Abstract
In inflammation, neutrophils and other leukocytes roll along the microvascular endothelium before arresting and transmigrating into inflamed tissues. Arrest requires conformational activation of the integrin lymphocyte function-associated antigen-1 (LFA-1). Mutations of the FERMT3 gene encoding kindlin-3 underlie the human immune deficiency known as leukocyte adhesion deficiency-III. Both kindlin-3 and talin-1, another FERM domain-containing cytoskeletal protein, are required for integrin activation, but their individual roles in the induction of specific integrin conformers are unclear. Here, we induce differential LFA-1 activation in neutrophils through engagement of the selectin ligand P-selectin glycoprotein ligand-1 or the chemokine receptor CXCR2. We find that talin-1 is required for inducing LFA-1 extension, which corresponds to intermediate affinity and induces neutrophil slow rolling, whereas both talin-1 and kindlin-3 are required for induction of the high-affinity conformation of LFA-1 with an open headpiece, which results in neutrophil arrest. In vivo, both slow rolling and arrest are defective in talin-1–deficient neutrophils, whereas only arrest is defective in kindlin-3–deficient neutrophils. We conclude that talin-1 and kindlin-3 serve distinct functions in LFA-1 activation.
Introduction
Integrins1 are adhesion molecules whose ligand binding function is regulated by conformational changes in their extracellular domain.2,3 There is structural and functional evidence for at least 3 global β2 integrin conformations: compact/bent, extended with a closed or open headpiece.3 The resting integrin with a bent conformation exhibits low affinity for ligand, whereas the extended conformations exhibit intermediate (closed headpiece) or high (open headpiece) binding affinities.3 In a process termed “inside-out” integrin activation, cellular signaling cascades lead to the direct binding of the FERM domain-containing talin-1 and kindlin-3 proteins to the integrin cytoplasmic domain resulting in conformational changes that are propagated across the plasma membrane.3,4 Talin-1 and kindlin-3 bind via 2 different NXX(Y/F) motifs found in most integrin β-cytoplasmic domains.5 In the β2 integrin subunit, expressed in all leukocytes, the corresponding sequences are NPLF and NPKF. In various adhesion assays, both talin-1 and kindlin-3 are required for integrin adhesive function,6,7 but it is not known whether they serve the same or different functions in regulating conformational rearrangement of the integrin ectodomain.
All leukocyte adhesion deficiency-III (LAD-III) patients have premature stop codons or nonsense mutations in both alleles of their FERMT3 gene.8-11 Their leukocytes express normal amounts of the integrin lymphocyte function-associated antigen-1 (LFA-1, also known as αLβ2 and CD11a/CD18), yet are unable to migrate to sites of infection or inflammation, thus causing severe recurrent bacterial infections.12,13 In addition, LAD-III patients exhibit defective activation of platelet β3 integrins and thus have a bleeding disorder resembling Glanzmann thrombasthenia.12 Limited investigations into leukocyte adhesion under flow9,14 have revealed that the adhesion defect of LAD-III leukocytes is at the level of arrest, the transition from rolling to firm adhesion. Patients with defective talin-1 (gene name, TLN1) have not been described, probably because a TLN1 null mutation would lead to early embryonic lethality, as it does in Tln1−/− mice.15 By contrast, Fermt3−/− mice and fetal liver cell-derived chimeras are viable and show a clear platelet6 and leukocyte14 adhesion deficiency and are therefore considered a faithful model of LAD-III.
Under inflammatory conditions, neutrophils roll along the wall of small venules by interacting with E- and P-selectins as well as with the LFA-1 ligand intercellular adhesion molecule-1 (ICAM-1).16,17 Neutrophil slow rolling is mediated by partially activated LFA-1 with an extended ectodomain and intermediate ligand binding affinity.18,19 Neutrophils transition from rolling to firm adhesion when LFA-1 is fully activated by chemokine signaling through G protein-coupled receptors.16,17 LFA-1 activation involves separation of the αL and β2 cytoplasmic tails,20 which induces integrin extension from the bent to the extended conformation.3 Even when LFA-1 is extended, its affinity for ICAM-1 is still so low that no binding of soluble ICAM-1 is detectable.21 LFA-1 then undergoes further conformational changes resulting in an open headpiece.3 Like all β2 integrins, the LFA-1 αL subunit contains an I-domain inserted between blades 2 and 3 of the β propeller.3 The conformational opening of this I-domain involves a “bell-rope” downward movement of its α7 helix, generating the high-affinity conformer that binds soluble ICAM-1.2
Although both talin-1 and kindlin-3 are involved in inside-out integrin activation, it is not known whether they bind sequentially or simultaneously.5 The talin F3 domain has been shown to bind to the β3 integrin cytoplasmic domain to facilitate cytoplasmic tail separation.22 In a reconstituted in vitro system, talin head domain binding is sufficient to induce αIIbβ3 integrin extension.23 Kindlins also bind integrin β subunits through their F3 domain,24 but no further structural information about kindlin-3 binding to integrins is available.
The current study was designed to determine the relationship between talin-1 and kindlin-3 binding and the affinity states of LFA-1 (bent, extended, with closed or open headpiece). Using functional ex vivo and in vivo assays, we find that talin-1 is essential for neutrophil slow rolling and arrest, whereas kindlin-3 is required only for neutrophil arrest, thus demonstrating that talin-1 and kindlin-3 have distinct roles in LFA-1 activation. Reporter antibodies are available for human LFA-1 that bind to epitopes in extended (NKI-L16 and KIM127) or high-affinity (2E8 and mAb 24) LFA-1. We directly assessed the roles of talin-1 and kindlin-3 in structural changes to LFA-1 during activation using these conformation-specific antibodies. Confirming our results in primary mouse neutrophils, we find that talin-1 is required for LFA-1 extension, whereas both talin-1 and kindlin-3 are needed for inducing headpiece opening associated with the high-affinity state of LFA-1.
Methods
Mixed chimeric mice
Mice harboring floxed Tln1 alleles25 were crossed with Mx1-Cre mice in which Cre recombinase expression is controlled by the Mx1 promoter and can be induced by interferon production after administration of synthetic double-stranded RNA.26 Fermt3+/− and Fermt3−/− mice have been described.6 To generate mixed chimeras, 8- to 12-week-old C57BL/6 mice were lethally irradiated (12 Gy) and then reconstituted by intravenous injection of hematopoietic stem cells at a 1:1 ratio obtained from the bone marrow of LysMGFP mice, in which mature neutrophils express GFP,27 and from either the bone marrow of Tln1fl/flMx1-Cre mice or the liver of Fermt3−/− E17-20 fetuses. For Tln1fl/flMx1-Cre/LysMGFP chimeras, deletion of talin-1 was achieved by 3 intraperitoneal injections of 250 μg polyinosinic-polycytidylic acid (poly I/C; InvivoGen), each 2 days apart starting 4 weeks after hematopoietic reconstitution. Poly I/C–treated Tln1fl/flMx1-Cre/LysMGFP chimeras were used for experiments 4 to 6 weeks after poly I/C administration. Fermt3−/−/LysMGFP chimeras were used for experiments 6 to 8 weeks after hematopoietic reconstitution. Mice were housed in a specific pathogen-free facility. The Animal Care and Use Committee of the La Jolla Institute for Allergy and Immunology approved all animal experiments. Before all experiments, mice were anesthetized with an intraperitoneal injection of ketamine hydrochloride (125 mg/kg), atropine sulfate (25 μg/kg), and xylazine (12.5 mg/kg).
Soluble ICAM-1 binding assay
Bone marrow cells were isolated from mixed chimeric mice and suspended in Hanks Balanced Salt Solution containing 1mM CaCl2 and MgCl2 (Invitrogen). Cells were exposed to 10 nM CXCL1 (PeproTech), or an equal volume of PBS, in the presence of ICAM-1/Fc (20 μg/mL; R&D Systems) and allophycocyanin-conjugated anti–human IgG1 (Fc-specific; Southern Biotechnology) for 3 minutes at 37°C. In all experiments, an anti-CD11b (clone M1/70, 10 μg/mL) blocking antibody against integrin Mac-1 was used to block Mac-1–dependent ICAM-1 binding. An anti-CD11a (clone M17/4, 10 μg/mL) blocking antibody was used to determine the dependence of ICAM-1 binding on LFA-1. Cells were fixed on ice and then labeled with PE-conjugated anti-Ly6G (Biolegend) to identify neutrophils. GFP+ (wild-type) and GFP− (Tln1−/− or Fermt3−/−) neutrophils were identified, and ICAM-1 binding was measured using flow cytometry measurement of allophycocyanin mean fluorescence intensity.
Autoperfused microfluidic flow chambers
Flow chambers were assembled using 20 × 200-μm rectangular glass capillaries.28 Flow chambers were coated with E-selectin/Fc (15 μg/mL; R&D Systems) alone or in combination with ICAM-1/Fc (15 μg/mL), and then blocked with 1% casein (Thermo Scientific). Polyethylene-10 tubing (Intramedic) was inserted into the carotid artery of anesthetized mice and then connected to the flow chamber. A wall shear stress of 6 dyne/cm2 was used in all experiments. Neutrophil rolling was recorded under dual bright field and stroboscopic fluorescence illumination. For neutrophil arrest assays, flow chambers were co-coated with E-selectin, ICAM-1, and CXCL1 (10 μg/mL) and autoperfused for 5 minutes before the number of adherent cells was counted in each field of view. Data were normalized according to the ratio of GFP+ (wild-type) to GFP− (gene-deficient) circulating neutrophils.
Intravital microscopy
Mice were injected intrascrotally with 500 ng TNF-α (PeproTech) 2 hours before anesthetization, intubation, carotid artery catheterization, and exteriorization of the cremaster muscle. Neutrophil rolling in postcapillary venules 20 to 50 μm in diameter was recorded under dual bright field and stroboscopic fluorescence illumination.29 All mice received intravenous injections of an anti–P-selectin antibody (clone RB40.34, 30 μg) to isolate E-selectin-induced slow rolling. Neutrophil slow rolling was blocked using an anti-CD11a (clone M17/4, 30 μg) antibody against LFA-1. Neutrophil arrest was measured as the number of arrested cells within 30 seconds after intravenous injection of 600 ng CXCL1 in mice that did not receive TNF-α or anti–P-selectin pretreatment. Data were normalized according to the ratio of GFP+ (wild-type) to GFP− (gene-deficient) circulating neutrophils before CXCL1 injection.
Thioglycollate-induced peritonitis
To assess neutrophil recruitment in a model of inflammation, 1 mL of 4% thioglycollate medium (Sigma-Aldrich) was injected into the peritoneum of mice 8 hours before flushing the peritoneal cavity with 5 mL PBS containing 2mM EDTA. The total cell number in peritoneal lavage was counted using a hemacytometer. The fraction of GFP+ (wild-type) and GFP− (Tln1−/− or Fermt3−/−) neutrophils in the peritoneal lavage was determined by flow cytometry using a PE-conjugated anti-Ly6G antibody.
LFA-1 reporter mAb binding to HL-60 cells
HL-60 cells (ATCC) were maintained in RPMI 1640 media supplemented with 10% FBS and antibiotics. Lentiviral vectors encoding shRNAs against talin-1 and kindlin-3 were obtained from shRNA libraries. For lentivirus production, 293T cells were transiently transfected using calcium precipitation. To achieve a stable knockdown, HL-60 cells were transduced with lentiviral supernatant and then selected and maintained in the presence of 3 μg/mL puromycin (Sigma-Aldrich). The cell-permeable wild-type and constitutively active Rap1a constructs have been described previously.30 HL-60 cells were incubated with 1μM Rap1a constructs for 20 minutes followed by a 5-minute incubation with isotype, anti-αL NKI-L16 mAb (provided by Carl Figdor, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands),31,32 anti-β2 KIM127 mAb (ATCC),33 anti-αL 2E8 mAb (provided by Qing Ma, MD Anderson Cancer Center, University of Texas, Houston, TX),34 or anti-β2 mAb 24 (provided by Nancy Hogg, Cancer Research UK, London Research Institute, London, United Kingdom).35 Antibody binding was measured as mean fluorescence intensity by flow cytometry.
LFA-1 activation in K562 cells
K562 cells (ATCC) were maintained in RPMI 1640 media supplemented with 10% FBS and antibiotics. Cells were transfected with cDNAs encoding human integrin αL and β2 using Amaxa nucleofection solution V and program T-16 (Lonza). β2F732A and β2F744A point mutations were introduced using a QuikChange site-directed mutagenesis kit (Agilent Technologies). Stable transfectants were obtained by FACS sorting (anti-αL TS2/4; BioLegend) and selection with 1 mg/mL geneticin (Invitrogen). K562 cells were incubated with 1μM phorbol 12-myristate 13-acetate (PMA) for 10 minutes followed by a 5-minute incubation with KIM127 mAb on ice. KIM127 binding to cells with similar expression of LFA-1 was measured by flow cytometry.
Statistical analysis
For comparison between 2 groups, 2-tailed Student t tests were performed. For comparison between more than 2 groups, 1-way ANOVA and Tukey posttest were used. P values less than .05 were considered significant. All analyses were performed using Prism GraphPad Version 5.0d software.
Results
Talin-1 and kindlin-3 are required for CXCL1-induced high-affinity LFA-1
To address the function of talin-1 and kindlin-3, we constructed mixed chimeric mice in which a fraction of the circulating neutrophils were deficient in either talin-1 (Tln1fl/flMx1-Cre, deletion induced by poly-I/C administration)25,36 or kindlin-3 (Fermt3−/−),6 and the remaining neutrophils were wild-type expressing green fluorescent protein (Lyz2GFP)27 for detection by flow cytometry or live cell imaging. Deletion of talin-1 or kindlin-3 protein in Ly6G+GFP− neutrophils was assessed by Western blot (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This approach ensures that sufficient numbers of healthy platelets (no bleeding) and leukocytes (no infections) are present in each mouse, thus avoiding compensatory or adaptive phenotypes. Both types of mixed chimeric mice exhibited mild to moderate neutrophilia, as has been observed previously in kindlin-3–deficient mice,14 and the fraction of circulating talin-1 or kindlin-3–deficient neutrophils was typically 50% to 80% of the total circulating neutrophil population (data not shown).
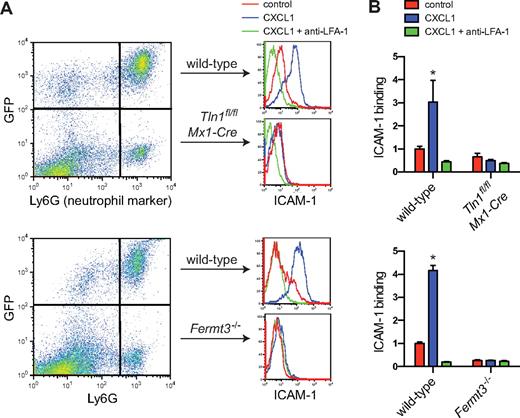
The binding of soluble ICAM-1 is a commonly used test to assay the high-affinity state of LFA-1.2,21 To assess the roles of talin-1 and kindlin-3 in chemokine-stimulated induction of high-affinity LFA-1, bone marrow from mixed chimeric mice was incubated with soluble murine ICAM-1/Fc. Wild-type bone marrow neutrophils (identified by expression of Ly6G and GFP; Figure 1A) showed minimal ICAM-1 binding when resting but significant binding after activation with the chemokine CXCL1, which was completely blocked by preincubation with a blocking antibody to LFA-1 (Figure 1B) or ICAM-1 (data not shown). ICAM-1 binding in response to CXCL1 was not detectable in neutrophils that were deficient in talin-1 or kindlin-3 (Ly6G+GFP−; Figure 1B), demonstrating that both talin-1 and kindlin-3 are required for chemokine-induced high-affinity LFA-1. This is consistent with data reporting a requirement for talin-1 and kindlin-3 to activate αIIbβ3 integrin in platelets6,25,36 and defective LFA-1–dependent arrest of pre-B cells and reduced expression of the β2 integrin extension reporter antibody KIM127 when talin-1 is silenced.37
Impaired induction of high-affinity LFA-1 in talin-1 and kindlin-3–deficient neutrophils. (A) Flow cytometric measurement of soluble murine ICAM-1/Fc binding to bone marrow neutrophils isolated from Tln1fl/flMx1-Cre/LysMGFP and Fermt3−/−/LysMGFP mixed chimeric mice. Bone marrow cells exposed to ICAM-1 (20 μg/mL) were stimulated with CXCL1 (10nM, 3 minutes). (B) CXCL1 up-regulates ICAM-1 binding to LFA-1 in wild-type, but not in talin-1 or kindlin-3–deficient neutrophils. Preincubation with a blocking anti–LFA-1 mAb (clone M17/4; 10 μg/mL) was used to determine the dependence of ICAM-1 binding on LFA-1. Data are mean ± SEM fold increase over control (n = 3 each).
Impaired induction of high-affinity LFA-1 in talin-1 and kindlin-3–deficient neutrophils. (A) Flow cytometric measurement of soluble murine ICAM-1/Fc binding to bone marrow neutrophils isolated from Tln1fl/flMx1-Cre/LysMGFP and Fermt3−/−/LysMGFP mixed chimeric mice. Bone marrow cells exposed to ICAM-1 (20 μg/mL) were stimulated with CXCL1 (10nM, 3 minutes). (B) CXCL1 up-regulates ICAM-1 binding to LFA-1 in wild-type, but not in talin-1 or kindlin-3–deficient neutrophils. Preincubation with a blocking anti–LFA-1 mAb (clone M17/4; 10 μg/mL) was used to determine the dependence of ICAM-1 binding on LFA-1. Data are mean ± SEM fold increase over control (n = 3 each).
Deficiency of talin-1, but not kindlin-3, results in loss of slow rolling
In addition to the chemokine/G protein-coupled receptor signaling cascade, neutrophils also possess a chemokine-independent pathway of LFA-1 activation that is triggered by E-selectin binding to P-selectin glycoprotein ligand-1 (PSGL-1).18,19,29,38,39 To investigate whether talin-1 and kindlin-3 have the same or different functions in LFA-1 activation, we took advantage of a set of in vitro and in vivo neutrophil adhesion assays in which rolling on E-selectin induces the extended conformation of LFA-1, leading to slow rolling but not arrest.18,19 Rolling on E-selectin induces LFA-1 extension18 as reported by the extension reporter antibodies NKI-L16 (for αL)32 and KIM127 (for β2)33 but does not induce the open headpiece conformation as reported by mAb 24.35 Chemokine-induced neutrophil arrest, but not LFA-1–dependent slow rolling, is blocked by an allosteric antagonist that induces LFA-1 extension but stabilizes a closed I-domain, further supporting the idea that distinct LFA-1 conformers mediate slow rolling and arrest events.19 Therefore, the ability of neutrophils to undergo LFA-1–dependent slow rolling provides a functional readout of the ability of LFA-1 to be extended in response to physiologic stimuli.
When whole blood from mixed chimeric mice was directly perfused through miniaturized flow chambers28 coated with E-selectin, neutrophils deficient for talin-1 or kindlin-3 (GFP− cells) rolled at the same velocity as wild-type neutrophils (Figure 2B), indicating that selectin-dependent rolling is unaffected by loss of talin-1 or kindlin-3. When flow chambers were coated with E-selectin in combination with ICAM-1, wild-type neutrophils exhibited normal LFA-1–mediated slow rolling behavior with a significantly reduced velocity compared with the E-selectin substrate, but talin-1–deficient neutrophils were unable to undergo slow rolling (Figure 2A-B; supplemental Video 1). By contrast, neutrophils lacking kindlin-3 showed normal slow rolling indistinguishable from wild-type neutrophils (Figure 2B; supplemental Video 2). These data suggest that kindlin-3 is dispensable, whereas talin-1 is required, for LFA-1 extension to an intermediate affinity state.
Neutrophil slow rolling and arrest inex vivoflow chambers. (A) Time-lapse images of GFP+ wild-type and GFP−Tln1fl/flMx1-Cre (top) or Fermt3−/− (bottom) neutrophils rolling side-by-side in an autoperfused flow chamber coated with E-selectin and ICAM-1. Scale bar represents 20 μm. (B) Mean rolling velocities of neutrophils in flow chambers with immobilized E-selectin or E-selectin and ICAM-1. A wall shear stress of 6 dyne/cm2 was used in all experiments. Data are mean ± SEM of at least 25 cells per condition (n = 3 mice each). (C) Neutrophil arrest in flow chambers with immobilized E-selectin, ICAM-1, and CXCL1 after 5 minutes of perfusion. Data are mean ± SEM (number of adherent cells in each of at least 3 fields of view per flow chamber; n = 3 mice each).
Neutrophil slow rolling and arrest inex vivoflow chambers. (A) Time-lapse images of GFP+ wild-type and GFP−Tln1fl/flMx1-Cre (top) or Fermt3−/− (bottom) neutrophils rolling side-by-side in an autoperfused flow chamber coated with E-selectin and ICAM-1. Scale bar represents 20 μm. (B) Mean rolling velocities of neutrophils in flow chambers with immobilized E-selectin or E-selectin and ICAM-1. A wall shear stress of 6 dyne/cm2 was used in all experiments. Data are mean ± SEM of at least 25 cells per condition (n = 3 mice each). (C) Neutrophil arrest in flow chambers with immobilized E-selectin, ICAM-1, and CXCL1 after 5 minutes of perfusion. Data are mean ± SEM (number of adherent cells in each of at least 3 fields of view per flow chamber; n = 3 mice each).
Previous studies of LFA-1 activation in leukocyte arrest have found that high-affinity LFA-1 mediates T lymphocyte arrest in vivo.40 When CXCL1, a ligand for the neutrophil-expressed chemokine receptor CXCR2, was coimmobilized with E-selectin and ICAM-1 in flow chambers, talin-1–deficient neutrophils were unable to arrest, as were kindlin-3–deficient neutrophils (Figure 2C). As a positive control, wild-type (GFP+) neutrophils in the same experiments exhibited the expected arrest behavior (Figure 2C). This result indicates that talin-1 and kindlin-3 are critical for activation of LFA-1 to a high-affinity state, supporting the finding that both proteins are needed for soluble ICAM-1 binding to LFA-1 in response to chemokine stimulation.
Talin-1, but not kindlin-3, is essential for neutrophil slow rolling in vivo
To confirm the observation that talin-1 and kindlin-3 play divergent roles in LFA-1–dependent neutrophil slow rolling in a more relevant in vivo model, we investigated venules in the cremaster muscle, a thin muscle in the scrotum of male mice41 (Figure 3A). To induce endothelial cell E-selectin expression, the mixed chimeric mice were pretreated with TNF-α. Similar to previous studies,19,29 a blocking antibody to P-selectin was used to focus on E-selectin–mediated rolling. In inflamed venules, talin-1–deficient neutrophils rolled significantly faster than wild-type neutrophils, suggesting that LFA-1 extension was defective in these cells (Figure 3B). Indeed, when a blocking mAb to LFA-1 was injected into these mice, the rolling velocity of the talin-1–deficient cells did not increase significantly, whereas that of wild-type neutrophils did, as expected (Figure 3B). By contrast, rolling of kindlin-3–deficient neutrophils was indistinguishable from that of wild-type neutrophils, both under control conditions and after LFA-1 blockade (Figure 3C). These data provide in vivo confirmation that talin-1 is required for LFA-1–dependent neutrophil slow rolling that is mediated by the extended/closed LFA-1 conformer.
Neutrophil slow rolling and arrest in vivo. (A) Dual bright field and fluorescence imaging of rolling neutrophils in postcapillary venules of the mouse cremaster muscle. (B-C) Rolling velocities of neutrophils after TNF-α pretreatment of the mouse cremaster muscle in (B) Tln1fl/flMx1-Cre/LysMGFP and (C) Fermt3−/−/LysMGFP mixed chimeric mice. All mice received an intravenous injection of an anti–P-selectin mAb (clone RB40.34, 30 μg/mouse) at least 15 minutes before image acquisition. A blocking anti–LFA-1 mAb (30 μg/mouse) was injected to determine the dependence of rolling velocity on LFA-1. Data are presented as cumulative velocity histograms and as mean rolling velocities ± SEM (n = 3 mice each). (D) Arrested neutrophils were counted after intravenous injection of CXCL1 (600 ng). Data are mean ± SEM (number of arrested cells per unit area of the blood vessel wall; n = 3 mice each), normalized according to the ratio of circulating wild-type to gene-deficient neutrophils before CXCL1 injection. (E) Neutrophil influx into the peritoneal cavity was measured 8 hours after 1 mL intraperitoneal injection of 4% thioglycollate medium. Data are mean ± SEM (number of neutrophils in the peritoneal lavage; n = 3 mice), normalized according to the ratio of circulating wild-type (GFP+) to gene-deficient (GFP−) neutrophils before thioglycollate injection.
Neutrophil slow rolling and arrest in vivo. (A) Dual bright field and fluorescence imaging of rolling neutrophils in postcapillary venules of the mouse cremaster muscle. (B-C) Rolling velocities of neutrophils after TNF-α pretreatment of the mouse cremaster muscle in (B) Tln1fl/flMx1-Cre/LysMGFP and (C) Fermt3−/−/LysMGFP mixed chimeric mice. All mice received an intravenous injection of an anti–P-selectin mAb (clone RB40.34, 30 μg/mouse) at least 15 minutes before image acquisition. A blocking anti–LFA-1 mAb (30 μg/mouse) was injected to determine the dependence of rolling velocity on LFA-1. Data are presented as cumulative velocity histograms and as mean rolling velocities ± SEM (n = 3 mice each). (D) Arrested neutrophils were counted after intravenous injection of CXCL1 (600 ng). Data are mean ± SEM (number of arrested cells per unit area of the blood vessel wall; n = 3 mice each), normalized according to the ratio of circulating wild-type to gene-deficient neutrophils before CXCL1 injection. (E) Neutrophil influx into the peritoneal cavity was measured 8 hours after 1 mL intraperitoneal injection of 4% thioglycollate medium. Data are mean ± SEM (number of neutrophils in the peritoneal lavage; n = 3 mice), normalized according to the ratio of circulating wild-type (GFP+) to gene-deficient (GFP−) neutrophils before thioglycollate injection.
To determine whether talin-1 or kindlin-3 play a role in neutrophil arrest in vivo, CXCL1 was intravenously injected during intravital imaging of cremaster muscle venules. Although nearly all wild-type neutrophils transitioned from rolling to firm adhesion, very few kindlin-3–deficient or talin-1–deficient neutrophils arrested (Figure 3D; supplemental Videos 3 and 4). Furthermore, when the entire neutrophil recruitment cascade (including cell rolling, arrest, adhesion strengthening, migration, and diapedesis) was tested in a model of sterile peritonitis induced by intraperitoneal injection of thioglycollate into mixed chimeric mice, both talin-1– and kindlin-3–deficient neutrophils were completely unable to enter into the peritoneal cavity (Figure 3E). Therefore, both talin-1 and kindlin-3 are necessary for effective neutrophil recruitment in vivo.
Rap1-induced LFA-1 extension occurs in the absence of kindlin-3
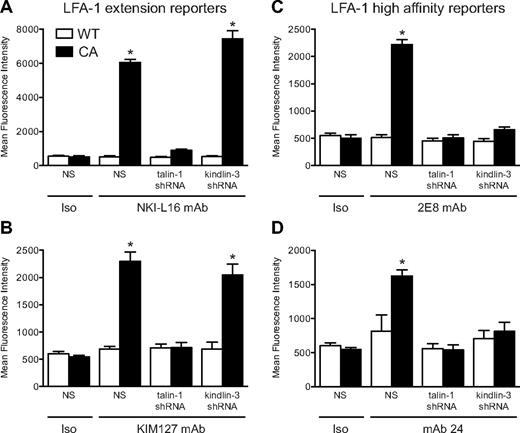
The functional assays in primary murine neutrophils test the effects of LFA-1 activation in response to chemokine receptor or PSGL-1 engagement. Chemokine- and chemoattractant-induced β2 integrin activation is dependent on activation of the GTPase Rap1.37,42 In human cells, reporter antibodies are available that recognize extended LFA-1 (NKI-L16 for αL and KIM127 for β2) or high-affinity LFA-1 (2E8 for αL and mAb 24 for β2) conformations. To take advantage of these tools and more directly assess LFA-1 conformation, we used RNA interference to stably knock down either talin-1 or kindlin-3 in the human HL-60 cell line. RNA interference-mediated silencing resulted in 84% and 82% knockdown of talin-1 and kindlin-3 protein levels, respectively (supplemental Figure 2). HL-60 cells were then incubated with a cell-permeable constitutively active Rap1a construct and the activation of LFA-1 to extended and extended/open conformations was assessed by flow cytometry. The Rap1a peptides had no effect on expression of endogenous talin-1 or kindlin-3 (data not shown). In wild-type HL-60 cells, we found that constitutively active Rap1a induced both LFA-1 extension, as reported by NKI-L16 (Figure 4A) and KIM127 binding (Figure 4B), and high-affinity LFA-1 activation, as reported by 2E8 (Figure 4C) and mAb 24 (Figure 4D). Exposure of the NKI-L16 and KIM127 epitopes in response to constitutively active Rap1a was blocked in talin-1–deficient cells but was induced in kindlin-3–silenced cells to a similar extent as in wild-type HL-60 cells (Figure 4A-B). In HL-60 cells in which talin-1 or kindlin-3 was silenced, 2E8 and mAb 24 binding in response to active Rap1a did not increase above baseline levels (Figure 4C-D). These data directly demonstrate that talin-1 is essential for LFA-1 extension, whereas kindlin-3 is required for the transition of LFA-1 from the intermediate (extended/closed) to the high (extended/open) affinity state.
Rap1a-induced LFA-1 activation. (A-D) HL-60 cells with stable expression of shRNA to knock down talin-1 or kindlin-3, or nonspecific shRNA (NS), were incubated with cell-permeable wild-type (WT; open bars) or constitutively active (CA; closed bars) Rap1a peptides (1μM) in the presence of fluorescently labeled conformation-specific reporter mAbs. Flow cytometry was used to measure the binding of isotype (Iso) or (A) NKI-L16 mAb, (B) KIM127 mAb, (C) 2E8 mAb, and (D) mAb 24. Data are mean fluorescence intensity ± SEM (n = 4). *Significantly different from all conditions without the asterisk.
Rap1a-induced LFA-1 activation. (A-D) HL-60 cells with stable expression of shRNA to knock down talin-1 or kindlin-3, or nonspecific shRNA (NS), were incubated with cell-permeable wild-type (WT; open bars) or constitutively active (CA; closed bars) Rap1a peptides (1μM) in the presence of fluorescently labeled conformation-specific reporter mAbs. Flow cytometry was used to measure the binding of isotype (Iso) or (A) NKI-L16 mAb, (B) KIM127 mAb, (C) 2E8 mAb, and (D) mAb 24. Data are mean fluorescence intensity ± SEM (n = 4). *Significantly different from all conditions without the asterisk.
β2 integrin-binding is required for regulation of LFA-1 by talin-1
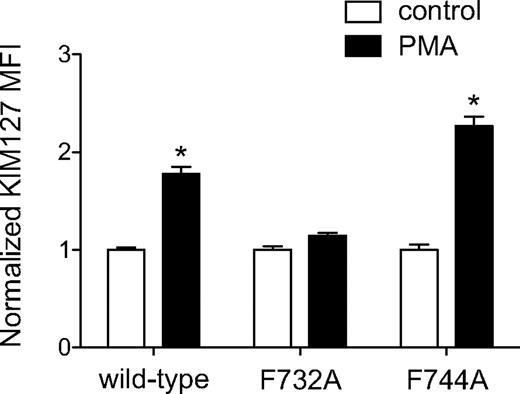
To show that the association of talin-1 with LFA-1 is required for the induction of conformational changes, we performed experiments using the human myeloid leukemic K562 cell line, which lacks endogenous expression of αL and β2 integrins. We analyzed the activation of wild-type LFA-1 and LFA-1 harboring mutations of either the talin-1 (β2-F732A)43 or kindlin-3 (β2-F744A)24 binding motifs. As has been shown previously,44 the protein kinase C activator PMA induced a significant increase in KIM127 binding to K562 cells expressing wild-type LFA-1 (Figure 5). K562 cells expressing the talin-1 binding-deficient mutant of LFA-1 did not exhibit an increase in KIM127 binding in response to PMA (Figure 5). In contrast, exposure of the KIM127 epitope in the kindlin-3 binding-deficient mutant of LFA-1 was enhanced by PMA to a similar extent as with wild-type LFA-1 (Figure 5). These results show that the reported binding site for talin-1 in the β2 integrin tail is necessary for the induction of LFA-1 extension, but the kindlin-3 binding site is not.
Integrin binding is required for talin-1–dependent LFA-1 activation. K562 cells expressing wild-type αL chain and wild-type or mutant β2 chain were incubated with PMA (1μM) and then labeled with KIM127 mAb. Flow cytometry was used to measure the binding of KIM127. Data are the fold increase mean fluorescence intensity (MFI) ± SEM over control untreated cells (n = 3).
Integrin binding is required for talin-1–dependent LFA-1 activation. K562 cells expressing wild-type αL chain and wild-type or mutant β2 chain were incubated with PMA (1μM) and then labeled with KIM127 mAb. Flow cytometry was used to measure the binding of KIM127. Data are the fold increase mean fluorescence intensity (MFI) ± SEM over control untreated cells (n = 3).
Discussion
There is mounting evidence that the FERM domain proteins of the talin and kindlin families are critical regulators of integrin affinity common to the activation of most, if not all, of the 24 different integrin heterodimers.5 The results presented here indicate that talin-1 and kindlin-3 play distinct roles in the induction of global conformational changes involved in activation of the integrin LFA-1 in myeloid cells. Using a unique set of in vitro and in vivo assays that allow the putative intermediate- and high-affinity states of LFA-1 to be analyzed separately, we found that talin-1 is required for LFA-1 extension (also reported by NKI-L16 and KIM127 mAbs) and neutrophil slow rolling, and both talin-1 and kindlin-3 are needed to induce the high-affinity LFA-1 conformer (also reported by 2E8 mAb and mAb 24) and neutrophil arrest.
Our results suggest a model where signaling downstream of chemokine receptor supports both talin-1 and kindlin-3 recruitment to LFA-1. Our study indicates that talin-1 must associate with the β2 integrin cytoplasmic tail to induce LFA-1 extension, a finding that is consistent with models postulating either the sequential or simultaneous binding of talin-1 and kindlin-3 to the integrin tail. Whether talin-1 is actively involved in the transition from a closed to an open headpiece or is simply needed to achieve integrin extension that is a prerequisite for headpiece opening remains unknown. There is evidence that ligands can bind to bent integrins,45 indicating that integrin extension and conformational changes within the ligand binding headpiece can occur independently. In the context of the current study showing that talin-1 is required for induction of both LFA-1 extension and headpiece opening, this would suggest that talin-1 plays a role in both types of structural rearrangements. The sequence of talin-1 and kindlin-3 binding to LFA-1 in response to chemokine receptor activation is currently unknown. Here, we show that kindlin-3 is required for induction of high-affinity LFA-1. One explanation for the failure of signaling downstream of PSGL-1 to induce high-affinity LFA-1 and neutrophil arrest may be that this pathway does not induce the recruitment or binding of kindlin-3 to the β2 integrin cytoplasmic tail. We predict that LAD-III patient leukocytes (FERMT3−/−) may be able to extend LFA-1 in response to chemokine stimulation during inflammation and undergo slow rolling but are unable to arrest because of an inability to transition to the high-affinity conformation of LFA-1.
Because integrin activation precedes integrin outside-in signaling and because kindlin-3 is required for the high-affinity conformation of LFA-1, the complete lack of neutrophil recruitment in thioglycollate-induced peritonitis supports the suggestion that kindlin-3 is indeed required for β2 integrin outside-in signaling.6,46 In addition to inside-out signaling, integrin outside-in signaling is involved in post-arrest adhesion strengthening47 and probably also in transendothelial migration.48,49 Thus, the combination of impaired integrin activation and outside-in signaling provides a plausible explanation for the complete defect of Fermt3−/− neutrophil recruitment in thioglycollate-induced peritonitis, even though slow rolling is preserved in kindlin-3–deficient neutrophils. This is also consistent with the severe innate immune deficiency in LAD-III patients, suggesting that inducing the extended conformation of LFA-1 is not sufficient for effective neutrophil recruitment in vivo, but high-affinity LFA-1 must be induced by kindlin-3 binding. The potential defects in Mac-1 (integrin αMβ2) activation in the absence of talin-1 or kindlin-3 would not be expected to contribute to the blockade of neutrophil recruitment, as Mac-1 deficiency does not impair neutrophil influx in experimental peritonitis.50
The mechanisms by which talins and kindlins are recruited to the plasma membrane to bind β integrin cytoplasmic tails remain poorly understood. In vitro experimental systems have found a role for Rap1 interacting adaptor molecule (RIAM) in forming an “integrin activation complex” by directly binding to both Rap1 and talin.51,52 Thus, membrane targeting sequences in Rap1 mediate recruitment of talin to the integrin. However, the mechanisms of Rap1-dependent β2 integrin activation in leukocytes appear to be more complex, with Rap1 also playing a role in integrin trafficking.53,54 How kindlins are recruited to the cytoplasmic face of the integrin is even less clear. The kindlin-2 pleckstrin homology domain, distinct from the integrin-binding F3 subdomain, has been shown to be involved in β1 and β3 integrin activation by interacting with phosphoinositides.55 Kindlin-2 preferentially binds phosphatidylinositol 3,4,5-trisphosphate,55 suggesting a potential role for PI3K in kindlin recruitment to the plasma membrane. Our findings that talin-1 and kindlin-3 are involved in distinct steps of conformational integrin activation suggest that the 2 proteins may also have different mechanisms for recruitment to the integrin cytoplasmic tail, although this remains to be elucidated in future studies.
We were able to test the respective roles of talin-1 and kindlin-3 in activation of LFA-1 to an extended/closed conformation, the putative intermediate affinity state, because neutrophils functionally use this LFA-1 conformer in a manner qualitatively distinct from that of the extended/open conformation (slow rolling vs firm adhesion).18,19 Such an assay has thus far been experimentally intractable in other cell types. For example, interpretation of adhesion or ligand binding assays must take both integrin affinity and valency into account. Nevertheless, because integrins are thought to exist in a conformational equilibrium,3 it is probable that the regulation of transitions between extended integrin conformers is a common feature among integrin subtypes rather than a phenomenon specific to LFA-1. Whether talins and kindlins exhibit distinct roles in the regulation and stabilization of specific conformations in other integrin subtypes and in response to other stimuli remains to be determined.
In the current study, we have shown that LFA-1 integrin activation through engagement of the selectin ligand PSGL-1 or through constitutively active Rap1a requires talin-1, but not kindlin-3, to induce LFA-1 extension to an intermediate affinity state that mediates neutrophil slow rolling. In addition to these functional data, we have shown that binding of NKI-L16 and KIM127, reporters of LFA-1 extension, requires talin-1, but not kindlin-3, whereas binding of high-affinity reporters 2E8 and mAb 24 requires both. These findings represent the first evidence for a differential role of talin-1 and kindlin-3 in integrin inside-out activation in rolling and arresting leukocytes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Carl Figdor (Radboud University Nijmege Medical Centre, Nijmegen, The Netherlands) for the NKI-L16 antibody, Dr Qing Ma (MD Anderson Cancer Center, University of Texas, Houston, TX) for the 2E8 antibody, and Hui Ouyang and Kathleen Lloyd for technical assistance.
This work was supported by the National Institutes of Health (T32 AI060536, C.T.L.; and R01 EB002185, K.L.), the German Research Foundation (AZ 428/3-1 and AZ 428/6-1, A.Z.; and SFB914, M.M.), and the Interdisciplinary Clinical Research Center (IZKF Muenster, Germany, Za2/001/10, A.Z.).
National Institutes of Health
Authorship
Contribution: C.T.L. designed and performed studies, analyzed and interpreted data, and wrote the manuscript; J.R. and A.Z. designed and performed experiments with HL-60 cells; M.M., B.G.P., S.J.M., D.R.C., M.H.G., and R.F. provided animals and other materials; and K.L. designed studies, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Klaus Ley, La Jolla Institute for Allergy and Immunology, 9420 Athena Circle, La Jolla, CA 92037; e-mail: klaus@liai.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal