Abstract
Apoptotic caspases, including caspase-9, are thought to facilitate platelet shedding by megakaryocytes. They are known to be activated during platelet apoptosis, and have also been implicated in platelet hemostatic responses. However, the precise requirement for, and the regulation of, apoptotic caspases have never been defined in either megakaryocytes or platelets. To establish the role of caspases in platelet production and function, we generated mice lacking caspase-9 in their hematopoietic system. We demonstrate that both megakaryocytes and platelets possess a functional apoptotic caspase cascade downstream of Bcl-2 family-mediated mitochondrial damage. Caspase-9 is the initiator caspase, and its loss blocks effector caspase activation. Surprisingly, steady-state thrombopoiesis is unperturbed in the absence of caspase-9, indicating that the apoptotic caspase cascade is not required for platelet production. In platelets, loss of caspase-9 confers resistance to the BH3 mimetic ABT-737, blocking phosphatidylserine (PS) exposure and delaying ABT-737–induced thrombocytopenia in vivo. Despite this, steady-state platelet lifespan is normal. Casp9−/− platelets are fully capable of physiologic hemostatic responses and functional regulation of adhesive integrins in response to agonist. These studies demonstrate that the apoptotic caspase cascade is required for the efficient death of megakaryocytes and platelets, but is dispensable for their generation and function.
Introduction
Apoptotic caspases are a family of aspartate-specific cysteinyl proteases that are activated during, and facilitate the execution of, programmed cell death. They cleave a range of cellular substrates, thereby causing the morphologic and biochemical signatures of apoptosis, such as DNA fragmentation, membrane blebbing, and phosphatidylserine (PS) externalization. In addition to their role in cell death, apoptotic caspases have been ascribed an increasing number of functions. These include critical roles in the differentiation of erythroid cells,1 osteoblasts,2 keratinocytes,3 lens fiber cells,4 skeletal muscle,5 and embryonic stem (ES) cells.6 Apoptotic caspases have also been suggested to regulate B-lymphocyte proliferation,7 HSC quiescence,8 activation of microglia,9 and the reprogramming of fibroblasts into iPS (induced pluripotent stem cells).10
There are 2 pathways to apoptosis, the intrinsic and the extrinsic. Both ultimately converge on the apoptotic effector caspases, caspase-3 and caspase-7. The intrinsic (or mitochondrial) apoptosis pathway is regulated by the interaction between Bcl-2 family proteins, which are divided into prosurvival and prodeath subsets. The critical mediators of the pathway are the prodeath proteins Bak and Bax, which, in viable cells are restrained by one or more of the 5 prosurvivals: Bcl-2, Bcl-xL, Mcl-1, Bcl-w, and A1. In response to apoptotic signals such as DNA damage or growth factor deprivation, a third group of Bcl-2 family members, the “BH3-only” proteins, trigger the activation of Bak and Bax.11,12 Active Bak and Bax cause mitochondrial outer membrane permeabilization (MOMP), which allows apoptogenic factors, including cytochrome c, to enter the cytosol.13 Cytochrome c interacts with the scaffolding protein Apaf-1. Subsequently, the initiator caspase, caspase-9, is recruited to the cytochrome c/Apaf-1 complex to form the apoptosome.14 This leads to the activation of caspase-9 and triggering of the caspase cascade. The importance of the caspase cascade for cellular homeostasis is underscored by the observation that constitutive deletion of Apaf-1 or caspase-9 in mice results in embryonic lethality, analogous to mice lacking the mitochondrial “gatekeepers” Bak and Bax. In some cell types, particularly those of the central nervous system, deletion of Apaf-1 or caspase-9, or ablation of cytochrome c's apoptotic function, effectively blocks apoptosis and results in cellular accumulation.15-17 In contrast, while loss of caspase-9 in fibroblasts and thymocytes can slow the rate at which apoptosis occurs, it cannot prevent cell death, and does not impede cell clearance.18
Megakaryocytes are large polyploid cells that develop primarily in the bone marrow (BM) and spleen. They are responsible for the production of platelets, small anucleate cells essential for hemostasis. During platelet shedding, megakaryocytes undergo a massive cellular reorganization that leads to the formation of proplatelets, large cytoplasmic extensions along which organelles and granules are transported. Proplatelets are extruded into the circulation where shear forces trigger their fragmentation, resulting in the release of platelets. A substantial body of evidence has led to the view that during this process, megakaryocytes deliberately activate caspases.19-24 Active apoptotic caspases have been observed in cultured megakaryocytes and megakaryocyte cell lines, and fluoromethylketone-based caspase inhibitors such as z-VAD.fmk impair the ability of mature megakaryocytes to form proplatelet extensions in vitro.20,21 This is believed to reflect a requirement for apoptotic caspase activity in cleaving and activating substrates that facilitate cytoskeletal remodeling.
Multiple studies have supported the notion that apoptotic caspase activity in platelet-shedding megakaryocytes is the result of intrinsic apoptosis pathway activation. Cytochrome c was detected in the cytoplasm of proplatelet-bearing megakaryocytes in vitro,21 and in humans, a variant of cytochrome c with enhanced apoptotic activity has been linked to autosomal-dominant thrombocytopenia.22 Mice overexpressing Bcl-2 in the hematopoietic compartment,23 or mice lacking the BH3-only protein Bim,24 are thrombocytopenic. Furthermore, overexpression of Bcl-2 in cultured megakaryocytes abrogates proplatelet formation.21
Emerging evidence has begun to call into question the idea that platelet production is an apoptotic process. We recently demonstrated that mature megakaryocytes contain a functional intrinsic apoptosis pathway, the key components of which are prosurvival Bcl-xL and prodeath Bak and Bax.25 Restraint of Bak and Bax by Bcl-xL is required for megakaryocytes to progress safely through proplatelet formation and platelet shedding in vivo. Genetic deletion or pharmacologic inhibition of Bcl-xL triggered megakaryocyte apoptosis, failure of proplatelet formation, and cell death. Conversely, disabling the pathway by knocking out Bak and Bax had no impact on thrombopoiesis. This is consistent with data showing that platelet counts in Bcl-2 transgenic mice are not significantly different to wild type when the spleen is removed,26 and that Bim−/− mice on a C57BL/6 background are no longer thrombocytopenic.27 Thus, it appears that Bcl-2 family–mediated apoptosis is not used by megakaryocytes to produce platelets. Activation of the intrinsic pathway is detrimental to megakaryocytes.
The role of apoptotic caspases in platelet production, however, remains unclear. In contrast to z-VAD.fmk, the difluorophenoxy-methylketone–based inhibitor Q-VD-OPh did not impair proplatelet formation by cultured megakaryocytes.25 Active caspases were found in megakaryocytes lacking Bcl-xL, or megakaryocytes treated with the BH3 mimetic compound ABT-737,28 but their contribution to the cell-death process was not determined. Whether megakaryocytes possess a classic apoptotic caspase cascade, with caspase-9 at its apex, has never been established. It is possible that in megakaryocytes, apoptotic caspases can be triggered independent of Bak/Bax-mediated mitochondrial damage, to facilitate platelet shedding.
In addition to their role in megakaryocytes, apoptotic caspases have been implicated in various aspects of platelet function.29 Although it is now well established that Bcl-xL and Bak regulate platelet survival and lifespan at steady state,30-34 the requirement for caspases in mediating platelet turnover is unclear. Certainly, induction of platelet apoptosis results in caspase activation, and caspase inhibition can block PS exposure,33 but the physiologic significance of this phenomena remains to be clarified. Intriguingly, caspases have also been linked to agonist-induced platelet functional responses. Several groups have reported caspase activation in response to agonist, and caspase inhibitors have been suggested to impair platelet aggregation.35-38 Evidence suggests that platelet apoptosis is distinct from platelet activation,33,39 but the contribution of caspases to both processes remains to be formally defined.
In this study, we have examined the consequences of caspase-9 deletion on the development and function of megakaryocytes and platelets. Using mice reconstituted with wild-type or caspase-9–deficient fetal liver cells (FLCs), we show that both megakaryocytes and platelets possess a functional apoptotic caspase cascade. Caspase-9 is the initiator caspase, and its loss blocks effector caspase activation in response to apoptotic stimuli. Surprisingly, steady-state thrombopoiesis is unperturbed in the absence of caspase-9, indicating that the apoptotic caspase cascade is not required for platelet production. In platelets, loss of caspase-9 confers resistance to ABT-737, blocking PS exposure and delaying ABT-737–induced thrombocytopenia in vivo. Despite this, steady-state platelet lifespan is normal. Casp9−/− platelets are fully capable of physiologic hemostatic responses and functional regulation of adhesive integrins in response to agonist.
Methods
Experimental animals
The generation and genotyping of Casp9−/− and Bak−/−Bax−/− mice used in this study has been described previously.40,41 Constitutive deletion of caspase-9 or Bak and Bax in mice results in embryonic lethality. To examine the consequence of their deletion on the megakaryocyte compartment in vivo, we generated hematopoietic chimeric mice by fetal liver transplantation. To generate caspase-9–deficient and Bak/Bax doubly deficient E13.5 donor fetal livers, timed intercrosses of Casp9+/− and Bak−/−Bax+/− mice were performed and embryos were harvested from pregnant females 13.5 days postconception. FLCs were then used to reconstitute lethally irradiated C57BL/6 CD45.1+ adult male recipients. All mouse strains used as donors were on an inbred C57BL/6 CD45.2+ background or had been backcrossed at least 10 generations. All animal experiments complied with the regulatory standards of, and were approved by, The Walter and Eliza Hall Institute or Alfred Medical Research and Education Precinct Animal Ethics Committees.
Megakaryocyte culture, purification, and proplatelet formation assay
E13.5 embryos were harvested into cold DME Kelso, 10% FCS, and fetal livers were dissected out and transferred to fresh media. FLC suspensions were prepared in PBS and used fresh or were frozen in 10% DMSO. A total of 5 × 105 cells/mL were seeded in a 6-well plate into serum-free media supplemented with 100 ng/mL murine thrombopoietin (TPO) produced in-house. Cells were cultured for 3 days at 37°C, 5% CO2, and overlaid on a discontinuous BSA density gradient (3%, 1.5%, 0%) in PBS, and allowed to stand for 35 minutes at room temperature. Large megakaryocytes from the 3% bottom layer were harvested and reseeded in 96-well plates 300-500 cells/well into serum-free media supplemented with TPO. Megakaryocytes forming proplatelets were counted the following days on a light microscope.
Cell-death assays
Washed platelets, at a concentration of 108 platelets/mL, were incubated at 37°C with ABT-737 for the indicated time with or without coincubation with 50μM Q-VD-OPh for 15 minutes at room temperature before ABT-737 treatment. Gradient-purified megakaryocytes, were seeded at 25 000 cells/mL in a 96-well U-bottom culture plate, followed by incubation at 37°C with ABT-737, staurosporine, or etoposide for the indicated time. Cell death was assayed by the addition of CellTiter-Glo, caspase-3/7–Glo, or caspase-9–Glo (Promega) reagent according to the manufacturer's instructions using a LumiSTAR Galaxy luminometer (BMG Labtech).
Measurement of PS exposure
PS exposure was measured by flow cytometry on a FACSCalibur (BD Biosciences) as described previously.33
Platelet clearance analysis
Platelet lifespan in vivo was quantified as described previously.27
ABT-737–induced thrombocytopenia
ABT-737 (1 mg/mL in DMSO) was diluted in a mixture of 30% propylene glycol, 5% Tween 80, 65% D5W (5% dextrose in water), pH 4-5; the final concentration of DMSO was < 1%. Mice were injected intraperitoneally with a single dose of 75 mg/kg ABT-737. Blood was collected from the retro-orbital plexus into tubes containing potassium EDTA (Sarstedt) and peripheral blood platelet number was determined using an Advia 2120 automated hematologic analyzer (Siemens).
Platelet function and hemostasis
Platelets were stimulated for 15 minutes at 22°C with thrombin or a combination of thrombin and convulxin. Activation was assessed by flow cytometry with Abs specific to activation markers (anti-CD62P for P-selectin exposure, anti-GPIIb/IIIa [clone JON/A] for the active integrin αIIbβ3) and annexin V protein to measure PS exposure. A list of Abs can be found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Template tail bleeding was performed as previously described.42 To assess hemostasis in a sensitized assay, mice were injected with 1 mg/kg hirudin via the jugular vein 10 minutes before template incision. For tail transection, the tail was transected at 3 mm from the tip and immediately immersed in 37°C saline. The bleeding time was determined as the time from the tail transection to the cessation of blood flow. Aggregation was performed using washed platelet suspensions (2.0 × 108/mL) supplemented with 1mM calcium and 0.5 mg/mL human fibrinogen. Aggregation was initiated by addition of ADP, thrombin, or collagen-related peptide (CRP), at the indicated concentrations, and monitored for 10 minutes at 37°C (150g) in a 4-channel automated platelet analyzer (AggRAM; Helena Laboratories). The extent of platelet aggregation was defined as the percentage change in optical density as measured by the automated platelet analyzer.
Statistical analysis
Analyses were performed using Prism software (GraphPad). P values were calculated by Student 2-tailed t test with Bonferroni correction for multiple comparisons.
Miscellaneous methods
Miscellaneous methods including materials and Abs, preparation of washed platelets, gradient purification of cultured megakaryocytes, serum thrombopoietin analysis, and megakaryocyte ploidy can be found in supplemental Methods.
Results
Caspase-9 is dispensable for platelet production
To determine the role of caspase-9 in the megakaryocyte lineage, we generated chimeric animals in which the hematopoietic system had been reconstituted with Casp9−/− FLCs. Livers were collected from Casp9+/+, Casp9+/−, or Casp9−/− C57BL/6 CD45.2 mice at embryonic day (E) 13.5, and transplanted into lethally irradiated wild-type C57BL/6 congenic CD45.1 recipients. Eight to 10 weeks posttransplant, donor engraftment was confirmed by flow cytometric analysis of peripheral blood leukocytes (supplemental Figure 1A). Casp9−/− FLCs engrafted efficiently and, as previously reported, recipients of Casp9−/− FLCs were outwardly healthy.18 Western blot analysis demonstrated that caspase-9 was deleted in purified platelets derived from Casp9−/− FLC-reconstituted mice (supplemental Figure 1B). Only chimeric mice exhibiting > 90% donor contribution to peripheral blood leukocytes at 8-10 weeks posttransplantation were used in this study.
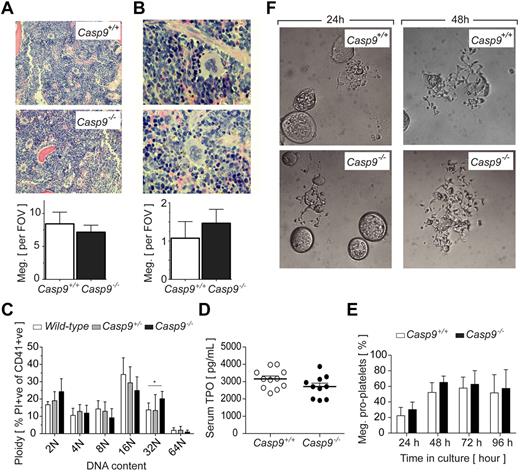
Peripheral blood platelet counts in recipients of Casp9+/+ or Casp9−/− FLCs were comparable (Table 1). Histologic examination of megakaryocytes in BM and spleen revealed no differences in number or gross morphology (Figure 1A-B, supplemental Figure 2). An analysis of DNA content showed a modest but statistically significant shift toward higher ploidy in the Casp9−/− megakaryocyte population (Figure 1C). Serum levels of TPO, the major cytokine regulator of megakaryopoiesis, were equivalent in recipients of Casp9+/+ and Casp9−/− FLCs (Figure 1D). Based on previous reports of apoptotic caspase activity in the cytoplasm of proplatelet-bearing megakaryocytes,20,21 we investigated the role of caspase-9 in this process. Casp9+/+ and Casp9−/− FLC-derived megakaryocytes were purified from a BSA gradient and reseeded in serum-free media containing TPO. Over the course of 4 days in culture, no obvious alterations in the formation of proplatelets by Casp9−/− megakaryocytes were detected (Figure 1E-F). Collectively, these data indicate that caspase-9 is not required for steady-state megakaryopoiesis or platelet production.
Peripheral blood parameters in FLC-reconstituted mice
| . | Casp9+/+, n = 13 . | Casp9−/−, n = 11 . |
|---|---|---|
| Lymphocytes, 106/mL | 9.86 ± 1.27 | 5.88 ± 1.09* |
| Neutrophils, 106/mL | 0.83 ± 0.20 | 0.92 ± 0.22 |
| Platelets, 106/mL | 929 ± 183 | 851 ± 208 |
| Mean platelet volume, fL | 8.26 ± 0.28 | 8.41 ± 0.27 |
| Erythrocytes, 109/mL | 10.92 ± 1.39 | 11.10 ± 0.28 |
| Hematocrit, % | 52.04 ± 6.61 | 50.33 ± 1.43 |
| Reticulocytes, % | 2.93 ± 0.21 | 3.58 ± 0.49* |
| . | Casp9+/+, n = 13 . | Casp9−/−, n = 11 . |
|---|---|---|
| Lymphocytes, 106/mL | 9.86 ± 1.27 | 5.88 ± 1.09* |
| Neutrophils, 106/mL | 0.83 ± 0.20 | 0.92 ± 0.22 |
| Platelets, 106/mL | 929 ± 183 | 851 ± 208 |
| Mean platelet volume, fL | 8.26 ± 0.28 | 8.41 ± 0.27 |
| Erythrocytes, 109/mL | 10.92 ± 1.39 | 11.10 ± 0.28 |
| Hematocrit, % | 52.04 ± 6.61 | 50.33 ± 1.43 |
| Reticulocytes, % | 2.93 ± 0.21 | 3.58 ± 0.49* |
FLC indicates fetal liver cell.
indicates P < .05.
Platelet production proceeds normally in the absence ofcaspase-9. (A) BM megakaryocyte number is not altered in caspase-9–deficient FLC-reconstituted mice. Average number of megakaryocytes in histologic sections of BM per 10 high-powered fields of view (FOV; original magnification, ×200). Data represent mean ± SD; n = 10-12 mice per genotype. (B) Spleen megakaryocyte number is not altered in Casp9−/− FLC-reconstituted mice. Average number of megakaryocytes in histologic sections of spleen per 5 high-powered FOV (original magnification, ×600). Data represent mean ± SD; n = 4-6 mice per genotype. (C) The megakaryocyte ploidy distribution profile of CD41+ BM cells is moderately altered by caspase-9 deficiency. Data represent mean ± SD; n = 4-8 mice per genotype; **P < .01. (D) Casp9−/− FLC-reconstituted mice have serum TPO levels equivalent to wild type. Data represent mean ± SEM; n > 10 mice per genotype. (E) Loss of caspase-9 does not perturb proplatelet formation. FLC-derived megakaryocytes were visually scored for proplatelet formation over the time indicated. Data represent the mean ± SD; n = 4-5 fetal livers per genotype. (F) Representative microscopic image of Casp9+/+ and Casp9−/− FLC-derived mature megakaryocytes forming proplatelets in culture. Original magnification, ×400.
Platelet production proceeds normally in the absence ofcaspase-9. (A) BM megakaryocyte number is not altered in caspase-9–deficient FLC-reconstituted mice. Average number of megakaryocytes in histologic sections of BM per 10 high-powered fields of view (FOV; original magnification, ×200). Data represent mean ± SD; n = 10-12 mice per genotype. (B) Spleen megakaryocyte number is not altered in Casp9−/− FLC-reconstituted mice. Average number of megakaryocytes in histologic sections of spleen per 5 high-powered FOV (original magnification, ×600). Data represent mean ± SD; n = 4-6 mice per genotype. (C) The megakaryocyte ploidy distribution profile of CD41+ BM cells is moderately altered by caspase-9 deficiency. Data represent mean ± SD; n = 4-8 mice per genotype; **P < .01. (D) Casp9−/− FLC-reconstituted mice have serum TPO levels equivalent to wild type. Data represent mean ± SEM; n > 10 mice per genotype. (E) Loss of caspase-9 does not perturb proplatelet formation. FLC-derived megakaryocytes were visually scored for proplatelet formation over the time indicated. Data represent the mean ± SD; n = 4-5 fetal livers per genotype. (F) Representative microscopic image of Casp9+/+ and Casp9−/− FLC-derived mature megakaryocytes forming proplatelets in culture. Original magnification, ×400.
Caspase-9 is the apoptotic initiator caspase in megakaryocytes
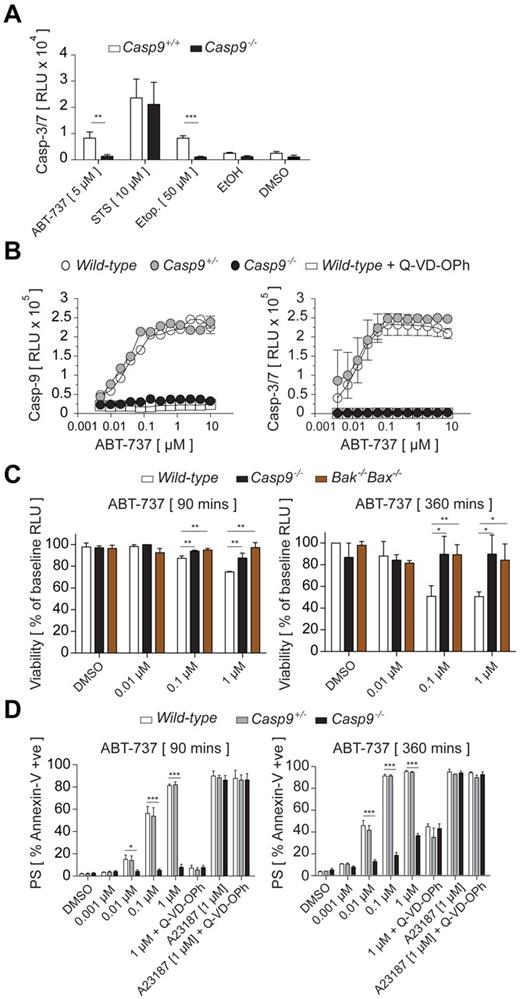
Given that caspase-9 is dispensable for the generation of platelets, we sought to clarify its role in megakaryocyte apoptosis. In many nucleated cell types, caspase-9 functions as the initiator of the caspase cascade. It becomes activated in response to apoptotic mitochondrial damage, subsequently triggering the downstream effector caspases-3 and -7.16 Mature Casp9+/+ and Casp9−/− FLC-derived megakaryocytes were treated with 3 compounds that are known to activate the intrinsic apoptosis pathway in other cell types: ABT-737, a BH3 mimetic compound that antagonizes Bcl-xL and the related prosurvival proteins Bcl-2 and Bcl-w,28 the topoisomerase II inhibitor etoposide, and the broad-spectrum kinase inhibitor staurosporine (STS). Subsequently, activation of the apoptotic effector caspases-3/7 was measured. In Casp9+/+ megakaryocytes, treatment with ABT-737, etoposide, or STS triggered rapid caspase-3/7 activation (Figure 2A). In the absence of caspase-9, caspase-3/7 activation in response to ABT-737 or etoposide was attenuated (Figure 2A). These data demonstrate that megakaryocytes possess an apoptotic caspase cascade, with caspase-9 functioning as the initiator, operating downstream of mitochondrial permeabilization. Interestingly, loss of caspase-9 did not prevent caspase-3/7 activation in response to STS (Figure 2A). Together with the recent demonstration that deletion of Bak and Bax in megakaryocytes also fails to prevent STS-induced death,25 it appears that in contrast to other cell types such as mouse embryonic fibroblasts,43 STS appears to activate additional cell-death pathways megakaryocytes.
Caspase-9 promotes megakaryocyte and platelet apoptosis. (A) Loss of caspase-9 inhibits activation of downstream caspase-3/7 in megakaryocytes. FLC-derived megakaryocytes were treated with staurosporine (STS), etoposide (Etop.), ABT-737, or ethanol (EtOH) or DMSO vehicle controls for 5 hours. Lysates were prepared and assayed for their ability to cleave the specific substrate for caspase-3/7 (z-DEVD-pNA). Data represent the mean ± SD; n = 3-4 livers per genotype (except EtOH and DMSO vehicle controls, n = 2); **P < .01, ***P < .001. (B) Loss of caspase-9 inhibits activation of downstream caspase-3/7 following Bcl-2 antagonism. Lysates were prepared and assayed for their ability to cleave specific substrates for caspase-9 (z-LEHD-pNA) or caspase-3/7 (z-DEVD-pNA). Data represent the mean ± SD; n = 3 mice per genotype (except wild type + Q-VD-OPh, n = 1). (C) Comparable attenuation of apoptosis in Casp9−/− platelets and those lacking Bak/Bax. Washed platelets were treated with varying amounts of ABT-737 for the time indicated. ATP content, proportional to light signal output, was determined by luminescence. Data represent the mean ± SD; n = 3 mice per genotype; *P < .05, **P < .01. (D) Loss of caspase-9 perturbs PS externalization. Washed platelets were treated with varying amounts of ABT-737 for 90 minutes (left panel) or 360 minutes (right panel), or the calcium ionophore A23187, and stained with annexin V before flow cytometric analysis. Data represent the mean ± SD, n = 3-5 mice per genotype; *P < .05, ***P < .001.
Caspase-9 promotes megakaryocyte and platelet apoptosis. (A) Loss of caspase-9 inhibits activation of downstream caspase-3/7 in megakaryocytes. FLC-derived megakaryocytes were treated with staurosporine (STS), etoposide (Etop.), ABT-737, or ethanol (EtOH) or DMSO vehicle controls for 5 hours. Lysates were prepared and assayed for their ability to cleave the specific substrate for caspase-3/7 (z-DEVD-pNA). Data represent the mean ± SD; n = 3-4 livers per genotype (except EtOH and DMSO vehicle controls, n = 2); **P < .01, ***P < .001. (B) Loss of caspase-9 inhibits activation of downstream caspase-3/7 following Bcl-2 antagonism. Lysates were prepared and assayed for their ability to cleave specific substrates for caspase-9 (z-LEHD-pNA) or caspase-3/7 (z-DEVD-pNA). Data represent the mean ± SD; n = 3 mice per genotype (except wild type + Q-VD-OPh, n = 1). (C) Comparable attenuation of apoptosis in Casp9−/− platelets and those lacking Bak/Bax. Washed platelets were treated with varying amounts of ABT-737 for the time indicated. ATP content, proportional to light signal output, was determined by luminescence. Data represent the mean ± SD; n = 3 mice per genotype; *P < .05, **P < .01. (D) Loss of caspase-9 perturbs PS externalization. Washed platelets were treated with varying amounts of ABT-737 for 90 minutes (left panel) or 360 minutes (right panel), or the calcium ionophore A23187, and stained with annexin V before flow cytometric analysis. Data represent the mean ± SD, n = 3-5 mice per genotype; *P < .05, ***P < .001.
Caspase-9 mediates platelet apoptosis and PS externalization
Platelets treated with ABT-737 undergo Bak/Bax-mediated death, exhibiting the classic hallmarks of mitochondrial apoptosis: cytochrome c release, activation of caspase-3, and exposure of PS on the outer leaflet of the plasma membrane.30,33,34,44,45 To determine the role of caspase-9 in this process, we treated platelets from wild-type, Bak−/−Bax−/−, or Casp9−/− FLC-reconstituted mice with ABT-737 for 90 minutes and measured apoptotic caspase activity, and ATP levels (an indicator of mitochondrial function). In wild-type platelets, a strong dose-dependent activation of caspase-9, and of the effector caspases-3 and -7, was observed (Figure 2B). This was completely blocked in Casp9−/− platelets, or in Casp9+/+ platelets preincubated with the pan-caspase inhibitor Q-VD-OPh46 (Figure 2B). Interestingly, ATP levels in Casp9−/− platelets did not significantly decline in response to ABT-737, remaining similar to those in Bak−/−Bax−/− cells (Figure 2C). A caveat to this ATP assay may be that the decline in ATP levels during apoptosis was underestimated because of the presence of a high background concentration of platelet-dense granule ATP. Nevertheless, this suggests that, over the course of 90 minutes, loss of caspase-9 preserves mitochondrial function to a similar degree as the combined absence of Bak and Bax.
To see what effect loss of caspase-9 might have on PS exposure, platelets were treated with ABT-737 for 90 minutes and cell-surface PS levels determined by flow cytometric analysis of annexin V binding. In the absence of caspase-9, PS externalization was abolished (Figure 2D). Consistent with previous studies, the same effect was achieved by coincubation with Q-VD-OPh.33 In contrast, neither caspase inhibition nor deletion of caspase-9 had any effect on PS exposure induced by the calcium ionophore A23187 (Figure 2D). To determine whether apoptotic PS exposure is completely dependent on caspase-9, the experiment was repeated with an incubation time of 360 minutes. Under these conditions, a proportion of Casp9−/− platelets did expose PS, although the positive fraction was significantly reduced relative to preparations of Casp9+/+ or Casp9+/− platelets (Figure 2D). Casp9−/− platelets exhibited a similar degree of PS exposure to wild-type platelets coincubated with Q-VD-OPh, suggesting that the mechanism is independent of caspases. Collectively, these data demonstrate that caspase-9 is the key initiator caspase downstream of apoptotic mitochondrial damage in platelets.
Caspase-9 mediates ABT-737–induced thrombocytopenia but is dispensable for normal platelet lifespan at steady state
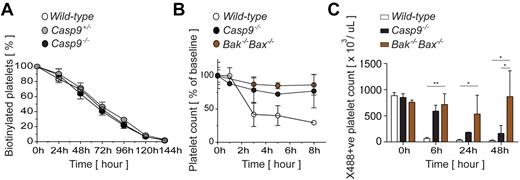
In the absence of Bak, steady-state platelet lifespan in vivo is almost doubled.34 Our findings raised the possibility that loss of caspase-9 might have the same effect. We examined the kinetics of platelet clearance by injecting FLC-reconstituted mice with biotin and tracking the disappearance of platelets from the circulation. Surprisingly, platelet survival curves in recipients of Casp9+/+ and Casp9−/− FLCs were indistinguishable (Figure 3A). Thus, unlike Bak- and Bax-mediated mitochondrial damage, the initiator function of caspase-9 is not required for the cellular process that mediates removal of platelets from the circulation to proceed.
Loss ofcaspase-9 modifies the kinetics of ABT-737–induced thrombocytopenia but not platelet lifespan at steadystate. (A) Steady-state platelet clearance can proceed independently of caspase-9. Survival of in vivo biotinylated platelets was determined by pulse-chase experiments. After whole-blood labeling with biotin, blood samples were obtained by tail bleeding at time intervals and the disappearance of circulating platelets. Data represent the mean ± SD; n = 4 mice per genotype. (B) Pre- and postmitochondrial blocks in apoptosis modify the kinetics of ABT-737–induced thrombocytopenia. Mice were treated with a single dose of ABT-737, killed at time intervals, and platelet counts were determined at the indicated time intervals by automated counting. Data represent the mean ± SD; n = 2-6 mice/genotype per time point. (C) Caspase-9 deletion does not confer long-term resistance to ABT-737 in vivo. Circulating platelets were prelabeled with X488. Mice were then injected with a single dose of ABT-737. At the time points indicated, blood samples were obtained by tail bleeding and the presence of X488+ platelets was determined by flow cytometry. Data represent the mean ± SD; n = 5-7 mice per genotype; *P < .05, **P < .01.
Loss ofcaspase-9 modifies the kinetics of ABT-737–induced thrombocytopenia but not platelet lifespan at steadystate. (A) Steady-state platelet clearance can proceed independently of caspase-9. Survival of in vivo biotinylated platelets was determined by pulse-chase experiments. After whole-blood labeling with biotin, blood samples were obtained by tail bleeding at time intervals and the disappearance of circulating platelets. Data represent the mean ± SD; n = 4 mice per genotype. (B) Pre- and postmitochondrial blocks in apoptosis modify the kinetics of ABT-737–induced thrombocytopenia. Mice were treated with a single dose of ABT-737, killed at time intervals, and platelet counts were determined at the indicated time intervals by automated counting. Data represent the mean ± SD; n = 2-6 mice/genotype per time point. (C) Caspase-9 deletion does not confer long-term resistance to ABT-737 in vivo. Circulating platelets were prelabeled with X488. Mice were then injected with a single dose of ABT-737. At the time points indicated, blood samples were obtained by tail bleeding and the presence of X488+ platelets was determined by flow cytometry. Data represent the mean ± SD; n = 5-7 mice per genotype; *P < .05, **P < .01.
Given that deletion of caspase-9 can protect platelets from the effects of ABT-737 in vitro, we therefore asked what role it plays in the rapid-onset thrombocytopenia triggered by ABT-737 in vivo.34,45 Cohorts of Casp9+/+, Casp9−/−, and Bak−/−Bax−/− FLC-reconstituted mice were administered a single dose of ABT-737 and platelet counts were monitored. As expected, acute thrombocytopenia was observed in recipients of Casp9+/+ FLCs, with platelet counts dropping to ∼ 25% of baseline by 8 hours (Figure 3B). In contrast, platelet counts in Casp9−/− and Bak−/−Bax−/− FLC-reconstituted animals remained stable over this time frame (Figure 3B). We hypothesized that the protection afforded by loss of caspase-9 might be short-term, and so repeated the experiment. To circumvent the masking effect of new platelet production, we firstly injected FLC-reconstituted mice with X488, a fluorescent label specific for platelets. After the labeling step, a single dose of ABT-737 was given, and platelet numbers were tracked by flow cytometric analysis of peripheral blood. As expected, at 6 hours we observed minimal clearance of labeled Casp9−/− and Bak−/−Bax−/− platelets (Figure 3C). However, at 24 and 48 hours post-ABT-737 treatment, most Casp9−/− platelets had been removed from the circulation. This was in contrast to Bak−/−Bax−/− platelets, numbers of which were largely stable. Thus, it appears that platelets exposed to ABT-737 undergo commitment to apoptotic clearance at the level of Bak- and Bax-mediated mitochondrial damage. Deletion of caspase-9 can provide temporary protection by slowing the rate at which apoptosis occurs, but does not ultimately prevent clearance.
Caspase-9–deficient platelets are hemostatically functional
Several previous studies have suggested that platelets activate caspases in response to classic agonists, and that broad-spectrum caspase inhibition can interfere with platelet function.35-38 We therefore treated Casp9+/+ and Casp9−/− platelets with a range of agonists and measured their response. Flow cytometric analysis of P-selectin expression, conformational change in integrin GPIIb/IIIa, and PS exposure did not reveal any major abnormalities in the activation response of Casp9−/− platelets compared with wild type (Figure 4A). Aggregation assays conducted with a range of stimuli revealed no defects (Figure 4B). To explore a possible role for caspase-9 in hemostasis, bleeding times were measured by the template method (Figure 4C). No differences were observed between Casp9+/+ and Casp9−/− FLC-reconstituted mice; however, given the spread in bleeding times of the Casp9−/− animals, we performed additional sensitized bleeding assays. Pretreating mice with hirudin extended the overall bleeding time in both Casp9+/+ and Casp9−/− transplant recipients, but did not reveal an underlying difference (Figure 4D). This was also true when the alternative transection tail-bleeding method was used (Figure 4E). Taken together, these data indicate that Casp9−/− FLC-reconstituted mice are not hemostatically compromised.
Hemostasis and platelet function is unaltered by the absence ofcaspase-9. (A) Caspase-9 is not essential for normal platelet activation in vitro. Washed platelets were stimulated in vitro with activation agonists. The cell-surface profile of platelets was examined by measuring the platelet activation markers P-selectin, integrin GpIIb/IIIa, and PS exposure by flow cytometry. Data represent the mean ± SD; n = 4-5 mice per genotype (except Thr. [0.5 U/mL] + Conv. [500 ng/mL], n = 2). (B) Platelet aggregation kinetics is not altered by the absence of caspase-9. Aggregation of washed platelets derived from Casp9+/+ and Casp9−/− FLC-reconstituted mice was measured using an aggregometer. Data represent the mean ± SD; n > 3 mice/genotype per condition (except ADP 5.0μM, n = 2). (C) Loss of caspase-9 does not alter tail-bleeding time. Bleeding time was measured using the template method. Data represent the mean ± SEM; n = 5-7 mice per gentoype. (D) Template tail-bleeding time following administration of hirudin anticoagulant. Data represent the mean ± SEM; n = 6-8 mice per gentoype. (E) Bleeding time of transected tail. Data represent the mean ± SEM; n = 5-6 mice per genotype.
Hemostasis and platelet function is unaltered by the absence ofcaspase-9. (A) Caspase-9 is not essential for normal platelet activation in vitro. Washed platelets were stimulated in vitro with activation agonists. The cell-surface profile of platelets was examined by measuring the platelet activation markers P-selectin, integrin GpIIb/IIIa, and PS exposure by flow cytometry. Data represent the mean ± SD; n = 4-5 mice per genotype (except Thr. [0.5 U/mL] + Conv. [500 ng/mL], n = 2). (B) Platelet aggregation kinetics is not altered by the absence of caspase-9. Aggregation of washed platelets derived from Casp9+/+ and Casp9−/− FLC-reconstituted mice was measured using an aggregometer. Data represent the mean ± SD; n > 3 mice/genotype per condition (except ADP 5.0μM, n = 2). (C) Loss of caspase-9 does not alter tail-bleeding time. Bleeding time was measured using the template method. Data represent the mean ± SEM; n = 5-7 mice per gentoype. (D) Template tail-bleeding time following administration of hirudin anticoagulant. Data represent the mean ± SEM; n = 6-8 mice per gentoype. (E) Bleeding time of transected tail. Data represent the mean ± SEM; n = 5-6 mice per genotype.
Discussion
Since the late 1990s, several studies have produced evidence suggesting that the intrinsic apoptosis pathway is deliberately activated by megakaryocytes to facilitate proplatelet formation and platelet shedding.20-24 However, the precise requirement for this pathway had not been specifically determined. We recently demonstrated that megakaryocytes do indeed possess a functional intrinsic apoptosis pathway, but that it is not required for platelet production.25 In fact, activation of Bak and Bax in megakaryocytes resulted in failure of proplatelet formation and cell death. In mature megakaryocytes, Bak and Bax are restrained by the Bcl-2 family prosurvival protein Bcl-xL. Genetic deletion or pharmacologic inhibition of Bcl-xL triggered Bak/Bax-mediated mitochondrial damage, caspase activation, and premature death of megakaryocytes, resulting in a profound thrombocytopenia. Thus, it appears that in megakaryocytes, as in many other cell types, the interplay between Bcl-2 family proteins dictates whether a cell lives or dies. Bak/Bax-mediated apoptosis does not facilitate platelet production. Instead, megakaryocytes must restrain apoptosis to survive and progress safely through proplatelet formation and platelet shedding.
Although the role of the Bcl-2 family proteins and mitochondrial apoptosis in megakaryocytes is now clearer, the identity and function of apoptotic caspases in megakaryocytes remained to be addressed. In the current study, we have demonstrated that megakaryocytes possess an apoptotic caspase cascade that is activated in response to prosurvival protein inhibition by the BH3 mimetic compound ABT-737, or DNA damage induced by etoposide. Caspase-9 sits atop this cascade, functioning as the initiator. Genetic deletion of Caspase-9 in megakaryocytes impairs activation of the apoptotic effector caspases-3 and -7 over the time frame assayed. It has been previously shown that loss of Bak and Bax protects megakaryocytes from the effects of ABT-737, not only preventing mitochondrial damage and caspase activation, but also rescuing proplatelet formation.25 Our data support the notion that activation of apoptotic caspases in megakaryocytes is detrimental to platelet production. Whether inhibition of the caspase cascade would restore proplatelet formation in a megakaryocyte in which Bak and Bax were active remains to be determined. It may be that, even in the absence of the caspase cascade, Bak/Bax-mediated mitochondrial damage is sufficient to attenuate proplatelet formation.25 Certainly, it is likely that activation of Bak and Bax irreversibly commits the megakaryocyte to death.
Contrary to previous reports,20,21 our work demonstrates that, at steady state, caspase-9 is dispensable for proplatelet formation. This is supported by evidence that the broad-spectrum caspase inhibitor Q-VD-OPh does not impair proplatelet formation by cultured megakaryocytes.25 The possibility remains that one or more of the other apoptotic caspases has a nonapoptotic function that contributes to platelet shedding. Given our results and those of Josefsson et al,25 “nonapoptotic” in this context would be defined as operating independently of Bak/Bax-mediated mitochondrial damage and caspase-9 activation. In addition, our findings do not exclude nonapoptotic caspases, for example, the proinflammatory caspase, caspase-1, or other proteolytic enzymes, such as calpains or cathepsins, as potential mediators of proplatelet formation.
Analogous to megakaryocytes, we have shown that platelets possess an apoptotic caspase cascade, and that caspase-9 is the initiator caspase. When incubated with ABT-737 for 90 minutes in vitro, caspase-9–deficient platelets are protected from loss of mitochondrial function, and do not activate effector caspases or expose PS. This agrees with previous studies using Q-VD-OPh.33 In vivo, Casp9−/− FLC-reconstituted mice were protected from ABT-737–induced thrombocytopenia. However, in the latter experiment, protection was transient, and in vitro, extended incubation times demonstrated that loss of capase-9 merely delays platelet apoptosis, rather than preventing it. Consistent with this, platelet lifespan was normal in recipients of Casp9−/− FLCs. In contrast, platelet lifespan in Bak−/− or Bak−/−Bax−/− mice is almost doubled.25 So, while it is clear that activation of Bak and Bax is a critical step in the apoptotic death of platelets at steady state in vivo, activation of caspase-9 is not. Although further studies with mice lacking caspases-3 and -7 will be required for a definitive answer, it is therefore tempting to speculate that the entire apoptotic caspase cascade is dispensable for homeostatic platelet death and clearance in vivo. It may be that once Bak and Bax are activated, clearance of a platelet from the circulation occurs before apoptotic caspases are triggered. In this scenario, the elusive clearance signal would be caspase-independent, and, given our data, unlikely to be externalized PS.
In addition to their role in mediating platelet death, caspases have been proposed to be activated in response to platelet agonists, such as thrombin37,38 and calcium-ionophore,29,38 and caspase inhibitors have been suggested to impair platelet function.36 Our data indicate that activation of the initiator caspase-9 is not absolutely required for the functional response to platelet activation stimuli. We, and others, have previously shown that agonists do not induce platelet apoptosis.33,39 Platelets have evolved 2 distinct biochemical pathways for procoagulant function; one apoptotic and caspase-dependent and the other a calcium-dependent, caspase-independent pathway induced by physiologic agonists. We have further shown that hemostasis is also maintained in Casp9−/− FLC-reconstituted mice. There may still be a role for other caspases in platelet function, but not caspase-9, and it is unlikely that the intrinsic apoptotic caspase cascade is required for platelet function. Our data support the idea that intrinsic apoptotic machinery is important for controlling the survival and turnover of megakaryocytes and platelets. Inadvertent activation of apoptosis is likely to have profound consequences on the homeostasis of the megakaryocyte lineage.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rachael Lane and Jason Corbin for excellent technical assistance; Emma Lanera, Kelly Trueman, Shauna Ross, Chris Evans, Stephanie Green, and Louise McGregor for expert animal husbandry; and Abbott for ABT-737. They thank Profs Andreas Strasser, Richard Flavell, and Craig Thompson for the generous gift of the Casp9+/− and Bak−/−Bax+/− founder mice.
This work was supported by project grants (516725, 575535, 545848), program grant (461219), fellowships (S.P.J., D.C.S.H., and B.T.K.), and an Independent Research Institutes Infrastructure Support Scheme grant (361646) from the Australian National Health and Medical Research Council, fellowships from the Sylvia and Charles Viertel Charitable Foundation (B.T.K.), the Swedish Research Council (E.C.J.), the Leukemia & Lymphoma Society (E.C.J.), a scholarship from the Leukemia Foundation of Australia (M.J.W.), and a Victorian State Government Operational Infrastructure Support grant.
Authorship
Contribution: M.J.W., S.P.J., D.C.S.H., and B.T.K. designed the research; M.J.W., S.M.S., E.C.J., K.E.J., K.J.H., C.J., and M.A.D. performed experiments; and M.J.W. and B.T.K. analyzed results and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliations for C.J. are Centre de Référence des Pathologies Plaquettaires, Laboratoire d'hématologie, CHU de Bordeaux, Bordeaux, France; Université de Bordeaux Adaptation cardiovasculaire à l'ischémie, U1034, Pessac, France; and Inserm, Adaptation cardiovasculaire à l'ischémie, U1034, Pessac, France.
Correspondence: Benjamin T. Kile, Cancer and Hematology Division, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville 3052, Victoria, Australia; e-mail: kile@wehi.edu.au.

![Figure 4. Hemostasis and platelet function is unaltered by the absence of caspase-9. (A) Caspase-9 is not essential for normal platelet activation in vitro. Washed platelets were stimulated in vitro with activation agonists. The cell-surface profile of platelets was examined by measuring the platelet activation markers P-selectin, integrin GpIIb/IIIa, and PS exposure by flow cytometry. Data represent the mean ± SD; n = 4-5 mice per genotype (except Thr. [0.5 U/mL] + Conv. [500 ng/mL], n = 2). (B) Platelet aggregation kinetics is not altered by the absence of caspase-9. Aggregation of washed platelets derived from Casp9+/+ and Casp9−/− FLC-reconstituted mice was measured using an aggregometer. Data represent the mean ± SD; n > 3 mice/genotype per condition (except ADP 5.0μM, n = 2). (C) Loss of caspase-9 does not alter tail-bleeding time. Bleeding time was measured using the template method. Data represent the mean ± SEM; n = 5-7 mice per gentoype. (D) Template tail-bleeding time following administration of hirudin anticoagulant. Data represent the mean ± SEM; n = 6-8 mice per gentoype. (E) Bleeding time of transected tail. Data represent the mean ± SEM; n = 5-6 mice per genotype.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/18/10.1182_blood-2011-11-394858/4/m_zh89991288030004.jpeg?Expires=1769085936&Signature=kkv~CZASImaY4JgDQXctJENtNyBKvq77PHvkd9itLp83tZCkPHP2TqxqSrBqnTOCWgmAz3R1qQGKsacOGOl-nCKRiLyrZDzuR1KnLUTCwnIV5-RynYUuPHZHhdlYErH0plK7gHsJ-U~iYTB85nMj0AB4Otmv-jHKCk2ROA0u90ZaaigL0PhSP14vN~57WZkBsuoWZPiosxRtAV3mamqryFNQSa8QDXcMIzyvLeeqibJd7p3SSlUcz08~WItaUjzr9rFT6ewKdM8fNamRgn1PznbD-lGxnAcF8oYaZPW-~4Zn-HCWOIdXkPEo9VzS9x2RbVLpKb1yR9N5SFCtxzm9RQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal