Abstract
Identification of genes that regulate the development, self-renewal, and differentiation of stem cells is of vital importance for understanding normal organogenesis and cancer; such knowledge also underpins regenerative medicine. Here we demonstrate that chemical mutagenesis of mice combined with advances in hematopoietic stem cell reagents and genome resources can efficiently recover recessive mutations and identify genes essential for generation and proliferation of definitive hematopoietic stem cells and/or their progeny. We used high-throughput fluorescence-activated cell sorter to analyze 9 subsets of blood stem cells, progenitor cells, circulating red cells, and platelets in more than 1300 mouse embryos at embryonic day (E) 14.5. From 45 pedigrees, we recovered 6 strains with defects in definitive hematopoiesis. We demonstrate rapid identification of a novel mutation in the c-Myb transcription factor that results in thrombocythemia and myelofibrosis as proof of principal of the utility of our fluorescence-activated cell sorter–based screen. Such phenotype-driven approaches will provide new knowledge of the genes, protein interactions, and regulatory networks that underpin stem cell biology.
Introduction
Derived from the Greek word for blood (αιμα, haima), hematopoiesis describes the generation of fully differentiated effector blood cells from multipotential, self-renewing hematopoietic stem cells (HSCs).1 There are 2 waves of hematopoiesis in vertebrates (Figure 1A). The primitive wave is derived from hemangioblasts that form in the posterior primitive streak and migrate onto the yolk sac (YS) from embryonic day 7 (E7.0)2,3 where they give rise to primitive erythroid progenitors (EryP) in association with endothelial and vascular smooth muscle cells. EryP proliferate and differentiate synchronously to generate large nucleated red cells, which enter the circulation as the heart begins to beat at approximately E9.0.3,4 Platelets are also generated in the YS and enter the fetal circulation early, where they may or may not perform a classic hemostatic function.5 The definitive hematopoietic wave expands in the fetal liver (FL) from approximately E11.5, but HSCs are produced before this time in the ventral wall of the dorsal aorta and YS. Their potential to make at least 8 different mature blood cell lineages is not realized until the FL bud is formed from the gut tube and a suitable microenvironment is established for HSC seeding. From E11.5 to E15.5, there is a massive expansion of the FL, which is initially geared for production of definitive enucleated red blood cells to sustain rapid fetal growth (Figure 1A). From approximately E15.5, myeloid lineages are produced and hepatocytes begin to differentiate within the FL as it transforms from a hematopoietic organ into an adult liver.
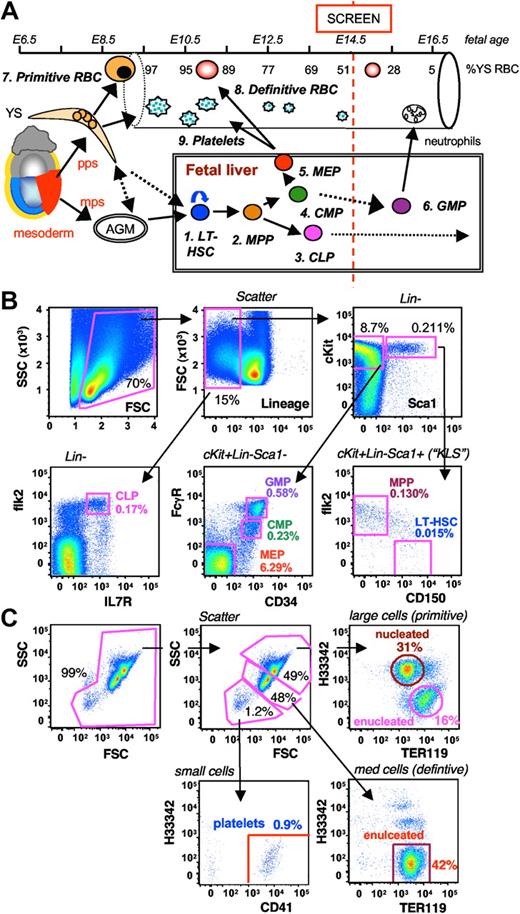
Development of FACS-based embryonic screening assays. (A) Schematic of hematopoietic development focusing on the circulating blood cell types and FL progenitor cell types assayed in our E14.5 screen. Early blood progenitors exit the posterior primitive streak (pps) and differentiate on the YS before entering the circulation from when the heart begins to beat at approximately E9.0. Large platelets also develop from the YS and enter the circulation early. Definitive HSCs enter and exit the midprimitive streak (mps) slightly later and migrate to the aorta-gonad-mesonephros (AGM) region where they develop with the ventral wall of the dorsal aorta where they lie dormant until the FL buds provides a receptive niche for HSC seeding and proliferation. In the FL, a hierarchy of progressively restricted progenitors is produced. Early in ontogeny, MEPs provide a high output of enucleated red cells and platelets. Later, myeloid progenitors generate neutrophils, and still later lymphoid progenitors (CLPs) seed the bone marrow and thymus where they provide B and T cells, respectively. We elected to screen the blood and FL at E14.5 so as to gain access to primitive and definitive blood cells (∼ 50:50 mix), and all definitive progenitor cell types. (B) The FL screen assays 6 functionally distinct HSC/progenitor cell subsets, LT-HSCs, MPPs, CLPs, CMPs, GMPs, and MEPs, by 8-color FACS analysis. (C) The FB screen assays 3 distinct subsets of megakaryo/erythroid cells, YS-derived primitive RBCs, FL-derived definitive RBCs, and platelets, by 3-color FACS analysis and cell size (on logarithmic scale). A representative plot of tissues from an E14.5 C57BL/6J embryo is shown, along with the percentage of nucleated blood cells (B) and percentage of total blood (C).
Development of FACS-based embryonic screening assays. (A) Schematic of hematopoietic development focusing on the circulating blood cell types and FL progenitor cell types assayed in our E14.5 screen. Early blood progenitors exit the posterior primitive streak (pps) and differentiate on the YS before entering the circulation from when the heart begins to beat at approximately E9.0. Large platelets also develop from the YS and enter the circulation early. Definitive HSCs enter and exit the midprimitive streak (mps) slightly later and migrate to the aorta-gonad-mesonephros (AGM) region where they develop with the ventral wall of the dorsal aorta where they lie dormant until the FL buds provides a receptive niche for HSC seeding and proliferation. In the FL, a hierarchy of progressively restricted progenitors is produced. Early in ontogeny, MEPs provide a high output of enucleated red cells and platelets. Later, myeloid progenitors generate neutrophils, and still later lymphoid progenitors (CLPs) seed the bone marrow and thymus where they provide B and T cells, respectively. We elected to screen the blood and FL at E14.5 so as to gain access to primitive and definitive blood cells (∼ 50:50 mix), and all definitive progenitor cell types. (B) The FL screen assays 6 functionally distinct HSC/progenitor cell subsets, LT-HSCs, MPPs, CLPs, CMPs, GMPs, and MEPs, by 8-color FACS analysis. (C) The FB screen assays 3 distinct subsets of megakaryo/erythroid cells, YS-derived primitive RBCs, FL-derived definitive RBCs, and platelets, by 3-color FACS analysis and cell size (on logarithmic scale). A representative plot of tissues from an E14.5 C57BL/6J embryo is shown, along with the percentage of nucleated blood cells (B) and percentage of total blood (C).
We know much about the genes that are critical for definitive stem cell generation and differentiation from reverse genetic approaches in mice, primarily gene knockouts.6 Most of the essential transcription factors were initially discovered as oncogenes, as proteins that bind to important cis-regulatory elements in other genes, or by sequence homology.1,7 Some factors play dual roles in primitive and definitive hematopoiesis, whereas others are relatively definitive-specific. c-Myb is an example of the latter,8 whereas Scl/Tal-1 and Gata2 play essential roles in both waves.9,10
Forward genetic screens using the mutagen ethylnitrosourea (ENU) offer several advantages over reverse genetics for gene discovery.11 Most importantly, they are phenotype-driven and so make no presumptions about which genes are involved in a particular process. Resulting point mutations also mimic those that characterize the majority of human inherited and acquired genetic diseases, and point mutations can lead to hypomorphic or antimorphic alleles in addition to loss-of-function alleles. The ability of mis-sense mutations to radically affect proteome regulatory networks and subsequent phenotypes has been well demonstrated in both yeast12 and bacteria.13 Mammalian examples are also beginning to be described from ENU mouse screens,14-16 including at the stem cell level.17 Gain-of-function (dominant mutations) and precise mutations in critical cis-regulatory elements are also possible outcomes from ENU screens. These are difficult to achieve using reverse engineering technologies without leaving small marks (such as LoxP sites) behind.
Several important genes for HSC and blood cell production have been discovered through ENU in zebrafish.18 Initial screens focused on embryonic anemia so were biased toward finding mutations in patterning or primitive hematopoiesis. Whereas some mutations were found in known hematopoietic genes,19 many more were in unexpected genes.20,21 Our screen builds on numerous successful dominant or haploinsufficient hematopoietic22,23 and immunologic11,14 screens to identify ENU phenodeviants in mice. We describe 2 complementary fluorescence-activated cell sorter (FACS)–based assays to detect perturbations in subsets of definitive HSC/progenitors, red cells, and platelets in midgestation (E14.5) mouse embyros. We conducted our screens on fetal tissues24,25 (liver and blood) rather than adult bone marrow to (1) minimize loss of lethal recessive phenotypes before birth, (2) access a hematopoietic organ (FL) containing quantifiable subsets of HSC/progenitors, (3) reduce time to screening of the animal, and (4) reduce project costs. To date, we have screened more than 1300 G3 embryos from 45 pedigrees for 9 hematopoietic parameters in parallel and identified 6 recessive mutations. We have given these pedigrees Australian Aboriginal names to reflect the Australian origin of the screen, and as acknowledgment that our Aboriginal peoples understand land sustainability just as stem cells maintain and repair adult tissues after damage or degeneration.
Methods
Mice
All 8 wild-type strains, C57BL/6J (B6), C57BL/10SnJ (B10), C57L/J, BALB/b, C3H/HeH, CBA/CaJ, FVB/NJ, and 129/SvEv, and ENU pedigrees (B6) were maintained at the Australian Phenomics Facility. The morning of vaginal plug observation was E0.5. ENU pedigree founder (G0) B6 male mice were treated weekly with 90 mg/kg ENU over 3 weeks. All mouse experiments were approved by the Institutional Review Board of Australian National University.
Flow cytometry
A full list of antibodies is available in supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All cells were analyzed on an LSRII (BD Biosciences) and FACS data analyzed using FlowJo Version 9.1 software (TreeStar).
Hematologic analyses
Whole blood (175 μL) from adult mice was collected into ethylenediaminetetraacetic acid tubes and run through an Advia 120/2120 Hematology System analyzer (Siemens AG). Cytospins were at 500g for 4 minutes in a Cytospin 3 (Shandon). Cells were fixed in 100% methanol and stained in May-Grünwald-Giemsa. Paraffin sections of spleen were stained with hematoxylin and eosin, Perl stain, or reticulin using standard protocols (supplemental data).
Genomic mapping and genotyping
DNA from (B6 × B10) F2 embryos was analyzed using a panel of 80 B6/B10 single nucleotide polymorphisms (SNPs) that on average span the mouse genome at 20-Mb intervals. Full details of SNP marker design and genotyping are listed in supplemental data.
Gene sequencing
Sequencing of candidate genes was to locate the causal ENU B6 nucleotide substitution. DNA was prepared from an individual affected mouse and primers designed for candidate genes to amplify all exons plus or minus 15 bp to cover splice junctions. Primers are available on request. Amplicons were then sequenced by the Sanger method on a 3730xl capillary sequencer (Applied Biosystems). This automated platform used Big Dye Terminator chemistry Version 3.1 (Applied Biosystems). The raw trace files were analyzed using Lasergene software (DNASTAR) against B6 reference genome. Mutations were confirmed in a second affected person.
Biochemistry
The E308G mutation on c-Myb that included an N-terminal HA tag was recreated using a polymerase chain reaction-based mutagenesis approach, after which the mutant was cloned into BamHI and XhoI sites of pcDNA3.1 (Invitrogen). Reporter and glutathione S-transferase (GST) pull-down assays to detect interaction between Myb and CBP KIX were performed as described in supplemental data.
Statistics
Data were analyzed for significance between groups using a 2-tailed Student t test. Differences were considered significant at P < .05.
Results
A screen for hematopoietic stem/progenitor cells and blood production
Our primary aim was to find recessive mutations causing abnormal definitive HSC production or turnover. A secondary aim was mutations that resulted in defective red cell and/or platelet production. Most of the knowledge about HSC frequencies and behavior has come from various assays using adult bone marrow. However, FL is a very rich source of HSCs that outcompete bone marrow stem cells in most scenarios.26,27 To efficiently screen for long-term HSC (LT-HSC) and progenitor cell phenotypes in FL, we first set up a high-throughput FACS assay using wild-type C57BL/6J (B6) mice (Figure 1B). We chose this strain for mutagenesis because we have extensive experience in using it for ENU screens,11,14 the mouse reference genome was built using B6 DNA making ENU-generated SNP detection easier, and many monoclonal antibodies have often been used specifically in B6. In particular, functional HSC assays, such as competitive repopulation assays, are easier using B6 congenic strains because of the availability of CD45 strain-specific antibodies.
Using an 8-color single-stain protocol, 6 functionally distinct hematopoietic stem and progenitor cell subsets, LT-HSCs, multipotent progenitors (MPPs), common lymphoid progenitors (CLPs), common myeloid progenitors (CMPs), granulocyte/macrophage progenitors (GMPs), and megakaryocyte/erythrocyte progenitors (MEPs), were quantified in parallel (Figure 1B). Self-renewing LT-HSCs were gated according to the 5-color cKit+Lin−Sca1+CD150+flk2− cell surface phenotype, whereas MPPs were cKit+Lin−Sca1+CD150−flk2+.26,27 The 4 subsets of lymphoid and myeloid progenitors were gated according to published protocols.28,29
By a separate 3-color FACS analysis and cell size measurement on a log scale, we also established a companion high-throughput screening assay for subsets of blood cells and platelets in fetal blood (FB) based on recently published work (Figure 1C).3,5 YS-derived primitive red blood cells (RBCs) were identified based on their large size, nucleus (ie, Hoechst 33342 positivity), and expression of the cell surface marker TER119. FL-derived definitive RBCs were medium-sized and expressed TER119 but were enucleated (Hoechst 33342-negative). Finally, platelets were very small in size and stained for CD41 but not Hoechst 33342. Together, these 9 subsets of cells assayed in parallel provided a high-resolution snapshot of early hematopoiesis in both a primary tissue and the periphery (Figure 1A). The use of 2 complementary screening assays each with multiple phenotypic readouts also provided internal controls to minimize the identification of false positives.
Ontogeny of hematopoietic stem and progenitor cell compartments
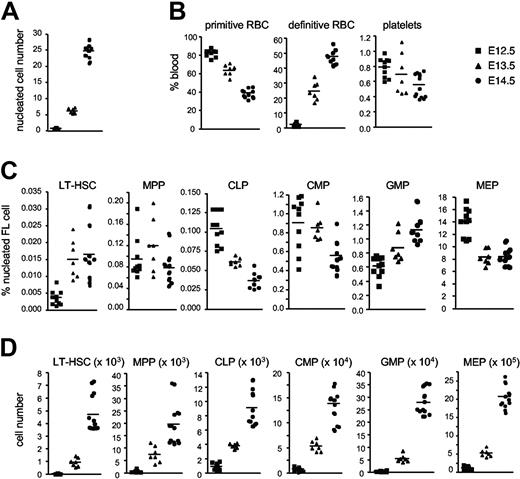
We analyzed FL and FB samples from B6 embryos aged E12.5, E13.5, and E14.5 to examine the dynamics of stem and progenitor cell proliferation. A 5-fold increase in nucleated cells in the FL was observed with each developmental day (Figure 2A). Primitive RBCs formed the majority (∼ 80%) of the circulating FB at E12.5, a frequency that dropped by approximately 20% with each developmental day (Figures 1A, 2B). This is consistent with the gradual switch from primitive to definitive erythroid cells in the circulation.3,4 We found that primitive erythroid cells underwent progressive enucleation in the circulation to greater levels than realized until recently,3 such that by E14.5 approximately 20% were enucleated (Figure 1C). The reduction in primitive RBCs was mirrored by a relative increase in definitive RBCs from approximately 5% at E12.5 to approximately 25% at E13.5 and approximately 50% at E14.5 (Figure 2B). By comparison, mean platelets counts of approximately 0.5% to 1% relative to RBCs were relatively constant across developmental days (Figure 2B), consistent with substantial platelet production from the YS as well as the FL.5
Ontogeny of hematopoietic compartments in FL and blood. (A) Total numbers of viable nucleated blood cells in E12.5, E13.5, and E14.5 FLs. (B) Percentages of primitive RBCs, definitive RBCs, and platelets in E12.5, E13.5, and E14.5 fetal blood. (C) Percentages of LT-HSCs and 5 progenitor populations in E12.5, E13.5, and E14.5 FLs. (D) Total numbers of HSCs and 5 progenitor populations in E12.5, E13.5, and E14.5 FLs. E12.5 (n = 29), E13.5 (n = 31), and E14.5 (n = 38) C57BL/6J embryos were analyzed in 3 separate experiments, with data from 1 representative experiment shown. Viable cells were determined by Trypan Blue exclusion.
Ontogeny of hematopoietic compartments in FL and blood. (A) Total numbers of viable nucleated blood cells in E12.5, E13.5, and E14.5 FLs. (B) Percentages of primitive RBCs, definitive RBCs, and platelets in E12.5, E13.5, and E14.5 fetal blood. (C) Percentages of LT-HSCs and 5 progenitor populations in E12.5, E13.5, and E14.5 FLs. (D) Total numbers of HSCs and 5 progenitor populations in E12.5, E13.5, and E14.5 FLs. E12.5 (n = 29), E13.5 (n = 31), and E14.5 (n = 38) C57BL/6J embryos were analyzed in 3 separate experiments, with data from 1 representative experiment shown. Viable cells were determined by Trypan Blue exclusion.
Frequencies (Figure 2C) and absolute numbers (Figure 2D) of the 6 HSC/progenitor cell subsets in FL were quantified across developmental days. MEP was the predominant progenitor population, with the E14.5 frequency progressing MEP > GMP > CMP > MPP > CLP > LT-HSC. LT-HSC, MPP, and CLP were very low in number at E12.5, composing only a few hundred cells but expanding to 5000 to 20 000 cells by E14.5.
Based on these data, the optimal developmental age to conduct our screen was E14.5, when the primitive and definitive RBC compartments in FB were at approximately 50% relative frequencies (Figures 1, 2), facilitating accurate quantification of both. In addition, mice harboring targeted deletions of genes essential for definitive hematopoiesis survive to approximately E14.5 because the primitive wave can sustain embryos until then.8,30 Furthermore, we reasoned that loss-of-function or hypomorphic alleles of genes involved in both primitive and definitive hematopoieis, such as Epo, EpoR, or SCL/tal-1, might be viable or recently dead at E14.5.31 Lastly, E14.5 FL is large and easy to process, whereas older FL is harder to dissociate into single cells.
HSC compartments in inbred mouse strains
Because an outcross to a non-B6 inbred strain is required to map the causative ENU mutation underlying any phenotype of interest, we needed to ensure that the FACS assays were robust and identical in the mapping strain eventually chosen. Numerous phenotyping surveys encompassing a broad range of biologic disciplines have been conducted in inbred strains,32,33 but none has specifically focused on the variability on HSC/progenitor cell surface profile34 and frequency35,36 across a comprehensive number of inbred mouse strains, particularly with recent advances in identified HSC markers.26,27
We analyzed 7 inbred strains (C57BL/10SnJ, C57L/J, BALB/b, C3H/HeH, CBA/CaJ, FVB/NJ, and 129/SvEv) alongside our ENU-mutagenized B6 stain for the baseline composition and enumeration of HSC/progenitor cell subsets in FL, and erythroid cells/platelets in FB. There were some major surprises. For example, a clear lack of Sca1 cell surface expression in BALB/c, C3H/HeH, and CBA/CaJ strains resulted in an inability to recognize and score LT-HSCs using Sca1 (Figure 3A; supplemental Figure 1A). FVB/NJ and 129/SvEv strains also showed a reduction in Sca1 expression relative to B6, C57BL/10SnJ, and C57L/J. One possible explanation for this apparent absence of Sca1 surface expression is that the epitope recognized by the Sca1 monoclonal antibody may have amino acid changes because of functionally conservative SNPs in non-B6 strains. Alternative antibodies raised against different epitopes or polyclonal antisera could give different results. Nevertheless, our choice of mapping strain to detect HSC phenodeviants was limited to B6-related and FVB/N strains. In addition, CLP frequency was similar to C57BL/6 levels in B6 variants and 129/SvEv mice, but all other strains had lower frequencies (Figure 3A). In addition, a shift in CD34 and FcγR cell surface staining resulted in ambiguous GMP frequencies for all but C57BL/10SnJ, whereas the CMP/MEP subsets were less variable across the strains tested (Figure 3A; supplemental Figure 1B).
Composition of hematopoietic compartments in FL and blood in inbred mouse strains. (A) Percentages of LT-HSC and 5 progenitor populations in FLs across inbred mouse strains. (B) Percentages of primitive RBCs, definitive RBCs, and platelets across inbred mouse strains. E14.5 embryos from C57BL/6J (n = 24), C57BL/10SnJ (n = 16), C57L/J (n = 14), BALB/b (n = 16), C3H/HeH (n = 24), CBA/CaJ (n = 17), FVB/NJ (n = 21), and 129/SvEv (n = 31) strains were analyzed in 3 separate experiments, with data from 1 representative experiment shown.
Composition of hematopoietic compartments in FL and blood in inbred mouse strains. (A) Percentages of LT-HSC and 5 progenitor populations in FLs across inbred mouse strains. (B) Percentages of primitive RBCs, definitive RBCs, and platelets across inbred mouse strains. E14.5 embryos from C57BL/6J (n = 24), C57BL/10SnJ (n = 16), C57L/J (n = 14), BALB/b (n = 16), C3H/HeH (n = 24), CBA/CaJ (n = 17), FVB/NJ (n = 21), and 129/SvEv (n = 31) strains were analyzed in 3 separate experiments, with data from 1 representative experiment shown.
FB was also tested (Figure 3B). Although precise developmental timing between mouse litters was one experimental variable that sometimes caused skewed ratios between circulating primitive and definitive RBCs (Figure 2), there was a distinct lack of TER119 expression on the surface of primitive RBCs of C3H/HeH and FVB/NJ strains (supplemental Figure 2). TER119 expression on definitive RBCs was more uniform across all mouse strains; a SNP in the gene encoding the surface protein recognized by the TER119 monoclonal antibody is thus unlikely (supplemental Figure 2).
Based on these observations, we outcrossed our ENU-mutagenized B6 pedigrees to the C57BL/10SnJ (B10) strain because it showed the least variability in hematopoietic profile relative to B6 across all parameters examined, an unsurprising finding given their common ancestry37 and close position on the mouse phylogenetic tree.38 Although this meant a reduction in polymorphisms between these 2 strains and mapping power,39 we thought this was superseded by the need to reliably identify phenodeviants on outcrossing.
Summary of ENU pedigrees screened
We chose a concentration of ENU based on many years of experience in the Australian Phenomics Facility. Previous experiments have determined that we generate approximately 30 mutations in coding genes in each founder G1 male using a standard 3 × 90 mg/kg ENU.40 Using our FACS assays, we screened 1375 G3 embryos at E14.5 from 45 B6 ENU pedigrees and identified 6 strains with phenotypes in HSC/progenitors and/or early blood cell production. Supplemental Table 1 documents the first 1107 embryos and 34 pedigrees screened. Average litter size was 8 and the embryo resorption rate was approximately 15%. Some pedigrees showed a consistently higher rate of embryo resorption possibly indicative of either a dominant or recessive early embryonic lethal phenotype. We found 6 ENU pedigrees with reproducible defects defined as present in at least 3 independent litters. ENU11.21 (kandarra), ENU11.28 (booreana), and ENU11.211 (mulkirri) have been outcrossed and heritability confirmed. A further 3 pedigrees, ENU11.208 (chokerre), ENU11.235 (wonggan), and ENU11.312 (kamu), are in the process of outcrossing for mapping (Table 1).
ENU mutant pedigrees
| Name (English translation) . | Phenotype . | Map location . | Mutation . | |
|---|---|---|---|---|
| Fetal liver . | Blood . | |||
| Booreana (white) | Increased HSC | Anemia and increased platelets | Chromosome 10: 33-57 Mb | c-Myb A923G |
| Kandarra (blood) | Normal at E13.5 | Anemia at E13.5 | Chromosome 2: 67-93 Mb | Undetermined |
| Mulkirri (plenty) | Normal | Severe anemia, normal platelets | Chromosome 7: 81-115 Mb | Undetermined |
| Chokerre (red) | Increased HSC | Anemia and increased platelets | Backcrossing | Undetermined |
| Kamu (blood red) | Normal | Anemia | Backcrossing | Undetermined |
| Wonggan (liver) | Increased HSC | Normal | Backcrossing | Undetermined |
| Name (English translation) . | Phenotype . | Map location . | Mutation . | |
|---|---|---|---|---|
| Fetal liver . | Blood . | |||
| Booreana (white) | Increased HSC | Anemia and increased platelets | Chromosome 10: 33-57 Mb | c-Myb A923G |
| Kandarra (blood) | Normal at E13.5 | Anemia at E13.5 | Chromosome 2: 67-93 Mb | Undetermined |
| Mulkirri (plenty) | Normal | Severe anemia, normal platelets | Chromosome 7: 81-115 Mb | Undetermined |
| Chokerre (red) | Increased HSC | Anemia and increased platelets | Backcrossing | Undetermined |
| Kamu (blood red) | Normal | Anemia | Backcrossing | Undetermined |
| Wonggan (liver) | Increased HSC | Normal | Backcrossing | Undetermined |
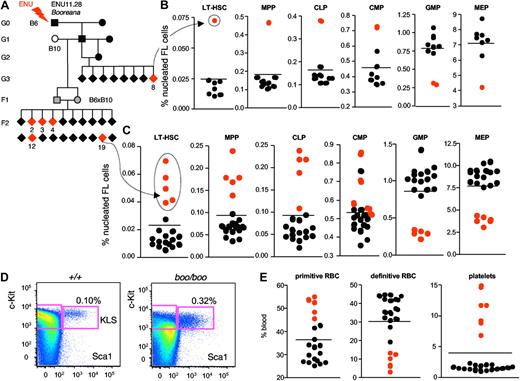
Identification of the Booreana mutant phenotype
The ENU strain booreana (from the Aboriginal word for white) was so named because embryo 8 from the first litter of pedigree ENU11.28 (Figure 4A) showed an expansion of LT-HSCs (Figure 4B,D). Booreana homozygous embryos (boo/boo) are indistinguishable from littermates by inspection (Figure 5A). However, boo/boo FL has an increased frequency of LT-HSC (4-fold), MPP (3-fold), CLP (3-fold), variable CMP numbers, but decreased GMP (3-fold) and MEP (2-fold; Figure 4B). That some cell subsets had expanded and others reduced strongly suggested a bona fide phenodeviant. The distinctive boo phenotype recurred in one embryo (no. 17) in the third litter of pedigree ENU11.28 (supplemental Figure 3). In this case, CMP numbers were normal, but the other subsets were like those in the founder embryo.
Booreana ENU mutant identification and heritability testing. Example of a variant embryo (mouse G3.8 from pedigree ENU11.28; A) identified by the FL screen as having atypical stem cell and progenitor FACS hematopoiesis (increased LT-HSC, MPP, CLP, and CMP; decreased GMP and MEP; B) at E14.5. The C57BL/6J G1 male founder was outcrossed to a C57BL/10SnJ female and then established as a true-breeding strain by intercrossing F1(B6 × B10) siblings and testing with the same screening protocol. Additional variant embryos (mutant F2.2, F2.3, F2.4, F2.12, and F2.19) showed an identical abnormal increase in KLS (cKit+Lin−Sca1+) and other stem cell subsets (C-D), and a perturbed fetal blood FACS profile (increased primitive RBC and platelets; decreased definitive RBC; E) at E14.5. Red dots represent putative recessive mutants; and black dots, phenotypic wild-type littermates.
Booreana ENU mutant identification and heritability testing. Example of a variant embryo (mouse G3.8 from pedigree ENU11.28; A) identified by the FL screen as having atypical stem cell and progenitor FACS hematopoiesis (increased LT-HSC, MPP, CLP, and CMP; decreased GMP and MEP; B) at E14.5. The C57BL/6J G1 male founder was outcrossed to a C57BL/10SnJ female and then established as a true-breeding strain by intercrossing F1(B6 × B10) siblings and testing with the same screening protocol. Additional variant embryos (mutant F2.2, F2.3, F2.4, F2.12, and F2.19) showed an identical abnormal increase in KLS (cKit+Lin−Sca1+) and other stem cell subsets (C-D), and a perturbed fetal blood FACS profile (increased primitive RBC and platelets; decreased definitive RBC; E) at E14.5. Red dots represent putative recessive mutants; and black dots, phenotypic wild-type littermates.
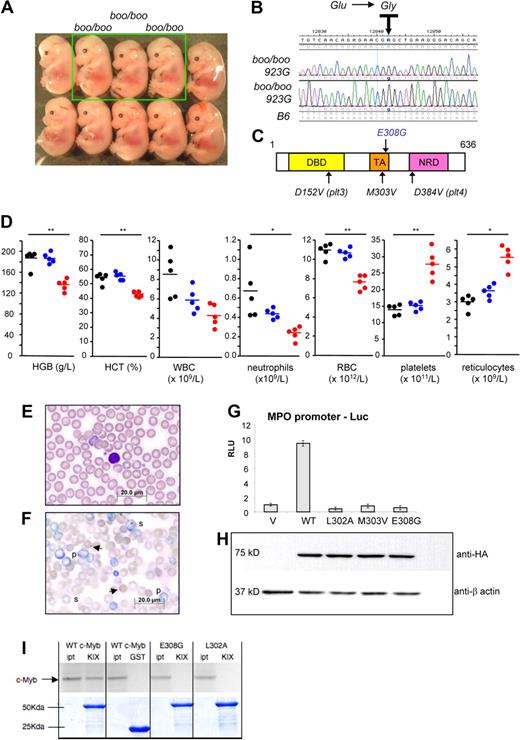
The booreana ENU strain harbors a point mutation in the transactivation domain of c-Myb. (A) Homozygous booreana mutants (boo/boo) appear normal at E14.5. (B) Resequencing of c-Myb (ENSMUSG00000019982) from 2 phenotype-positive embryos identified an A-to-G transition in both cases in exon 8 at base pair 923, changing amino acid 308 from glutamic acid to glycine. (C) Domain structure of c-Myb showing the E308G point mutation in the transactivation domain. DNB indicates DNA binding; TA, transactivation/acidic; and NRD, negative regulatory. (D) Complete blood counts of 8-week-old booreana homozygous and heterozygous mutants and wild-type littermate controls as measured by ADVIA 2120 machine. *Statistically significant differences (P < .05) between boo/boo and +/+ groups. **Significance between boo/boo and both +/+ and boo/+ groups. (E-F) Blood films of 12-week-old boo/boo mice (E) and boo/+ littermates (F). The mutant animals have increased platelets, polychromasia (p), and Howell-Jolly bodies (arrowheads) and basophilic stippling (s). For panels E and F, images were generated using an Olympus BX51 microscope with a U PlansApo 60× lens, 1.35 NA, under Olympus imersion oil. Images were collected using an Olympus DP70 camera and DP controller software. Digital images were adjusted and labeled using Photoshop CS4. (G) Reporter assays in 293T cells cotransfected with the myeloperoxidase gene promoter linked to lacZ and various HA-tagged c-Myb constructs, including wild-type (WT), L302A, M303V, and E308G (Booreana) mutations within the TA domain. Bars represent mean normalized relative light units (RLU), and error bars represent SD of the mean of triplicate biologic assays. (H) Western blot of transfected 293T cells for the HA tag and for endogenous β-actin showing equivalent transfection efficiency. (I) GST pull-down assays showing interactions between GST-CBP-KIX (KIX) and in vitro translated c-Myb mutants. Input (ipt) and bound wild-type (WT), E308G, or L302A radiolabeled c-Myb proteins eluted from the indicated GST fusions are shown in top panel; the bottom panel shows the presence of the relevant GST proteins, visualized by Coomassie blue staining, in each binding reaction.
The booreana ENU strain harbors a point mutation in the transactivation domain of c-Myb. (A) Homozygous booreana mutants (boo/boo) appear normal at E14.5. (B) Resequencing of c-Myb (ENSMUSG00000019982) from 2 phenotype-positive embryos identified an A-to-G transition in both cases in exon 8 at base pair 923, changing amino acid 308 from glutamic acid to glycine. (C) Domain structure of c-Myb showing the E308G point mutation in the transactivation domain. DNB indicates DNA binding; TA, transactivation/acidic; and NRD, negative regulatory. (D) Complete blood counts of 8-week-old booreana homozygous and heterozygous mutants and wild-type littermate controls as measured by ADVIA 2120 machine. *Statistically significant differences (P < .05) between boo/boo and +/+ groups. **Significance between boo/boo and both +/+ and boo/+ groups. (E-F) Blood films of 12-week-old boo/boo mice (E) and boo/+ littermates (F). The mutant animals have increased platelets, polychromasia (p), and Howell-Jolly bodies (arrowheads) and basophilic stippling (s). For panels E and F, images were generated using an Olympus BX51 microscope with a U PlansApo 60× lens, 1.35 NA, under Olympus imersion oil. Images were collected using an Olympus DP70 camera and DP controller software. Digital images were adjusted and labeled using Photoshop CS4. (G) Reporter assays in 293T cells cotransfected with the myeloperoxidase gene promoter linked to lacZ and various HA-tagged c-Myb constructs, including wild-type (WT), L302A, M303V, and E308G (Booreana) mutations within the TA domain. Bars represent mean normalized relative light units (RLU), and error bars represent SD of the mean of triplicate biologic assays. (H) Western blot of transfected 293T cells for the HA tag and for endogenous β-actin showing equivalent transfection efficiency. (I) GST pull-down assays showing interactions between GST-CBP-KIX (KIX) and in vitro translated c-Myb mutants. Input (ipt) and bound wild-type (WT), E308G, or L302A radiolabeled c-Myb proteins eluted from the indicated GST fusions are shown in top panel; the bottom panel shows the presence of the relevant GST proteins, visualized by Coomassie blue staining, in each binding reaction.
We outcrossed the heterozygous boo/+ G1 founder male from pedigree ENU11.28 to a wild-type B10 female and then established booreana as a heritable breeding line by intercrossing F1(B6xB10) siblings and testing with the same FL FACS screening protocol. Fulfilling our expectation from baseline phenotyping of non-B6 strains (Figure 3), the introduction of B10 genome did not suppress the booreana phenotype, which was observed at an expected Mendelian ratio (5/20; Figure 4C). There was a consistent increase in LT-HSC and CLP, with reduction in MEP and GMP. CMP were variable because of difficulties in gating these cells accurately in boo/boo embryos (supplemental Figure 5). FB from boo/boo embryos showed an expansion in their primitive RBC subset and dramatic reduction in their definitive RBCs (Figure 4E; supplemental Figure 4), consistent with the decreased MEP frequency. However, boo/boo embryos showed a striking 5- to 10-fold increase in circulating platelets (Figure 4E; supplemental Figure 4).
Booreana embryos harbor a mutation in the transactivation domain of c-Myb
We generated a boo/boo DNA pool (n = 12) alongside an unaffected pool of ENU11.28 embryos (n = 20) and analyzed these against a panel of 80 B6/B10 SNPs to map the underlying booreana mutation to a 57-Mb region on chromosome 10 containing approximately 300 genes. The proto-oncogene c-Myb resides in the interval and was a strong candidate for mutation given previous reports describing hematopoietic defects in mice harboring c-Myb mutations.22 Resequencing of all c-Myb exons identified an A-to-G transition at nucleotide 923 in exon 8 only in those embryos that displayed the characteristic booreana phenotype (Figure 5B). The DNA mutation results in a glutamic acid to glycine substitution at amino acid 308 (E308G) in the transactivation (TA) domain of c-Myb (Figure 5C). Interestingly, the c-MybE308G/E308G mutation is only 5 amino acids from another ENU mutant, c-MybM303V/M303V (also an A-to-G transition), which resulted multilineage hematopoietic abnormalities (see “c-Myb and hematopoiesis” below).23
To test the biochemical function of c-MybE308G/E308G, we cloned the mutant cDNA into an expression vector and tested its ability to transactivate the myeloperoxidase gene promoter in reporter assays. Compared with wild-type c-Myb, the E308G mutation is completely inert (Figure 5G). There was slight residual function in the M303V TA domain mutation, suggesting that the E308G mutation is more severe. Because c-Myb has been reported to bind p300 and CBP via its TA domain,23 we also asked whether the mutation in the acidic residue disrupted interaction. We found complete absence of binding of CBP to E308G and L302A mutations in GST pull-down assays in 293T cells (Figure 5I).
Adult c-MybE308G/E308G mice are a model for human essential thrombocythemia with myelofibrosis
Like c-MybM303V/M303V mice, we found that 8-week old boo/boo mice had markedly increased platelets (P < .001), anemia (P = .003), and increased reticulocytes (P < .001; Figure 5D). Boo/boo homozygotes also have neutropenia (P = .01), which was not reported in c-MybM303V/M303V mice, suggesting some biochemical differences between these alleles. There were red cell abnormalities, including polychromasia and Howell-Jolly body inclusions (Figure 5E-F). There was moderate splenomegaly in boo/boo adults (254 ± 46 mg, n = 6) compared with heterozygous littermates (115 ± 15 mg, n = 5) because of expansion of the red pulp (Figure 6A-B). On the other hand, the white pulp/lymphocyte component of spleen was reduced in boo/boo mice. The overall splenic architecture was disorganized in many cases with pink amorphous material in the red pulp, increased iron staining, and markedly increased reticulin staining (Figure 6C-F).
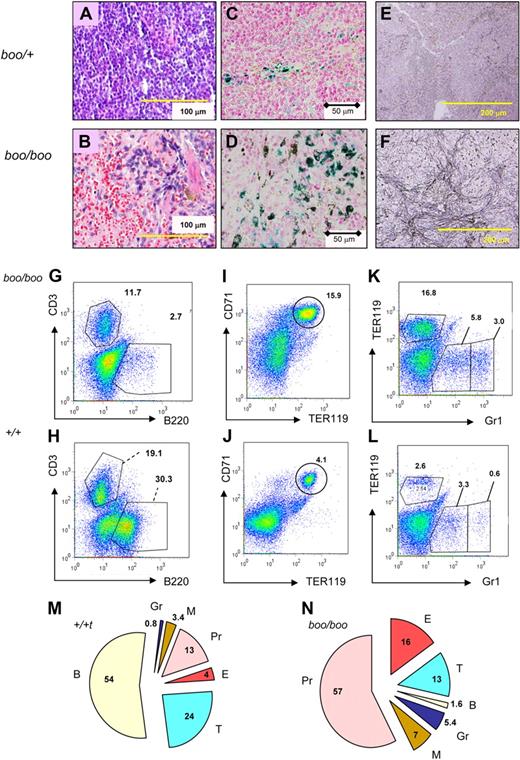
Reduced B lymphopoiesis and abnormal myeloid differentiation and myelofibrosis in boo/boo mice. (A-B) Hematoxylin and eosin stain of spleen shows reduced white pulp and increased and disorganized red pulp with amorphous pink material in boo/boo mice. (C-D) Perl stain showing increased iron accumulation in red pulp macrophages within boo/boo spleen. (E-F) Increased reticulin (fibrosis) in boo/boo mice. (G-H) Absent B cells and relatively reduced T cells in boo/boo spleen. Numbers indicate percentage of cells falling within the indicated FACS gates. (I-J) Increased frequency of CD71+/TER119+ mature erythroid cells and also CD71lo immature cells in boo/boo spleen. (K-L) Increased Gr1hi neutrophils, Gr1int monocytes, and TER119+ erythroid cells in boo/boo spleen. (M-N) Pie graphs show percentage contribution of B220+ B cells, CD3+ T cells, TER119+ erythroid cells, Gr1 bright granulocytes, Gr1 intermediate macrophages, and “other” cells (mostly CD71 weak early erythroid lineage cells) to the spleen. Numbers represent the mean of FACS analyses from 6 boo/boo mice and 6 wild-type (+/+) littermate controls.
Reduced B lymphopoiesis and abnormal myeloid differentiation and myelofibrosis in boo/boo mice. (A-B) Hematoxylin and eosin stain of spleen shows reduced white pulp and increased and disorganized red pulp with amorphous pink material in boo/boo mice. (C-D) Perl stain showing increased iron accumulation in red pulp macrophages within boo/boo spleen. (E-F) Increased reticulin (fibrosis) in boo/boo mice. (G-H) Absent B cells and relatively reduced T cells in boo/boo spleen. Numbers indicate percentage of cells falling within the indicated FACS gates. (I-J) Increased frequency of CD71+/TER119+ mature erythroid cells and also CD71lo immature cells in boo/boo spleen. (K-L) Increased Gr1hi neutrophils, Gr1int monocytes, and TER119+ erythroid cells in boo/boo spleen. (M-N) Pie graphs show percentage contribution of B220+ B cells, CD3+ T cells, TER119+ erythroid cells, Gr1 bright granulocytes, Gr1 intermediate macrophages, and “other” cells (mostly CD71 weak early erythroid lineage cells) to the spleen. Numbers represent the mean of FACS analyses from 6 boo/boo mice and 6 wild-type (+/+) littermate controls.
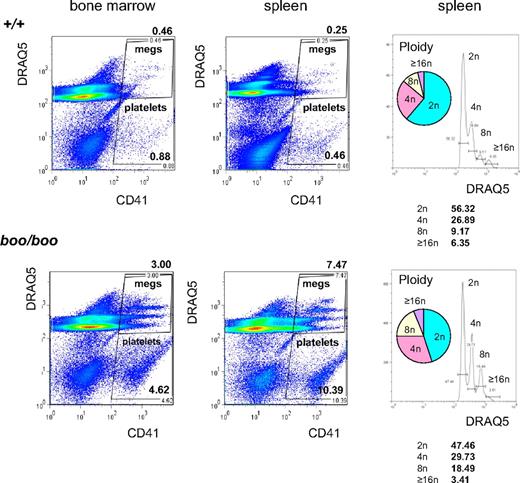
Megakaryocytes in boo/boo mutants were also increased approximately 8-fold in the bone marrow and approximately 20-fold in the spleen as determined by CD41 staining (Figure 7). In addition to an increase in number, there was an increase in nuclear ploidy of boo/boo megakaryocytes as determined by DRAQ5 incorporation in CD41+ cells (Figure 7). Together, these changes are very reminiscent of human myeloproliferative neoplasms (MPNs) such as essential thrombocythemia (ET) with myelofibrosis (MF; see “Homozygous Booreana mice are a model for human essential thrombocythemia and myelofibrosis” below).
Increased and aberrant megakaryopoiesis in boo/boo mice. FACS for CD41 and the DNA intercalating dye DRAQ5 in bone marrow and spleen of boo/boo and wild-type (+/+) littermates. There is a marked increase in large CD41+ cells (megakarocytes) in spleen (∼ 20-fold) and bone marrow (∼ 8-fold). Ploidy analysis shows a “right shift” in boo/boo spleen (ie, more megakaryocytes have > 8n ploidy and less have 2n ploidy).
Increased and aberrant megakaryopoiesis in boo/boo mice. FACS for CD41 and the DNA intercalating dye DRAQ5 in bone marrow and spleen of boo/boo and wild-type (+/+) littermates. There is a marked increase in large CD41+ cells (megakarocytes) in spleen (∼ 20-fold) and bone marrow (∼ 8-fold). Ploidy analysis shows a “right shift” in boo/boo spleen (ie, more megakaryocytes have > 8n ploidy and less have 2n ploidy).
Splenic FACS analysis of booreana adult mice revealed markedly reduced B-cell lymphopoiesis in homozygous mutants (Figure 6G-H), as well as an increased frequency of CD71+/TER119+ cells (Figure 6I-J), Gr1hi neutrophils, Gr1int monocytes, and TER119+ erythroid cells (Figure 6K-L). Gr1int cells were confirmed to be monocytes by coexpression of Ly6G (data not shown). The percentage of splenic CD3+ T cells was reduced to approximately 50% of wild-type littermates (Figure 6M-N), but absolute splenic T-cell numbers are probably close to equivalent given the 50% increase in spleen weight.
Discussion
Forward genetics offers the potential to find novel genes involved in any biologic process if a robust assay is established for reliable detection of phenodeviants. Dominant and recessive screens using high-throughput blood cell counting assays have successfully identified mutations in many hematopoietic genes.11,14,22-25 Some were well known from previous studies, whereas others were unexpected players. Embryonic recessive screens have simply relied on identification of visible phenodeviants.24,25 To our knowledge, this is the first attempt to use quantitative FACS assays to detect perturbations in all the major definitive HSC/progenitor cell subsets. Such an undertaking would be difficult in adult mice, but our father-daughter mating strategy and fetal screen of G3s have enhanced the feasibility of such an undertaking; with a small team, we have performed a pilot screen of 45 pedigrees and found 6 recessive mutations. The rapid cloning of a novel point mutation in the c-Myb oncogene provides proof of principle that informative mutations in key definitive stem cell genes can be identified by using FACS and a recessive breeding strategy.
Strain differences in HSC and RBC antigen expression
In the process of searching for an appropriate mapping strain, we uncovered differences in surface expression of stem cell markers. For example, Sca1 is not expressed on the cell surface of HSCs in several commonly used mouse strains, such as BALB/c, C3H, and CBA, and is weak in 129/Sv. This has implications for using Sca1 for the study of HSC biology in mice generated from gene-targeted ES cell lines from a 129/Sv genetic background. One possibly trivial explanation is that the epitope recognized by the Sca1 monoclonal antibody (D7) is slightly divergent in the non-B6 strains because of an SNP that results in a conservative amino acid change. Thus, Sca1 might be present in a variant form on HSCs in these strains. Further studies with different hybridomas or polyclonal antisera to Sca1 would be necessary to resolve this.
The TER119 red blood cell epitope does not seem to be present on embryonic red cells of some strains (C3H and FVB). TER119 is associated with glycophorin A, but the precise epitope has not been identified.41 Its absence in the primitive erythroid wave of certain strains is intriguing; it is present in the definitive wave of these strains so the genes coding for TER119 must be present in the genomes, and the SNP argument is not likely to be valid in this case. Our results suggest failure of TER119 detection in certain strains is either the result of a lack of a key regulatory protein in the primitive wave of these strains or of a partner protein or processing pathway component, which normally leads to stable protein accumulation at cell surface. These results suggest TER119 has limited utility for the study of primitive erythroid cell differentiation in certain mouse strains.
c-Myb and hematopoiesis
c-Myb is a well-known critical regulator of hematopoiesis and stem cell biology in other organ systems.42 Gene knockout causes embryonic lethality by E15.5 from anemia.8 Several ENU c-Myb alleles have been discovered using screens to detect blood abnormalities. The M303V mutation impairs interaction with transcriptional coactivators, such as p300 and CBP.23 Our mutation in an acidic residue of the TA domain leads to more severe transcriptional crippling and complete failure to interact with CBP. The phenotype of adult c-MybE308G/E308G mice is probably slightly more severe than the phenotype of c-MybM303V/M303V mice, consistent with the in vitro gene reporter data. Interestingly, a mutation in the KIX domain of p300, which disrupts interaction with c-Myb, leads to a similar phenotype, suggesting that the c-Myb-p300/CBP interaction is critical for aspects of hematopoiesis.43 Thus, because the phenotype of the c-Myb knockout is much more severe than the 2 ENU strains, which disrupt p300/CBP cofactor binding, there must be additional critical functions for c-Myb in vivo that do not depend on traditional coactivator binding. For example, c-Myb might have additional key roles as a transcriptional repressor or work via additional protein interactions to direct gene expression or chromatin architecture.
Carpinelli et al performed a dominant screen for perturbations in platelet counts in adult mice.22 They found 2 alleles of c-Myb, which they named plt3 and plt4 because they displayed mildly increased platelet numbers in adult heterozygotes. c-Mybplt3/plt3 and c-Mybplt4/plt4 homozygote mice have very high platelet numbers (∼ 4000 × 106/mL) like c-MybE308G/E308G mice, and also mild anemia and reduced splenic B cells. The plt3 mutation resides in the DNA-binding domain of c-Myb, although it retains significant ability to transactivate a reporter gene so is likely to retain some DNA binding. Only a fraction of c-Mybplt3/plt3 mice survive the perinatal period so the phenotype is more severe than the c-MybE308G/E308G or c-MybM303V/M303V phenotypes as one might expect for a DNA-binding mutation. On the other hand, c-Mybplt4/plt4 mice survive at Mendelian ratios from a c-Mybplt4/wt cross, so is less severe than the DNA-binding mutation in plt3. The plt4 mutation results in mutation D384V within the negative regulatory domain (NRD) domain of c-Myb. The in vivo interactions of this NRD domain are still incompletely understood and probably shed important knowledge about protein networks in which c-Myb operates.
Homozygous Booreana mice are a model for human essential thrombocythemia and myelofibrosis
c-MybE308G/E308G adult mice develop splenomegaly and a phenotype reminiscent of the human MPN, ET, and MF. This is perhaps not surprising because fibrosis in humans with these diseases is thought to result from excess production of cytokines, such as PDGF via increased megakaryocyte mass in bone marrow and spleen.44 In approximately 50% of human cases, the underlying genetic defect in ET and MF is a point mutation in the JAK2 kinase (V617F), which leads to constitutive activity in the absence of stimulation from receptors, such as EpoR and MPL.45 There are also reported activating mutations in the cytoplasmic domain of the MPL receptor (often at amino acid 515) in some cases of MF46 and other described mutations, but in many cases of MPN the underlying mutation is unknown. Our results suggest that it might be worth resequencing c-Myb in cases of JAK2 V617F-negative and MPL515-wild-type ET and MF. In addition, c-MybE308G/E308G mice could be used to test new small molecules that inhibit JAKs or other signaling pathways that are overly active in MPN, such as PDGF receptor signaling in marrow fibroblasts.
A bottleneck on the pathway to gene identification
We were able to identify the c-Myb mutation in booreana quickly because the boo/boo phenotype was distinctive and previous mutations and gene targeting of c-Myb had been reported.8,23 However, identification of the causative mutation in our other pedigrees is taking longer for several reasons. First, there are limited SNPs between B6 and B10, so there is limited power to find meiotic recombinants in the intervals of interest. We have used newly described SNPs derived from deep sequencing of the B10 genome (Bruce Beutler, The Scripps Research Institute, written communication, September 2009) to limit the interval in mulkirri to 34 Mb on chromosome 7 and the interval in kandarra to 26 Mb on chromosome 2 (Table 1). However, these large intervals include 558 and 641 coding genes in the intervals of mulkirri and kandarra, respectively. Further meiotic recombinants will not help narrow the interval because the bottleneck is in SNPs, not recombination events. This is a consequence of using the B10 strain, but this is unavoidable because of the strain specificity of our assays. We have resequenced the coding exons of a few genes in these intervals, which have a history in the hematopoietic field and are yet to find the causative mutation in either strain. It is possible the causative mutations reside outside coding regions (eg, promoters or enhancers), but this has been described rarely and is not the experience of our colleagues.40 We think it is more likely the genes of interest are novel with respect to hematopoiesis.
There has been a remarkably rapid advance in fast and cheap deep sequencing technologies, which will aid mutation identification. These technologies are being used in large-scale cancer resequencing projects but are also ideally suited to detecting ENU mutations. One advantage of using B6 mice for mutagenesis is the reference genome was generated using this strain, so unknown and confounding mouse strain-specific SNPs are less likely to be troublesome, and the ENU-induced mutation is more likely to be obvious. There are different approaches in using these technologies, which are much cheaper than deep sequencing of the whole mutant genome. We are currently using solution capture and resequencing of coding exons within the mulkirri and kandarra intervals as an efficient and relatively cost-effective way to find the causative mutations.47
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chris Goodnow and Ed Bertram for scientific advice and financial support, Harpreet Vohra and Mick Devoy for assistance with flow cytometry, and Shelley-Mae Bolton, Ryan Dunstan, and Nadiah Roslan for animal husbandry.
This work was supported by the National Health and Medical Research Council (C.J. Martin Fellowship; P.P.), the Leukemia Foundation (scholarship; D.R.P.), and the University of Queensland (Postdoctoral Fellowship; P.Y.).
This article is dedicated to the memory of Jared Franklin Purton (1976-2009).
Wellcome Trust
Authorship
Contribution: P.P. conceived and designed the study, collected, assembled, analyzed, and interpreted the data, composed figures, wrote the manuscript, and gave final approval of manuscript; R.T., B.W., A.E.H., D.R.P., P.Y., S.O.C., and R.L. collected and assembled the data and gave final approval of the manuscript; T.J.G. analyzed and interpreted data, edited the manuscript, and gave final approval of the manuscript; and A.C.P. conceived and designed the study, analyzed and interpreted the data, composed figures, wrote the manuscript, and gave final approval of manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Papathanasiou, Australian Phenomics Facility, Australian National University, Acton ACT 0200, Australia; e-mail: peter.papathanasiou@gmail.com; or Andrew C. Perkins, Institute for Molecular Bioscience, University of Queensland, St Lucia QLD 4067, Australia; e-mail: a.perkins@imb.uq.edu.au.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal