 900 or 1300 cGy on day −1 and transplanted with B6.WT BM and splenic T cells on day 0. Data from 2 combined replicate experiments is shown, n = 10 per group. Day 4 vs 7: ****P < .0001; **P = .003. (B) Sera IL-6 levels over time in B6D2F1 recipients irradiated with 1100 cGy on day −1 and transplanted on day 0 with allo BMT grafts consisting of either WT or IL-6−/− T cells with B6.WT BM. Data from 2 combined replicate experiments is shown, n = 10 per group. (C) IFN-γ, TNF, or (D) IL-6 levels were measured in sera from B6D2F1 recipients treated with saline or CsA and transplanted with B6.WT BM and splenic T cells. CsA was administered at 50 mg/kg per dose daily for 7 days. Data from 3 to 7 combined replicate experiments is shown, n = 29 to 30 per group (day 4), 37 or 38 per group (day 7), and 11 to 16 per group (day 11). Day 4, saline vs CsA: ****P < .0001, IFN-γ; ***P = .005, IL-6; **P = .007, TNF. Day 7, saline vs CsA: ****P < .0001, IFN-γ and TNF; *P = .01, IL-6. Day 11, *P < .05. (E) Th17 B6.IL-17-eYFP fate map reporter cells were enumerated in spleen and lung harvested on day 7 from B6D2F1 recipients treated with saline or CsA, and transplanted with B6.WT BM and splenic T cells (n = 9 to 13 per group). CsA was administered at 5 or 50 mg/kg per dose daily for 7 days. Data from 3 combined replicate experiments is shown; *P = .04, saline vs CsA.
CsA effects on IFN-γ and IL-6 following experimental BMT. (A) IL-6 levels measured in sera of B6D2F1 recipients who received lethal irradiation doses of either 900 or 1300 cGy on day −1 and transplanted with B6.WT BM and splenic T cells on day 0. Data from 2 combined replicate experiments is shown, n = 10 per group. Day 4 vs 7: ****P < .0001; **P = .003. (B) Sera IL-6 levels over time in B6D2F1 recipients irradiated with 1100 cGy on day −1 and transplanted on day 0 with allo BMT grafts consisting of either WT or IL-6−/− T cells with B6.WT BM. Data from 2 combined replicate experiments is shown, n = 10 per group. (C) IFN-γ, TNF, or (D) IL-6 levels were measured in sera from B6D2F1 recipients treated with saline or CsA and transplanted with B6.WT BM and splenic T cells. CsA was administered at 50 mg/kg per dose daily for 7 days. Data from 3 to 7 combined replicate experiments is shown, n = 29 to 30 per group (day 4), 37 or 38 per group (day 7), and 11 to 16 per group (day 11). Day 4, saline vs CsA: ****P < .0001, IFN-γ; ***P = .005, IL-6; **P = .007, TNF. Day 7, saline vs CsA: ****P < .0001, IFN-γ and TNF; *P = .01, IL-6. Day 11, *P < .05. (E) Th17 B6.IL-17-eYFP fate map reporter cells were enumerated in spleen and lung harvested on day 7 from B6D2F1 recipients treated with saline or CsA, and transplanted with B6.WT BM and splenic T cells (n = 9 to 13 per group). CsA was administered at 5 or 50 mg/kg per dose daily for 7 days. Data from 3 combined replicate experiments is shown; *P = .04, saline vs CsA.
Lung parenchyma is an active source of cytokines, which favors Th17 cell accumulation in the lung during IPS
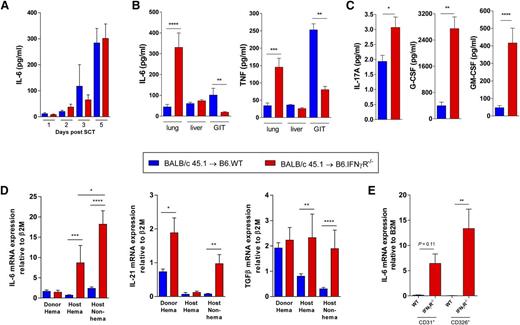
Given the dysregulation of IL-6 seen in patients and mice after alloSCT, we next examined the initial cytokine environment in allogeneic IFN-γR−/− SCT recipients who had not yet developed IPS (but were destined to do so) relative to wild-type (WT) recipients. We thus measured cytokine levels in serum and homogenized lung, liver, and GI tract tissue samples from these recipients 5 days after alloSCT. While serum IL-6 levels were comparable between IFN-γR−/− and WT recipients early after SCT (Figure 3A), soluble protein extracts from lung but not liver or GI tract tissue showed a marked increase in IL-6 and TNF levels at day 5 (Figure 3B). Concomitantly, IL-17A, G-CSF, and granulocyte macrophage (GM)-CSF were elevated in IFN-γR−/− recipients compared with WT mice (Figure 3C), a cytokine environment favoring both Th17 differentiation18 and mononuclear cell infiltration (GM-CSF, G-CSF) that is characteristic of IPS.5
IL-6 is generated by recipient lung epithelium and endothelium. G-CSF mobilized BALB/c.WT grafts were transplanted into lethally irradiated B6.WT and B6.IFN-γR−/− recipients (n = 5 to 12 per group combined from 2 experiments). Sera were obtained on days 1 to 5 and soluble protein extracts were prepared from homogenized lung, liver, and GI tract tissue harvested on day 5 post-SCT. (A) Serum IL-6 levels. (B) IL-6 and TNF levels measured in soluble protein extracts from lung, liver, and GI tract tissue. ****P < .0001; ***P < .001; **P < .01. (C) IL-17A, G-CSF, and GM-CSF levels measured in soluble protein extracts from lung tissue on day 5. ***P = .0008; G-CSF, **P = .002; *P = .011. (D) Lung tissue was harvested from B6.WT and B6.IFN-γR−/− recipients (n = 4 to 10 combined from 2 replicate experiments) of G-CSF mobilized BALB/c CD45.1+ grafts and cells were sort purified based on donor hematopoietic (CD45.1+), recipient hematopoietic (CD45.2+), or recipient nonhematopoietic (CD45.1negCD45.2neg) congenic markers at day 5 post-SCT. Cytokine expression was analyzed by real time quantitative reverse transcription PCR (qRT-PCR) for IL-6, TGFβ, and IL-21 transcripts. mRNA expression was determined relative to the expression of the housekeeping gene, β2M. IL-6: ***P = .0006, host hematopoietic WT vs IFN-γR−/−; ****P < .0001, host nonhematopoietic WT vs IFN-γR−/−; *P = .03, host hematopoietic IFN-γR−/− vs host nonhematopoietic IFN-γR−/−; IL-21: *P = .02, donor hematopoietic WT vs IFN-γR−/−; **P = .001, host nonhematopoietic WT vs IFN-γR−/−; TGFβ: **P = .003, host hematopoietic WT vs IFN-γR−/−; ****P < .0001, host nonhematopoietic WT vs IFN-γR−/−. (E) IL-6 mRNA expression quantified in sort purified pulmonary endothelial cells (CD45negCD31+) and epithelial cells (CD45negCD326+) from lung tissue harvested at day 5 post-SCT (n = 5 to 9 per group combined from 2 replicate experiments). **P = .007, epithelial cells, WT vs IFN-γR−/− recipients.
IL-6 is generated by recipient lung epithelium and endothelium. G-CSF mobilized BALB/c.WT grafts were transplanted into lethally irradiated B6.WT and B6.IFN-γR−/− recipients (n = 5 to 12 per group combined from 2 experiments). Sera were obtained on days 1 to 5 and soluble protein extracts were prepared from homogenized lung, liver, and GI tract tissue harvested on day 5 post-SCT. (A) Serum IL-6 levels. (B) IL-6 and TNF levels measured in soluble protein extracts from lung, liver, and GI tract tissue. ****P < .0001; ***P < .001; **P < .01. (C) IL-17A, G-CSF, and GM-CSF levels measured in soluble protein extracts from lung tissue on day 5. ***P = .0008; G-CSF, **P = .002; *P = .011. (D) Lung tissue was harvested from B6.WT and B6.IFN-γR−/− recipients (n = 4 to 10 combined from 2 replicate experiments) of G-CSF mobilized BALB/c CD45.1+ grafts and cells were sort purified based on donor hematopoietic (CD45.1+), recipient hematopoietic (CD45.2+), or recipient nonhematopoietic (CD45.1negCD45.2neg) congenic markers at day 5 post-SCT. Cytokine expression was analyzed by real time quantitative reverse transcription PCR (qRT-PCR) for IL-6, TGFβ, and IL-21 transcripts. mRNA expression was determined relative to the expression of the housekeeping gene, β2M. IL-6: ***P = .0006, host hematopoietic WT vs IFN-γR−/−; ****P < .0001, host nonhematopoietic WT vs IFN-γR−/−; *P = .03, host hematopoietic IFN-γR−/− vs host nonhematopoietic IFN-γR−/−; IL-21: *P = .02, donor hematopoietic WT vs IFN-γR−/−; **P = .001, host nonhematopoietic WT vs IFN-γR−/−; TGFβ: **P = .003, host hematopoietic WT vs IFN-γR−/−; ****P < .0001, host nonhematopoietic WT vs IFN-γR−/−. (E) IL-6 mRNA expression quantified in sort purified pulmonary endothelial cells (CD45negCD31+) and epithelial cells (CD45negCD326+) from lung tissue harvested at day 5 post-SCT (n = 5 to 9 per group combined from 2 replicate experiments). **P = .007, epithelial cells, WT vs IFN-γR−/− recipients.
We next sought to determine the source of IL-6 within the lung tissue posttransplant because our previous study suggested that the ability of IFN-γ to regulate IPS was mediated by effects on nonhematopoietic tissue.5 Using congenic markers, donor and host hematopoietic and nonhematopoietic cell populations were sorted from lung tissue at day 5 post-SCT and IL-6 messenger RNA (mRNA) expression levels were measured by qRT-PCR. IL-6 expression was largely limited to host tissue and was most highly expressed in the nonhematopoietic compartment within the lung of IFN-γR−/− recipients (Figure 3D). The levels of IL-6 mRNA in the host compartment were 10- to 20-fold that of IL-21 and transforming growth factor (TGF)-β (other cytokines critical to Th17 differentiation), and unlike IL-6, these cytokines were not restricted to host tissue, the known site of regulation by IFN-γ (Figure 3D). Moreover, IL-6 was highly transcribed predominantly in the pulmonary epithelium (CD45negCD326+), and to a lesser extent in the endothelium (CD45negCD31+), in the absence of IFN-γ signaling (Figure 3E). Taken together, these data demonstrate that the lung parenchyma represents an environment particularly suited to the induction of Th17 differentiation in the absence of IFN-γ signaling.
Given the role of IFN-γ in regulating the onset and severity of IPS5 and that of IL-6 in Th17 differentiation, we were interested in determining whether antigen-specific Th17 cells contributed to severe lung inflammation that occurs in the absence of IFN-γ signaling. We therefore analyzed the expression of IL-17A in the lung at this early time point (day 5) and found that IL-17A was detectable from both donor and recipient hematopoietic sources and that in the absence of IFN-γ signaling, the latter was significantly enhanced (Figure 4A). Since these data suggested an important source of recipient IL-17A, we analyzed the lungs of IL-17eYFP fate map reporter recipient mice. Cells from the IL-17eYFP fate map reporter mice express eYFP if they have produced IL-17A at any time, in the past and/or present.7 Interestingly, there was a significant population of IL-17A reporter cells present in the lungs of mice even before alloSCT that are predominantly (>70%) γ-δ T cells (Figure 4B). Intriguingly, 60% to 80% of all γ-δ (γδ) T-cell receptor+ (TCR+) T cells in the lung reported for IL-17A secretion early after alloSCT and these cells persisted for at least 5 days (Figure 4C). In contrast, <3% of αβTCR+ T cells in the lung reported for IL-17A over the same period (data not shown). Importantly, this IL-17A secreting γδ T-cell population persisted in increased numbers in the absence of IFN-γ signaling (Figure 4D-E).
The lung contains large numbers of preformed IL-17A secreting recipient γδ T cells. (A) G-CSF mobilized BALB/c.WT (CD45.1+) grafts were transplanted into lethally irradiated (CD45.2+) B6.WT and B6.IFN-γR−/− recipients (n = 8 to 10 per group combined from 2 experiments). Lung tissue was harvested and cells were sort purified based on donor hematopoietic (CD45.1+) or recipient hematopoietic (CD45.2+) congenic markers at day 5 post-SCT. IL-17A expression was analyzed by qRT-PCR and normalized to β2M. ****P < .0001; **P < .01. (B) G-CSF mobilized BALB/c.WT (CD45.1+) grafts were transplanted into B6.IL-17-eYFP fate map reporter recipients (n = 3 per group) and flow cytometry undertaken on lung cells before and after alloSCT. Representative plots demonstrating that 1% to 2% of lung cells report for IL-17A even prior to alloSCT (top panel) and that these cells are predominantly (72% to 86%) γδTCR+ T cells (middle panel). The majority of all γδTCR+ T cells (63% to 76%) report for IL-17A, even prior to alloSCT (bottom panel). (C) The recipient IL-17A reporter+ γδTCR+ T cells persist in the lung for at least 5 days after SCT. (D) Representative plots of recipient T cells in the lungs of B6.WT or B6.IFN-γR−/− recipients 3 days after alloSCT, and (E) total numbers (n = 5 per group), *P < .01.
The lung contains large numbers of preformed IL-17A secreting recipient γδ T cells. (A) G-CSF mobilized BALB/c.WT (CD45.1+) grafts were transplanted into lethally irradiated (CD45.2+) B6.WT and B6.IFN-γR−/− recipients (n = 8 to 10 per group combined from 2 experiments). Lung tissue was harvested and cells were sort purified based on donor hematopoietic (CD45.1+) or recipient hematopoietic (CD45.2+) congenic markers at day 5 post-SCT. IL-17A expression was analyzed by qRT-PCR and normalized to β2M. ****P < .0001; **P < .01. (B) G-CSF mobilized BALB/c.WT (CD45.1+) grafts were transplanted into B6.IL-17-eYFP fate map reporter recipients (n = 3 per group) and flow cytometry undertaken on lung cells before and after alloSCT. Representative plots demonstrating that 1% to 2% of lung cells report for IL-17A even prior to alloSCT (top panel) and that these cells are predominantly (72% to 86%) γδTCR+ T cells (middle panel). The majority of all γδTCR+ T cells (63% to 76%) report for IL-17A, even prior to alloSCT (bottom panel). (C) The recipient IL-17A reporter+ γδTCR+ T cells persist in the lung for at least 5 days after SCT. (D) Representative plots of recipient T cells in the lungs of B6.WT or B6.IFN-γR−/− recipients 3 days after alloSCT, and (E) total numbers (n = 5 per group), *P < .01.
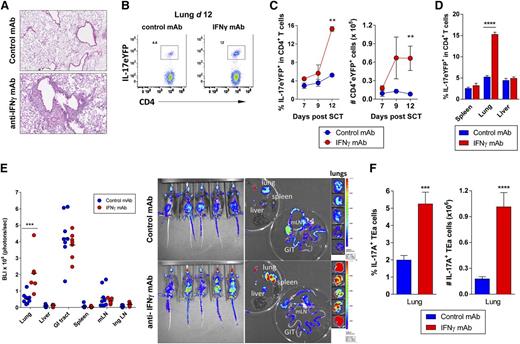
We next analyzed the relevance of the lung cytokine milieu to donor T-cell differentiation by transplanting G-CSF mobilized stem cell grafts from IL-17eYFP fate map reporter mice into B6D2F1 recipients with or without antibody blockade of IFN-γ signaling. alloSCT recipients receiving IFN-γ blockade developed respiratory distress (labored and rapid breathing) and histologic examination at day 12 demonstrated a dense pulmonary cellular infiltrate (Figure 5A). In this context, there was a marked increase in the proportion and absolute number of IL-17eYFP+ CD4+ donor T cells in the lungs of alloSCT recipients receiving IFN-γ blockade compared with control-treated mice (Figure 5B-C). Interestingly, this effect was restricted to lung tissue as elevated levels of IL-17eYFP+ CD4+ donor T cells were absent in other tissues examined (Figure 5D), suggesting a potential pathogenic role for Th17 cells specifically in the lung. To determine whether this response was alloantigen specific, donor grafts containing TEa luciferase+ transgenic CD4 T cells were transplanted into B6D2F1 recipients with or without IFN-γ blockade. The donor TEa T cells are specific for recipient I-Ed such that luciferase expression acts as a reporter for alloantigen-specific T-cell expansion. This revealed that in the absence of IFN-γ, T-cell expansion indeed occurs preferentially in the lung, specifically in response to recipient alloantigen (Figure 5E). In addition, Th17 differentiation was shown to be promoted in these allo-specific T cells in the absence of IFN-γ (Figure 5F). Thus, the lung represents a site in which IL-17 secreting donor αβTCR+ and recipient γδTCR+ T cells accumulate in the absence of IFN-γ signaling.
Th17 cells accumulate specifically in the lungs in the absence of IFN-γ signaling. Splenocytes from G-CSF mobilized B6.IL-17-eYFP fate map reporter donors (A-D) or TEa TCR transgenic donors (E-F) were transplanted into lethally irradiated B6D2F1 recipients (n = 3 to 6 per group). Control or anti–IFN-γ mAb (500 μg/dose) was administered every alternate day starting from day 0 and tissues harvested on days 7, 9, or 12. (A) Representative lung pathology in control vs IFN-γ blocked allograft recipients at day 12 post-SCT are shown. (B) Representative dot plots showing the frequency of donor CD4+ IL-17eYFP+ T cells in lung tissue at day 12 post-SCT with anti–IFN-γ or control mAb treatment. (C) Time course data showing accumulation of donor CD4+ IL-17eYFP+ T cells in the lung over time ± IFN-γ blockade. **P = .002, control vs IFN-γ treated groups at day 12. (D) Comparison of donor CD4+ IL-17eYFP+ T-cell frequencies at day 12 post-SCT in different tissues ± IFN-γ blockade. ****P < .0001, control vs IFN-γ treated groups. (E) Alloantigen-specific TEa transgenic T-cell expansion in whole body and individual organs at day 12 post-SCT. Quantified luciferin signals (left) and representative bioluminescence images (right) are shown (n = 7 to 9 per group). Light emission is presented as photons per second per cm2 per steer radiant (ph s−1 cm−2 sr−1). Total flux of organ is presented as photons per second (ph s−1). Lung BLI: ***P = .0003, control vs IFN-γ treated groups. (F) Frequency and absolute number of IL-17A+ TEa T cells in lung at day 12 post-SCT ± IFN-γ blockade (n = 10 per group). ***P = .0007, ****P < .0001, control vs IFN-γ treated groups. All data representative of at least 2 combined replicate experiments. Ing LN, inguinal lymph node; mLN = mesenteric lymph node.
Th17 cells accumulate specifically in the lungs in the absence of IFN-γ signaling. Splenocytes from G-CSF mobilized B6.IL-17-eYFP fate map reporter donors (A-D) or TEa TCR transgenic donors (E-F) were transplanted into lethally irradiated B6D2F1 recipients (n = 3 to 6 per group). Control or anti–IFN-γ mAb (500 μg/dose) was administered every alternate day starting from day 0 and tissues harvested on days 7, 9, or 12. (A) Representative lung pathology in control vs IFN-γ blocked allograft recipients at day 12 post-SCT are shown. (B) Representative dot plots showing the frequency of donor CD4+ IL-17eYFP+ T cells in lung tissue at day 12 post-SCT with anti–IFN-γ or control mAb treatment. (C) Time course data showing accumulation of donor CD4+ IL-17eYFP+ T cells in the lung over time ± IFN-γ blockade. **P = .002, control vs IFN-γ treated groups at day 12. (D) Comparison of donor CD4+ IL-17eYFP+ T-cell frequencies at day 12 post-SCT in different tissues ± IFN-γ blockade. ****P < .0001, control vs IFN-γ treated groups. (E) Alloantigen-specific TEa transgenic T-cell expansion in whole body and individual organs at day 12 post-SCT. Quantified luciferin signals (left) and representative bioluminescence images (right) are shown (n = 7 to 9 per group). Light emission is presented as photons per second per cm2 per steer radiant (ph s−1 cm−2 sr−1). Total flux of organ is presented as photons per second (ph s−1). Lung BLI: ***P = .0003, control vs IFN-γ treated groups. (F) Frequency and absolute number of IL-17A+ TEa T cells in lung at day 12 post-SCT ± IFN-γ blockade (n = 10 per group). ***P = .0007, ****P < .0001, control vs IFN-γ treated groups. All data representative of at least 2 combined replicate experiments. Ing LN, inguinal lymph node; mLN = mesenteric lymph node.
Recipient-derived IL-6 is critical for Th17 differentiation and development of IPS
We hypothesized that the high levels of recipient-derived IL-6 generated in the absence of IFN-γ signaling was essential for the differentiation of pathogenic donor Th17 cells within the lung and IPS. We therefore performed alloSCT experiments blocking IL-6 signaling systemically. A significant reduction in the absolute number of donor Th17 cells in lung and spleen was seen in the second week of alloSCT (Figure 6A) with donor CD4+ T cells isolated from lung tissue producing significantly less IL-17A in allograft recipients receiving anti–IL-6R inhibition (Figure 6B). IL-17A was produced predominantly by donor CD4+ T cells in the lung compared with other peripheral lymphoid organs, consistent with the IL-6 data demonstrating the same specificity for the lung. To ascertain the relationship between Th17 cells and TNF, an important cytokine in IPS, we analyzed TNF secretion from fate reported eYFP+ Th17 cells and their eYFPneg counterparts. Interestingly, Th17 cells were the highest TNF producers (Figure 6C). To confirm a role of host-derived IL-6 in promoting Th17 differentiation during IPS, we performed alloSCT using BALB/c.IFN-γ−/− stem cell grafts into either B6.WT or B6.IL-6−/− recipients. This revealed a significant reduction in the absolute number of donor Th17 cells in the lung in IL-6−/− recipients (Figure 6D-E). In addition, IL-17A production from donor CD4+ T cells isolated from the lung of IL-6−/− recipients was also significantly attenuated (Figure 6F). Interestingly, the lack of recipient-derived IL-6 also attenuated TNF production by donor T cells, consistent with the fact that Th17 cells produce significant quantities of this cytokine (Figure 6F). These observations were localized to lung tissue, as a similar reduction was not seen in the spleen of IL-6−/− recipients (Figures 6E-F). Thus, recipient-derived IL-6 is required for the preferential donor Th17 differentiation within the lung.
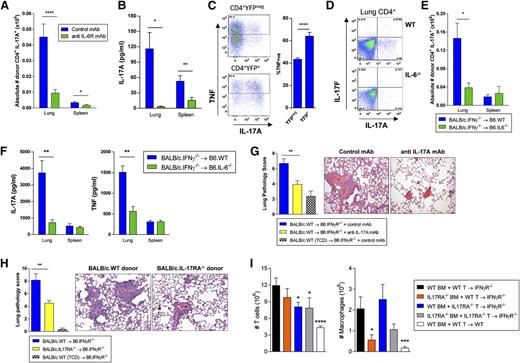
Recipient-derived IL-6 induces Th17-dependent IPS. Splenocytes from G-CSF mobilized BALB/c.WT donors were transplanted into B6.IFN-γR−/− recipients. Control or anti–IL-6R mAb (500 μg/dose) was administered on days −1 and +3. Lung and spleen tissue were harvested between days 7 to 11 after SCT. (A) Absolute number of donor CD4+ IL-17A+ T cells were calculated for both lung and spleen. Data from 2 replicate experiments is shown (n = 9 to 10 per group). Lung: ****P < .0001, Spleen: *P = .04, control vs anti–IL-6R mAb groups. (B) Donor CD4+ populations were isolated from lung and spleen tissues and cultured ex vivo with plate bound CD3/28 mAbs. IL-17A production in supernatants was measured using cytokine bead arrays. Data from 2 replicate experiments is shown ( n = 8 to 11 per group). Lung: *P = .03, Spleen: ****P < .0001, control vs anti–IL-6R mAb groups. (C) Splenocytes from G-CSF mobilized B6.IL-17-eYFP fate map reporter donors were transplanted into B6D2F1 recipients. Representative TNF production in fate reporter eYFP+ Th17 cells and eYFPneg T cells 7 days after SCT, mean ± SEM, n = 10 per group from 2 experiments, P < .0001. (D-F) G-CSF mobilized BALB/c.IFN-γ−/− grafts were transplanted into allogeneic B6.IL-6−/− recipients. (D) Representative plots of lung tissue showing intracellular IL-17A expression in donor CD4+ T cells in B6.WT or B6.IL-6−/− recipients are shown. (E) Absolute number of donor CD4+ IL-17A+ T cells in lung and spleen were calculated and data from 2 combined replicate experiments is shown (n = 9 to 12 per group). Lung: *P = .049, B6.WT vs B6.IL-6−/− recipients. (F) IL-17A and TNF production by ex vivo stimulated donor CD4+ populations isolated from lung and spleen (n = 5 to 7 per group). Lung: IL-17A, **P = .003; TNF, **P = .005, B6.WT vs B6.IL-6−/− recipients. (G) BALB/c.WT grafts (T-cell replete or TCD) were transplanted into B6.IFN-γR−/− recipients. Control or anti–IL-17A mAb (M210, 100 μg/dose) was administered every alternate day posttransplant starting from day 0 with lung tissue harvested on day 9. Data from 3 combined replicate experiments is shown (n = 13 to 16 per group). Lung pathology: **P = .002, control vs anti–IL-17A mAb treated groups. (H) BALB/c.WT or BALB/c.IL-17RA−/− donor grafts (T-cell replete or TCD) were transplanted into allogeneic B6.IFN-γR−/− recipients and lung tissue was harvested on days 9 to 11 post-SCT. Data from 3 combined replicate experiments is shown (n = 15 to 16 per T-cell replete group). Lung pathology: **P = .003, WT vs IL-17RA−/− grafts. (I) Mixing experiment utilizing grafts comprised of either BALB/c.IL-17RA−/− BM or T cells in combination with BALB/c.WT BM or T cells transplanted into allogeneic B6.IFN-γR−/− recipients. Lung tissue was harvested on days 7 and 8, digested, and T cells and macrophages enumerated. Data from 2 combined replicate experiments is shown (n = 8 to 10 per group). T cells: **** P < .0001, WT recipients (white bars) vs IFN-γR−/− recipients (black bars) of WT BM + WT T-cell grafts; *P = .01, WT BM + IL-17RA−/− T cell grafts (blue bars) vs WT BM + WT T-cell grafts (black bars) into IFN-γR−/− recipients; *P = .02, IL-17RA−/− BM + IL-17RA−/− T-cell grafts (gray bars) vs WT BM + WT T-cell grafts (black bars) into IFN-γR−/− recipients. Macrophages: ***P = .0004, WT recipients (white bars) vs IFN-γR−/− recipients (black bars) of WT BM + WT T-cell grafts; * P = .02, IL-17RA−/− BM + WT T-cell grafts (orange bars) vs WT BM + WT T-cell grafts (black bars) into IFN-γR−/− recipients.
Recipient-derived IL-6 induces Th17-dependent IPS. Splenocytes from G-CSF mobilized BALB/c.WT donors were transplanted into B6.IFN-γR−/− recipients. Control or anti–IL-6R mAb (500 μg/dose) was administered on days −1 and +3. Lung and spleen tissue were harvested between days 7 to 11 after SCT. (A) Absolute number of donor CD4+ IL-17A+ T cells were calculated for both lung and spleen. Data from 2 replicate experiments is shown (n = 9 to 10 per group). Lung: ****P < .0001, Spleen: *P = .04, control vs anti–IL-6R mAb groups. (B) Donor CD4+ populations were isolated from lung and spleen tissues and cultured ex vivo with plate bound CD3/28 mAbs. IL-17A production in supernatants was measured using cytokine bead arrays. Data from 2 replicate experiments is shown ( n = 8 to 11 per group). Lung: *P = .03, Spleen: ****P < .0001, control vs anti–IL-6R mAb groups. (C) Splenocytes from G-CSF mobilized B6.IL-17-eYFP fate map reporter donors were transplanted into B6D2F1 recipients. Representative TNF production in fate reporter eYFP+ Th17 cells and eYFPneg T cells 7 days after SCT, mean ± SEM, n = 10 per group from 2 experiments, P < .0001. (D-F) G-CSF mobilized BALB/c.IFN-γ−/− grafts were transplanted into allogeneic B6.IL-6−/− recipients. (D) Representative plots of lung tissue showing intracellular IL-17A expression in donor CD4+ T cells in B6.WT or B6.IL-6−/− recipients are shown. (E) Absolute number of donor CD4+ IL-17A+ T cells in lung and spleen were calculated and data from 2 combined replicate experiments is shown (n = 9 to 12 per group). Lung: *P = .049, B6.WT vs B6.IL-6−/− recipients. (F) IL-17A and TNF production by ex vivo stimulated donor CD4+ populations isolated from lung and spleen (n = 5 to 7 per group). Lung: IL-17A, **P = .003; TNF, **P = .005, B6.WT vs B6.IL-6−/− recipients. (G) BALB/c.WT grafts (T-cell replete or TCD) were transplanted into B6.IFN-γR−/− recipients. Control or anti–IL-17A mAb (M210, 100 μg/dose) was administered every alternate day posttransplant starting from day 0 with lung tissue harvested on day 9. Data from 3 combined replicate experiments is shown (n = 13 to 16 per group). Lung pathology: **P = .002, control vs anti–IL-17A mAb treated groups. (H) BALB/c.WT or BALB/c.IL-17RA−/− donor grafts (T-cell replete or TCD) were transplanted into allogeneic B6.IFN-γR−/− recipients and lung tissue was harvested on days 9 to 11 post-SCT. Data from 3 combined replicate experiments is shown (n = 15 to 16 per T-cell replete group). Lung pathology: **P = .003, WT vs IL-17RA−/− grafts. (I) Mixing experiment utilizing grafts comprised of either BALB/c.IL-17RA−/− BM or T cells in combination with BALB/c.WT BM or T cells transplanted into allogeneic B6.IFN-γR−/− recipients. Lung tissue was harvested on days 7 and 8, digested, and T cells and macrophages enumerated. Data from 2 combined replicate experiments is shown (n = 8 to 10 per group). T cells: **** P < .0001, WT recipients (white bars) vs IFN-γR−/− recipients (black bars) of WT BM + WT T-cell grafts; *P = .01, WT BM + IL-17RA−/− T cell grafts (blue bars) vs WT BM + WT T-cell grafts (black bars) into IFN-γR−/− recipients; *P = .02, IL-17RA−/− BM + IL-17RA−/− T-cell grafts (gray bars) vs WT BM + WT T-cell grafts (black bars) into IFN-γR−/− recipients. Macrophages: ***P = .0004, WT recipients (white bars) vs IFN-γR−/− recipients (black bars) of WT BM + WT T-cell grafts; * P = .02, IL-17RA−/− BM + WT T-cell grafts (orange bars) vs WT BM + WT T-cell grafts (black bars) into IFN-γR−/− recipients.
We next determined the specific contribution of IL-17A to the development of IPS. In this setting, B6.IFN-γR−/− recipients of G-CSF mobilized BALB/c.WT stem cell grafts were treated with either control or an anti–IL-17A neutralizing antibody. The blockade of systemic IL-17A significantly reduced lung pathology (Figure 6G), confirming a pathogenic role for IL-17A in IPS. We hypothesized that IL-17A may be signaling through donor cells to promote inflammatory responses, and in turn amplify IPS. We thus transplanted grafts lacking the α subunit of the IL-17 receptor (IL-17RA−/−), which renders IL-17A signaling incompetent. IL-17RA−/− grafts indeed had a major reduction in their ability to induce IPS (Figure 6H), confirming that IL-17A signaling through the donor graft promotes IPS. Thus, IL-6 derived from lung epithelia promotes pathogenic Th17 differentiation after alloSCT. Interestingly, despite the fact that prevention of IL-17A signaling through donor cells and broad IL-17A inhibition with antibody attenuated IPS, lung disease could still occur in IL-17A−/− recipients of WT grafts and WT recipients of IL-17A−/− grafts in the absence of IFN-γ (data not shown), confirming that both donor and recipient sources of this cytokine contribute to disease. IPS thus also developed in both WT and TCRδ−/−-deficient recipients receiving IFN-γ inhibition (pathology scores 10.1 ± 1.0 vs 9.8 ± 0.7). To determine which cell populations in the donor graft respond to IL-17A signaling and infiltrate the lung during IPS, we performed mixing experiments where the transplanted grafts comprised of either IL-17RA−/−-deficient BM or T cells. This demonstrated that both T cells and BM-derived macrophages require IL-17A signaling to migrate into the lung during IPS (Figure 6I). In contrast, there was no effect on neutrophils within the lung at these time points (data not shown).
IL-6 is dysregulated during the development of IPS
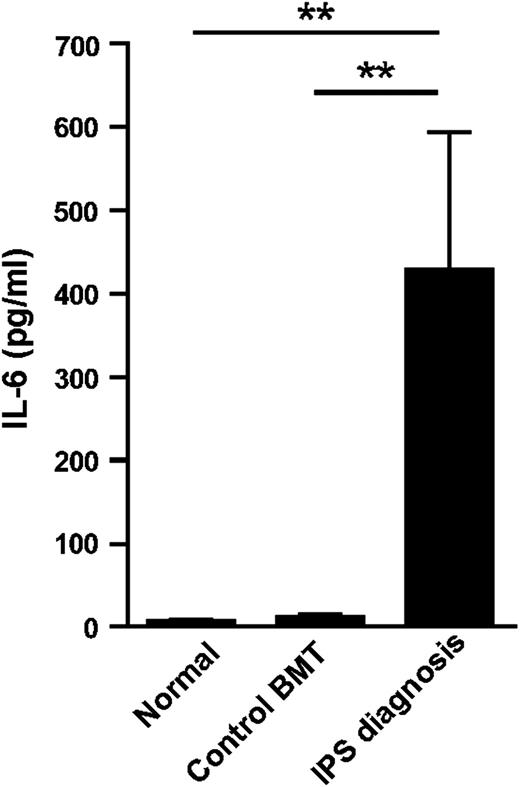
Having established a role for host-derived IL-6 in experimental models of IPS, we further investigated the translational relevance of our observations. We therefore measured plasma IL-6 levels in a large cohort of alloSCT recipients (n = 38) who developed IPS in US-based study cohorts. We found that at the time of diagnosis, typically in the second and third week after SCT, IL-6 levels were dramatically increased when compared with SCT recipients without complications at the same time point after SCT and normal volunteers (Figure 7). In this cohort of IPS, over 90% of recipients received ablative conditioning, which was TBI-based in approximately half of these patients. We reasoned that patients with IPS refractory to treatment may exhibit the most severe IL-6 dysregulation. Consistent with this, IL-6 concentrations were indeed highest in a subset of patients who did not respond to combination therapy with steroids and TNF neutralization with etanercept (340 ± 180 [responders] vs 643 ± 370 [nonresponders]; P = .038).
IL-6 is elevated in patients developing IPS. IL-6 levels in plasma from patients at the time of diagnosis of IPS after alloSCT (n = 38), control BMT recipients without evidence of IPS between days 14 and 21 after SCT (control BMT, n = 14) and normal volunteers (n = 13). **P < .01.
IL-6 is elevated in patients developing IPS. IL-6 levels in plasma from patients at the time of diagnosis of IPS after alloSCT (n = 38), control BMT recipients without evidence of IPS between days 14 and 21 after SCT (control BMT, n = 14) and normal volunteers (n = 13). **P < .01.
Discussion
We have previously shown that IFN-γ signaling through host lung parenchyma is important for regulating the development of IPS.5 In this study, we demonstrate that IFN-γ controls the expression of Th17 promoting cytokines in lung tissue following SCT. Critically, in the absence of IFN-γ, recipient IL-17 producing γδ T cells persist and IL-6 is secreted in high amounts in recipient lung tissue, promoting donor Th17 cell differentiation and accumulation, which culminates in fatal lung inflammation. Inhibition of the effector cytokine itself (IL-17A) or it’s signaling in donor cells (the IL-17 receptor) attenuates disease, thus defining the IL-6/IL-17 pathway as a critical determinant of IPS.
IL-6 is a pleiotropic cytokine that regulates multiple biological processes including acute-phase responses, inflammation, immune responses, and nervous and hematopoietic system development. In addition to its production by immune cells (eg, T cells, B cells, macrophages, neutrophils, and dendritic cells [DCs]), IL-6 has been shown to be secreted by nonimmune cells such as epithelial cells, endothelial cells, fibroblasts, and BM stromal cells.19 Interestingly, IL-6 expression has been shown to be upregulated in response to TBI in mice20 and our clinical data support the notion that TBI-induced IL-6 expression contributes to a localized microenvironment within the lung that promotes IPS. The high levels of IL-6 in the lung would appear to reflect the scale of epithelium and endothelium present at this site as a source that is inhibited by IFN-γ. In contrast, it is likely that in the GI tract, any IL-6 is derived from another cell source such as monocytes, which is instead enhanced by IFN-γ priming.21
In the lung, several potential antigen-presenting cells (APCs) exist including alveolar macrophages, tissue monocytes, endothelial/epithelial cells, and DCs. The majority of the antigen-loaded APCs remain within lung tissue rather than migrating to draining lymph nodes (LN), and their migration to the LN is not required for T-cell priming.22 We have previously reported that nonhematopoietic recipient APCs within target organs can induce alloreactive donor T-cell expansion and GVHD mortality.8 More recently, CD103+CD11b+ DCs producing IL-6 were shown to be required for Th17 differentiation within the GI tract.23 Together, these studies suggest a central role for tissue-specific APCs of both hematopoietic and nonhematopoietic lineages in the generation of mucosal Th cell responses. Our findings here demonstrate that during IPS, recipient nonhematopoietic lung tissue is an active source of cytokines that are able to modulate local immune responses by influencing alloantigen-specific donor T-cell differentiation. At this point, it is unclear whether nonhematopoietic tissue within the lung can act as an APC, as well as a source of cytokine to drive donor Th17 differentiation in isolation.
Calcineurin inhibitors such as CsA are the mainstay of immune suppression protocols in transplantation24-26 and have been demonstrated to inhibit cytokine production from memory T cells in vitro.27 More recently, CsA has actually been shown to promote Th17 differentiation in vivo, and subsequent bronchiolitis obliterans after experimental lung transplantation although effects on IL-6 were not studied.28 It was interesting to note in our clinical cohort, unlike IL-6, IFN-γ levels in human plasma did not significantly increase during the first 3 months of SCT, coinciding with concomitant immunosuppressive therapy (CsA and MTX) administered to these patients as standard GVHD prophylaxis. This suppression of IFN-γ secretion by calcineurin inhibition (and potentially MTX) represents an important contrast to preclinical models and the disease that develops therein, and may establish a cytokine environment more conducive to the development of IPS. Although calcineurin-free–based approaches may be advantageous in regard to regulatory subsets, this needs to be balanced against any potential reduction in potency on the inhibition of donor T-cell expansion or differentiation that may impact on the overall spectrum of GVHD. The addition of IL-6 inhibition to standard GVHD prophylaxis would be another potential approach and has demonstrated promising early results recently.29
The pathophysiology of IPS has been explored in several models wherein IFN-γ signaling is intact (reviewed in Panoskaltsis-Mortari et al3 ). In these models, an influx of cytolytic donor T cells and increased levels of TNF in broncho-alveolar lavage fluid are associated with the onset of experimental IPS, coincide with pulmonary endothelial and epithelial cell injury and activation, and regulate the recruitment of additional donor immune cells over time.16,30 Furthermore, inhibiting TNF either by the administration of soluble inhibitors or the genetic manipulation of donor cells significantly reduces endothelial cell apoptosis and the evolution of lung histopathology.30-33 These laboratory insights led to clinical trials using TNF inhibition to treat IPS, which has demonstrated encouraging responses.34-36 A large, prospective, phase 2 study combining etanercept with corticosteroids for children with IPS reported high early response rates and a 1-year survival of 63%, which is superior to all previous IPS reports.36 A recent phase 3 trial of corticosteroids with or without etanercept, did not demonstrate definitive effects of TNF inhibition on response or survival of patients with IPS above steroids alone. However, the trial accrued only 25% of its planned enrollment and was closed early, such that it was underpowered.37 Thus, a definitive clinical study confirming the efficacy of TNF inhibition for IPS is lacking. A previous murine study has suggested that Th17 differentiation is not involved in IPS, based on the ability of IFN-γ−/−IL-17−/− T cells to generate disease38 and we too have noted that IL-17−/− T cells can generate IPS. However, IL-17A neutralization or IL-17RA−/− donor cells significantly attenuates disease, highlighting the potential importance of recipient cells as a concurrent and nonredundant source of IL-17A.
In the data reported herein, IL-6 was found to be significantly dysregulated in both adult and pediatric patients with IPS. Consistent with our preclinical data revealing that lung parenchyma is a key producer of IL-6, we previously showed that bronchoalveolar fluid concentrations of IL-6 were 5 times higher than those measured in the plasma of SCT patients with IPS.34 Of particular interest, we now show that higher plasma concentrations of IL-6 at the time of diagnosis associate with lack of response to TNF inhibition. These findings have significant merit; not all patients with IPS respond to steroids and anti-TNF therapy, many relapse despite initial response, and being able to predict response is critical to the optimization of care for these patients. Anti–IL-6/IL-17 therapy may therefore be an alternative or useful adjunct therapy. We do not believe that systemic IL-6 levels are likely to be useful as a biomarker for IPS. This reflects the fact that IL-6 is dysregulated in most patients in the first week after SCT and peak levels at this time correlate with the development of grades 2 to 4 acute GVHD. Furthermore, in our patients with IPS, approximately 25% also had evidence of acute GVHD. Nevertheless, the presence of single nucleotide polymorphisms (SNP) in SCT donor and recipient pairs have been studied to help predict posttransplant complications. The presence of SNP in the IFN-γ and IL-6 genes have been reported in SCT recipients,39,40 and more recently, confirmed in a large genome-wide association study.41,42 Patients possessing the gain-of-function IL-6(-174)G SNP43 had a trend toward higher grades of acute GVHD, whereas those homozygous for SNP were more likely to develop chronic GVHD.39-41 These polymorphisms may thus prove to be useful in predicting patients at high risk of developing IPS, whereas IL-6 inhibition may itself be a useful therapeutic approach in the management of IPS in the clinic.
In conclusion, our results confirm and critically enhance our current understanding of the pathophysiology of IPS. Specifically, our observations underscore the contribution of IL-6 and IL-17A to lung injury in an IFN-γ low cytokine environment that is frequently seen after clinical SCT and appears conducive to lung inflammation. These findings identify novel therapeutic strategies that employ IL-6/IL-17A inhibition (alone or in combination) that may optimize outcomes for patients with IPS and help prevent the development of this lethal complication.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mark Bunting, Md Ashik Ullah, and Ping Zhang for assistance with some experiments, and Judy Avery for clinical data collection.
This study was supported by grants from the Australian National Health and Medical Research Council (NH&MRC) and Queensland Health. G.R.H. is an NH&MRC Australia Fellow and Queensland Health Senior Clinical Research Fellow; K.P.A.M. is a Cancer Council Queensland Senior Research Fellow; S.-K.T. and K.A.M. are NH&MRC Early Career Fellows; and M.K. is a Leukaemia Foundation of Australia Post-Doctoral Fellow.
Authorship
Contribution: A.V. designed and performed experiments, analyzed data, and wrote the manuscript; K.H.G. contributed to the design and execution of experiments, data analysis, and provided helpful discussion; E.K., S.D.O., R.D.K., M.L., M.C., K.E.L., B.E.T., N.C.R., K.A.A., K.A.M., and M.K. performed experiments and contributed to discussion; A.D.C. performed blinded histologic assessment; E.S. and J.L. coordinated the clinical study and collected data; P.R. provided critical reagents and intellectual input; G.A.K. and S.-K.T. enrolled patients in the Brisbane clinical study and provided intellectual input; K.P.A.M. provided intellectual input; G.A.Y., B.R.B., and K.R.C. contributed clinical data and provided intellectual input; and G.R.H. contributed to the design of experiments, provided intellectual input, and wrote the manuscript.
Conflict-of-interest disclosure: G.R.H. has received a paid consultancy from Amgen within the last 2 years. The remaining authors declare no competing financial interests.
Correspondence: Geoffrey R. Hill, QIMR Berghofer Medical Research Institute, 300 Herston Rd, Herston, QLD 4006, Australia; e-mail: geoff.hill@qimrberghofer.edu.au.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal