Abstract
We have quantified the frequency and proliferation of five subsets of primitive hematopoietic cells, using the cobblestone area forming cell (CAFC) assay, in marrow of five strains of mice with lifespans ranging from about 500 to 800 days. Stem cell characteristics were determined in young (6 weeks) and old (12 months) mice. We report striking effects of both intrinsic strain lifespan and individual mouse age on stem cell populations. First, the relative and absolute numbers of the most primitive stem cell subsets was threefold to fourfold higher in old than in young mice. Second, a considerable strain-to-strain variation in the number of primitive cells was observed: when absolute frequencies were calculated, there was a trend for longer lifespan to be correlated with a larger stem cell pool. Third, stem cells from old mice had a far lower cycling activity than cells from young mice. However, this was highly strain dependent: short-lived C3H/He and CBA/J mice showed a stronger reduction in cycling activity during aging than long-lived C57BL/6 mice. Finally, a significant negative correlation was demonstrated in young mice between maximal lifespan and proliferative activity. These data show that aging has a major impact on the frequency and cell-cycle kinetics of primitive hematopoietic cell compartments. In addition, the observation that cycling activity of stem cells is related to the maximal lifespan of the mouse strain may open ways to identify the genetic mechanisms of both strain- and age-dependent variation in the structure of primitive hematopoietic cell compartments.
THE HEMATOPOIETIC SYSTEM capably maintains normal numbers of mature blood cells during the entire lifespan of an organism.1,2 It is now established that a replenishment of relatively short-lived blood cells is maintained by a small population of primitive, self-renewing, stem cells. However, when Hayflick3,4 showed that at least some normal somatic cells are limited to an intrinsically defined number of cell divisions, this appeared difficult to reconcile with the idea of extensive, if not eternal, stem cell self-renewal. In support of the existence of an inherent mitotic clock in cycling cells is the observation that the proliferative capacity (number of potential population doublings) of fibroblasts, in vitro, strongly depended on the maximal lifespan of the donor species, including, for example, mice, dogs, and humans.5 Recently the interest in the aging of the hematopoietic system has been revitalized by the finding that telomere length may serve as Hayflick's mitotic clock.6,7 A shortening of the telomere with each cell division, until a critical length is achieved, may provide a molecular explanation of the observed divisional memory that many cells appear to have. A strong correlation has been observed between the proliferative capacity of stem cells and the developmental stage of maturation of the donor of these cells, fetal cells having far higher proliferative potential than their adult counterparts,8 a finding that could be linked to differences in telomere length in these cell types.7 Taken together, these results challenged the concept of continuous self-renewal of individual stem cells, and predicted that aging would affect stem cell population kinetics.
A large number of previous studies have been aimed at assessing to what extent, if any, aging affects primitive hematopoietic cell function; the results are conflicting. Proliferation of individual colony-forming unit-spleen (CFU-S) colonies of old mice was less than colonies from young9 or fetal mice.10,11 Moreover, long-term bone marrow (BM) cultures of young marrow produced more CFU-S than those of older marrow.12 However, in serial transplantation studies little effect of the age of the initial donor mice on engraftment potential was observed.13-15 In contrast, when the self-renewal (RS) of older marrow was compared with younger cells, an increase in RS with age was observed.16 When old and young marrow were compared in a competitive repopulation assay, old marrow of C57BL/6 mice competed better than young marrow17,18; however, the converse effect was observed when marrow of CBA/J mice was tested.17
A possibly related and strain-dependent effect of aging has been previously described in a series of publications by one of us.19-25 First, it was shown that the cycling activity of CFU-S varies among mouse strains, with C57BL/6 having a lower cycling activity than all others tested, including DBA/2.19 When chimeric (allophenic) mice were constructed, by combining C57BL/6 and DBA/2 embryos, initially a large percentage of mature blood cells in the chimeras was of DBA/2 origin. However, as the mice aged the DBA/2 cells vanished from the circulation and mature blood cells became entirely of C57BL/6 origin.20-23 It was hypothesized that DBA/2 stem cells cells have initially a numerical or proliferative advantage over C57BL/6 cells and therefore their offspring is most abundant in early adulthood and middle age. Subsequently, however, during the aging process, either DBA/2 cells lose their original properties, and/or C57BL/6 cells gain proliferative capacity, and outgrow DBA/2 cells.24
In an attempt to reevaluate the observations on aging of the hematopoietic system in general, and to explain the above findings in allophenic mice in particular, we have studied the frequency and cycling activity of various subsets of primitive hematopoietic cells using the cobblestone area forming cell (CAFC) assay. Five strains of mice, either 6 weeks or 12 months of age were tested, which were selected on the basis of their maximal lifespan; C3H/He and CBA/J having a short lifespan, DBA/2 being intermediate, and BALB/c and C57BL/6 having the longest lifespan.26
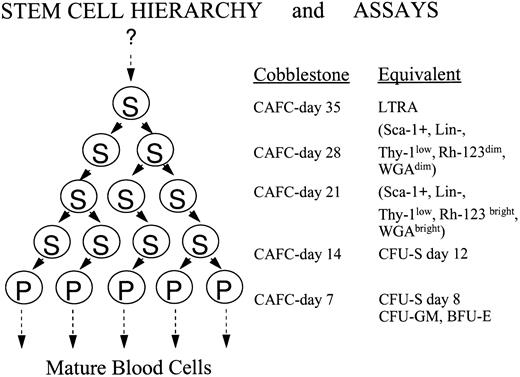
A major advantage of this in vitro stem cell assay is that it measures population sizes of individual stem cell subsets present27-29 and allows one to determine to what extent the primitive cells are in active cell cycle.30 In addition, BM cells from mice of different strains can be compared in a single-assay system, which is impossible in transplantation assays where donor and host immunologic status may affect engraftment. The CAFC assay is a limiting dilution type long-term BM culture. At various timepoints after initiation of the culture increasingly primitive stem cell subsets can be quantified.27-30 This assay dissects the heterogeneous, hierarchical stem cell population into various subcompartments. A general outline of the assay, in conjunction with its relation to several other stem cell assays, is presented in Fig 1.
Overview of the cobblestone area forming cell (CAFC) assay and its relationship with other stem/progenitor cell assays. The CAFC assay permits the dissection of the hierarchical, heterogeneous stem cell pool into several distinct subsets. Most essential is the fact that with increasing duration of the culture period, the primitiveness of the evaluated cell subsets increases correspondingly. Shown are the CAFC subsets, evaluated at weekly intervals, and their equivalents in other classic, functional or phenotypic stem cell assays. Evaluating the cultures for 35 days covers the major part of the hematopoietic stem cell hierarchy. The question mark on top of the figure indicates that it is still possible that even more primitive cells exist. If this is the case, it has no implications for the conclusions drawn in this study. Abbreviations: S, a stem cell that has multi-lineage potential; P, a progenitor cell that has restricted potential; LTRA, a cell which has long-term repopulating abilities; CFU-S, colony forming unit-spleen.
Overview of the cobblestone area forming cell (CAFC) assay and its relationship with other stem/progenitor cell assays. The CAFC assay permits the dissection of the hierarchical, heterogeneous stem cell pool into several distinct subsets. Most essential is the fact that with increasing duration of the culture period, the primitiveness of the evaluated cell subsets increases correspondingly. Shown are the CAFC subsets, evaluated at weekly intervals, and their equivalents in other classic, functional or phenotypic stem cell assays. Evaluating the cultures for 35 days covers the major part of the hematopoietic stem cell hierarchy. The question mark on top of the figure indicates that it is still possible that even more primitive cells exist. If this is the case, it has no implications for the conclusions drawn in this study. Abbreviations: S, a stem cell that has multi-lineage potential; P, a progenitor cell that has restricted potential; LTRA, a cell which has long-term repopulating abilities; CFU-S, colony forming unit-spleen.
Our studies indicate that aging has profound effects on stem cell characteristics, and showed that a correlation exists between the proliferative activity of primitive hematopoietic cells of young mice and the life expectancy of a particular mouse strain. These data may explain many of the aging observations previously reported, and suggest that genetic, strain-dependent factors are important in stem cell proliferation and aging.
MATERIALS AND METHODS
Mice.C3H/He, CBA/J, DBA/2, BALB/c, and C57BL/6 were obtained from either Iffa-Credo (through Broekman Instituut, Someren, The Netherlands), Harlan Sprague Dawley (Indianapolis, IN), or the National Institute of Aging (Bethesda, MD). Old C3H/He mice were only available as retired breeders. In repeated experiments no consistent differences were observed between mice obtained from different sources. All mice were female, and either 6 weeks or 12 months of age. Maximal lifespans of these mice are 500 days for C3H/He, 512 for CBA, 710 for DBA, 745 for BALB/c, and 789 days for C57BL/6.26
CAFC assay.In each experiment marrow cells flushed from the femora of four to five mice were pooled; experiments were repeated one to three times. The CAFC assay was essentially performed as described by Ploemacher et al.27 A stromal cell layer was grown in 96-well microtiter plates (Costar, Cambridge, MA) in Dulbecco's modified Eagle's medium (DMEM) (GIBCO-BRL, Life Technologies, Grand Island, NY), with each well containing 200 μL of 10% fetal calf serum, 5% horse serum, 10−5 mol/L hydrocortisone, 3.3 mmol/L L-glutamine, 80 U/mL penicillin, 80 μg/mL streptomycin, 10−4 mol/L β-mercaptoethanol, and 25 mmol/L NaHCO3 , in an incubator at 33°C, flushed with 5% CO2 in air. Instead of using fresh marrow cells as a source of the stromal layer, we used the FBMD-1 stromal cell line (derived from C57BL/6), which has been reported by Neben et al to result in similar CAFC-frequencies as stroma derived from fresh marrow and which we have used previously to the same effect.30-32 Stromal cell layers were allowed to grow to confluency and were then overlaid with freshly prepared BM cell suspensions at six dilutions, each cell concentration threefold apart (81,000 → 27,000 → 9,000 → 3,000 → 1,000 → 333 cells/well [200 μL]). When marrow cells were added the medium was switched to contain 20% horse serum as the sole serum source. For each cell dilution, 20 to 30 replicate wells were tested. Once a week half of the medium (100 μL) in a well was replaced with fresh medium. To assay the entire stem cell spectrum, the appearance of cobblestone areas was evaluated at weekly intervals for 5 weeks (only colonies growing underneath the stromal layer, consisting of at least five small nonrefractile cells, were counted at 100× magnification and phase contrast illumination on a Zeiss inverted microscope [Zeiss, Thornwood, NY]). As has been extensively described, the frequency of CAFC-day 7 correlates strongly with CFU-G(E)M/CFU-S-day 7, CAFC-day 14 correspond to CFU-S-day 12,27-29,33,34 and CAFC-day 35 coincide with cells that have long-term repopulating ability28 (Fig 1).
The limiting dilution analysis to determine the actual CAFC frequency was performed as described by Ploemacher et al.27 In short, individual wells were scored for the presence or absence of a cobblestone area. The percentage of negative wells as a function of the number of cells/well overlaid was used to estimate the frequency of the various stem cell subsets, using the maximum likelihood solution.35
Measurement of proliferative activity.The proliferative activity of the various primitive cells was assessed using two independent approaches. First, the fraction of cells killed by a 1-hour in vitro incubation with hydroxyurea was determined as we have reported before.30 For this measurement, all BM cell suspensions were diluted to a concentration of 1 × 107 cells in 1 mL. Hydroxyurea (HU; Sigma, St Louis, MO; 200 μg/mL, a total volume of 10 μL) was added to one sample, and incubated with the control sample for 1 hour at 33°C. Both cell suspensions were then washed twice, and a nucleated cell count was performed. Based on this count, the regular CAFC assay was then initiated. The fraction of cells killed by HU was calculated by dividing the estimated CAFC frequency in the HU-treated cell suspension by the control value (untreated marrow from the same group of mice).
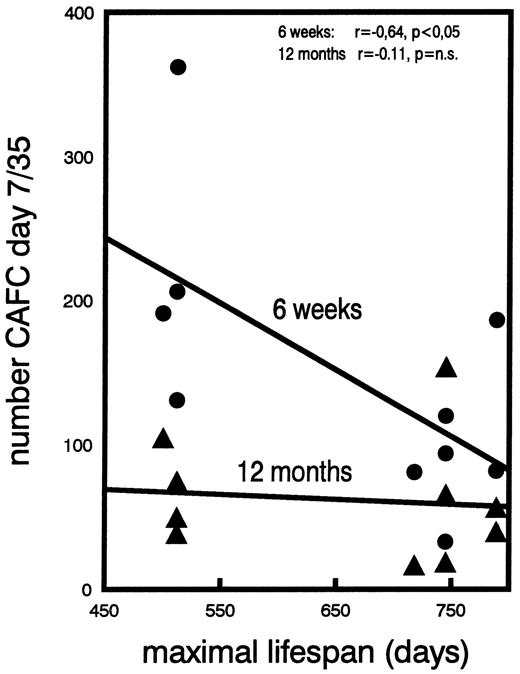
The second method used to estimate in vivo cell proliferation was performed by calculating the ratio of the number of CAFC day 7 to the number of CAFC day 35 (method used to construct Fig 5). This ratio is a reflection of the in vivo amplification that takes place between the most primitive (day 35) and the most differentiated (day 7) stem/progenitor cell stage that we assayed (Fig 1). We have previously used and reported the value of this parameter when evaluating the effect of long-term granulocyte colony-stimulating factor (G-CSF ) treatment on the kinetics of CAFC cell amplification.32
The correlation between the ratio of the numbers of CAFC day 7 to day 35, and the maximal lifespan of five mouse strains. Individual data points, obtained from young (•) or old (▴) mice are shown. The regression shows the relationship with the maximal lifespan of the respective strains. Lifespans for C3H/He is 500 days, for CBA/J 512 days, for DBA/2 710 days, for BALB/c 745 days, and for C57BL/6 789 days. The values of the correlation coefficients “r” are given, in addition to the level of significance (p).
The correlation between the ratio of the numbers of CAFC day 7 to day 35, and the maximal lifespan of five mouse strains. Individual data points, obtained from young (•) or old (▴) mice are shown. The regression shows the relationship with the maximal lifespan of the respective strains. Lifespans for C3H/He is 500 days, for CBA/J 512 days, for DBA/2 710 days, for BALB/c 745 days, and for C57BL/6 789 days. The values of the correlation coefficients “r” are given, in addition to the level of significance (p).
RESULTS
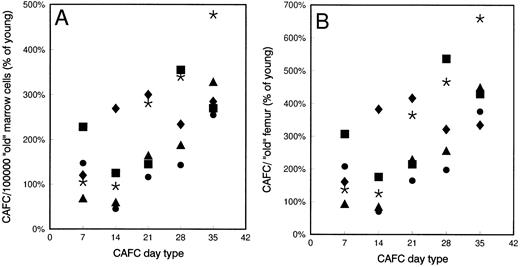
Old mice have more primitive stem cells than young mice.CAFC frequencies were determined weekly for 5 consecutive weeks in young and old mice. Figure 2 depicts the frequency of each CAFC subset of old mice, relative to the frequency in young mice. Figure 2A gives the frequency per 105 marrow cells, whereas Fig 2B gives the number per femur. Old mice had higher (50%) femoral cell counts than young mice (Table 1). In each individual experiment, old mice of all strains tested had significantly higher frequencies of primitive CAFC (day 28-35) subsets than young mice (P < .05, ie, nonoverlapping 95% confidence intervals), whereas these differences were less consistent, or not observed, for the less primitive CAFC day 7 and 14. Thus, the differences between young and old mice increased when more primitive cells were quantified.
Frequency of CAFC subsets in old mice compared with their young counterparts. Mean frequencies of CAFC day 7, 14, 21, 28, and 35 in old C3H/He (•, 1 experiment, 5 mice), CBA/J (*, 3 experiments, 15 mice), DBA/2 (▴, 1 experiment, 5 mice), BALB/c (▪, 3 experiments, 15 mice), and C57BL/6 (♦, 2 experiments, 9 mice) mice. Data are expressed as percentage of CAFC values found in young mice. (A) The CAFC frequencies per 105 marrow cells; (B) values per femur. Control frequencies in young mice for each cell type per 105 marrow cells were: C3H/He: day 7: 124, day 14: 61, day 21: 7.54, day 28: 2.14, day 35: 0.65. CBA/J: day 7: 110, day 14: 31.4, day 21: 5.34, day 28: 1.40, day 35: 0.50. DBA/2: day 7: 125, day 14: 77, day 21: 13.4, day 28: 7.61, day 35: 1.55. BALB/c: day 7: 80, day 14: 50.4, day 21: 11.8, day 28: 2.74, day 35: 0.87. C57BL/6: day 7: 123, day 14: 18.5, day 21: 4.96, day 28: 2.61, day 35: 1.01. Control values for (B) can be calculated using these data and the data given in Table 1.
Frequency of CAFC subsets in old mice compared with their young counterparts. Mean frequencies of CAFC day 7, 14, 21, 28, and 35 in old C3H/He (•, 1 experiment, 5 mice), CBA/J (*, 3 experiments, 15 mice), DBA/2 (▴, 1 experiment, 5 mice), BALB/c (▪, 3 experiments, 15 mice), and C57BL/6 (♦, 2 experiments, 9 mice) mice. Data are expressed as percentage of CAFC values found in young mice. (A) The CAFC frequencies per 105 marrow cells; (B) values per femur. Control frequencies in young mice for each cell type per 105 marrow cells were: C3H/He: day 7: 124, day 14: 61, day 21: 7.54, day 28: 2.14, day 35: 0.65. CBA/J: day 7: 110, day 14: 31.4, day 21: 5.34, day 28: 1.40, day 35: 0.50. DBA/2: day 7: 125, day 14: 77, day 21: 13.4, day 28: 7.61, day 35: 1.55. BALB/c: day 7: 80, day 14: 50.4, day 21: 11.8, day 28: 2.74, day 35: 0.87. C57BL/6: day 7: 123, day 14: 18.5, day 21: 4.96, day 28: 2.61, day 35: 1.01. Control values for (B) can be calculated using these data and the data given in Table 1.
Femoral Cellularities in Young and Aged Mice
| Age . | Mouse Strain . | ||||
|---|---|---|---|---|---|
| . | C3H/He . | CBA/J . | DBA/2 . | BALB/c . | C57BL/6 . |
| 6 wk | 12.6 | 13.2 | 10.3 | 16.7 | 21.7 |
| 12 mo | 17.8 | 17.8 | 14.0 | 26.7 | 31.1 |
| Age . | Mouse Strain . | ||||
|---|---|---|---|---|---|
| . | C3H/He . | CBA/J . | DBA/2 . | BALB/c . | C57BL/6 . |
| 6 wk | 12.6 | 13.2 | 10.3 | 16.7 | 21.7 |
| 12 mo | 17.8 | 17.8 | 14.0 | 26.7 | 31.1 |
Femoral cellularity (in millions) in five strains of mice, either 6 weeks or 12 months old. Each data point refers to the mean value per mouse of pooled marrow cells obtained from femora of 5 to 15 mice.
The number of stem cells varies from strain to strain.Absolute CAFC frequencies are presented for the two most primitive cell compartments. Figure 3 shows the CAFC day 28 and 35 frequencies in old and young mice of the five strains. Figure 3A and B give the frequency of CAFC day 28 and 35, respectively, per 105 marrow cells, whereas in Fig 3C and D the number of these cell types per femur was calculated. DBA/2 mice had a higher CAFC day 28 frequency than all other strains (twofold to threefold more than C57BL/6, threefold to fourfold more than C3H/He and CBA/J), both at a young and old age. However, when the various strains were compared with respect to the total number of CAFC day 28 per femur, this effect was not observed (Fig 3C). As a result, primarily of different femoral cellularities (Table 1), a (nonsignificant) trend existed toward higher total CAFC day 28 numbers with increased maximal lifespan. A similar pattern was observed when day 35 CAFC frequencies were quantified (Fig 3B and D); however, the strain-to-strain differences appeared to be somewhat smaller.
Frequencies of CAFC day 28 and 35 in young and old C3H/He, CBA/J, DBA/2, BALB/c, and C57BL/6 mice. (A and B) The mean CAFC frequencies per 105 marrow cells; (C and D) mean numbers of CAFC per femur. For the number of mice per group refer to the legend of Fig 2. Strains are plotted in order of increasing maximal lifespan (indicated below X-axis).
Frequencies of CAFC day 28 and 35 in young and old C3H/He, CBA/J, DBA/2, BALB/c, and C57BL/6 mice. (A and B) The mean CAFC frequencies per 105 marrow cells; (C and D) mean numbers of CAFC per femur. For the number of mice per group refer to the legend of Fig 2. Strains are plotted in order of increasing maximal lifespan (indicated below X-axis).
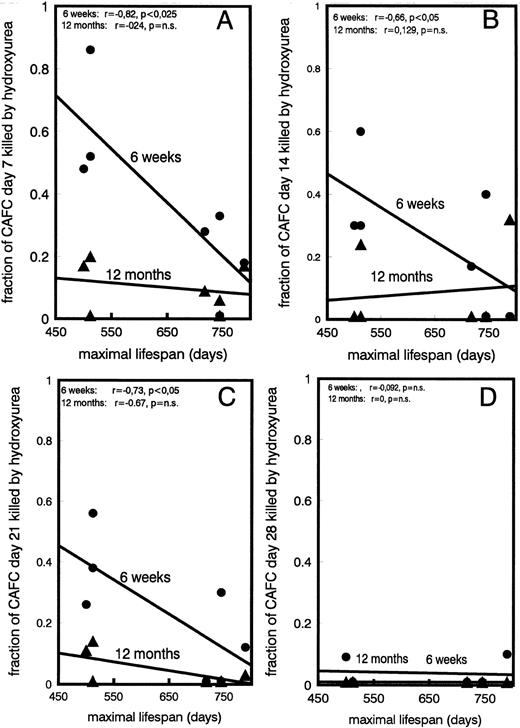
Proliferative activity of stem cells decreases with age and is negatively correlated with maximal lifespan of mouse strain.A surprising effect was observed when the cycling activity of the various CAFC subsets of young and old mice was assessed. Figure 4A, B, C, and D show the fraction of CAFC day 7, 14, 21, and 28, respectively, that was killed by in vitro HU incubation. Individual data of all strains are plotted as a function of the maximal lifespan and several results were obtained. First, as expected, CAFC day 7, the most differentiated cell type, were most susceptible to HU cytotoxicity (Fig 4A), whereas CAFC day 28, a far more primitive cell type, were not significantly affected by incubation with hydroxyurea (Fig 4D). CAFC day 35 were also not affected by HU incubation, but because results were identical to CAFC day 28 they are not shown. This is in full agreement with previous CAFC studies that have shown that CAFC day 7 correlate with cycling progenitors, typically measured by colony formation in semisolid medium, and are sensitive to 5-fluorouracil (5-FU) injected in vivo. CAFC day 28 correlate with quiescent long-term repopulating cells and are 5-FU resistant.29,30 More importantly, however, our data show that the cycling activity of the more committed CAFC cell types (day 7-21) decreases strongly during the aging process (Fig 4A through C). This is shown most strikingly in the shorter living strains, with the reduction in cycling activity between young and old mice marked in C3H/He and CBA/J mice, but undetectable in C57BL/6. A statistically significant negative correlation (P < .05) was observed between the maximal lifespan of the donor mouse strain and the cycling activity of CAFC day 7, 14, and 21, when obtained from young mice. This correlation could not be shown in old mice. Lastly, to assess if the differences found in the cycling activities were reflected in in vivo cell production differences, we calculated the ratio between CAFC day 7 and day 35, as we have reported before.32 This ratio reflects the amplification between CAFC day 35 and day 7 cell compartments. If, for example, the ratio was 1, it would imply that no cell amplification took place during differentiation (see Fig 1). The data plotted in Fig 5 show that this ratio correlates very well with the cycling activity data shown in Fig 4. Young mice had a substantially higher 7/35 ratio than old mice. Moreover, a statistically significant correlation was observed between this ratio and the maximal lifespan of the mouse strains (P < .05), but only in the young mice, not in old ones.
Cycling activity of CAFC subsets in old and young mice. Shown is the fraction of CAFC day 7 (A), 14 (B), 21 (C), and 28 (D), obtained from young (•) or old (▴) mice, that were killed by incubation, in vitro, with hydroxyurea (HU). The regression line, using individual data points obtained in young and old mice, shows the relationship with the maximal lifespan of the respective strains. Lifespans for C3H/He is 500 days, for CBA/J 512 days, for DBA/2 710 days, for BALB/c 745 days, and for C57BL/6 789 days. The correlation coefficient “r” of these lines is given in each figure, in addition to the level of significance (p). CAFC day 35 were not affected by HU (data are not shown).
Cycling activity of CAFC subsets in old and young mice. Shown is the fraction of CAFC day 7 (A), 14 (B), 21 (C), and 28 (D), obtained from young (•) or old (▴) mice, that were killed by incubation, in vitro, with hydroxyurea (HU). The regression line, using individual data points obtained in young and old mice, shows the relationship with the maximal lifespan of the respective strains. Lifespans for C3H/He is 500 days, for CBA/J 512 days, for DBA/2 710 days, for BALB/c 745 days, and for C57BL/6 789 days. The correlation coefficient “r” of these lines is given in each figure, in addition to the level of significance (p). CAFC day 35 were not affected by HU (data are not shown).
DISCUSSION
The data presented in this study show that aging significantly alters the primitive hematopoietic cell compartments. First, the proliferative activity of primitive cells is sharply reduced during the first year of murine life. Concomitantly however, the relative and absolute number of stem cells increases. Finally, these changes are strain-dependent, and appear to be related to the maximal lifespan of the mouse strain.
Historically, many of the studies that were aimed at identifying changes in stem cell characteristics during aging have made use of various types of in vivo transplantation experiments.1,9-11,13-18 Such an approach makes it cumbersome to compare different donor strains, because the host mice in each experiment must correspond to the donor to prevent graft rejection. In general in those studies, it was concluded that aging did not affect stem cell potential. Only when fetal cells were compared with adult BM, or when the proliferation of individual CFU-S colonies from young and old marrow was compared, a detrimental effect of aging was observed.8-11
However, most studies have evaluated the “repopulating ability” of populations of young and old marrow cells.1,13-18 The repopulating ability of a marrow graft is defined by both the number of stem cells present and their proliferative capacity (“quality”). In this study we have used the CAFC assay to separate these two variables, and find striking effects of aging. Our data may provide an explanation for at least some of the previously published results. Harrison,17 observing that old C57BL/6 marrow competed better than young marrow, concluded that no detrimental effect of aging occurred. However, in the same report he found the opposite to be true for CBA/J marrow. We show that the “extent” of aging is highly dependent on the strain tested. In fact, long-lived C57BL/6 appears to be a strain in which the proliferative activity of primitive cells remains largely constant through at least the first year of life. We find that old C57BL/6 mice have twofold to threefold more primitive cells (CAFC day 35) than young mice, which is in full agreement with Harrison's data.17,18 However, short-lived CBA/J, like C3H/He, seem more susceptible to aging, and although, like C57BL/6, old CBA/J mice have more primitive CAFC day 35 cells than their younger counterparts, the proliferation of the young cells is far higher. This may explain why in a competitive repopulation assay, in which the total CAFC pool is involved, young CBA/J marrow preferentially engrafted when competed with old marrow.17 This may also indicate a potential hazard in the evaluation of data obtained in competitive repopulation assays. Although this test probably is the best assay to measure the functional capacity of a marrow cell population, engraftment may not be solely dependent on stem cell numbers. It has previously been shown that an increased cycling status has detrimental effects on cell homing and subsequent engraftment of hematopoietic tissues.36 37 Thus, when two cell suspensions that differ intrinsically in their cycling activity are competed against each other, engraftment results may not properly reflect the number of stem cells present in the mixed grafts.
Indeed, it was this intrinsic difference in proliferative capacities of stem cells from different mouse strains that initiated the present study.19-25 In previous experiments it was found that DBA/2 marrow cells have a proliferative advantage compared with C57BL/6 cells, whether such is measured by cell-cycle kinetics, engraftment after transplant, or in chimeras. Most relevant to the initiation of the present study was the consistent finding that initially in allophenic mice, DBA/2 cells were most abundant in the peripheral blood. As the chimeras aged, however, C57BL/6 cells became predominant, although DBA/2 stem cells were still present but quiescent.23 It was speculated that DBA/2 cells had an initial numerical or growth advantage, which was lost during aging.20-24 Our present data may offer an explanation for this phenomenon, and in addition place this strain-dependent effect observed in allophenic mice in a broader context. Our finding that DBA/2 cells of young mice have a higher cycling activity than cells of young C57BL/6 mice had already been demonstrated in the initial report describing the DBA/2↔C57BL/6 allophenics.19 Importantly, however, we now show that during the first year of life the cycling activity of DBA/2 cells decreases, whereas this is not detectable in C57BL/6 stem cells. If this also occurs in allophenic mice, C57BL/6 cell proliferation during the first year of life is relatively low but stable, whereas DBA/2 cell proliferation is higher at first, but then decreases. It follows that C57BL/6 cells will consequently outnumber DBA/2 cells. In parental strain DBA/2 and C57BL/6 mice, however, our data show that aging is also accompanied by an increase in the relative and absolute stem cell frequency. It remains to be determined by the methods used in the current studies to what extent this effect takes place in allophenics and how it interferes with the decrease of cycling activity.
In the present study we were unable to detect higher numbers of primitive cells (CAFC day 28, 35) in C57BL/6 mice compared with DBA/2, as was suggested when rhodamine-123 dye uptake studies were performed.24 However, it is difficult to quantitatively compare CAFC data, which were obtained by using unfractionated whole BM, with FACS data obtained by sorting fractionated cell suspensions.
The observation of increased stem cell numbers in marrow in conjunction with a reduced proliferative activity during aging is worthy of comment. Although in the present study we did not quantify stem cell numbers in the spleen, it is very unlikely that the increase in stem cell numbers in the femur during aging is caused by a migration of cells from the spleen to the marrow. We have previously shown that spleens of normal young mice contain less than 1% (about 90 per spleen) of the total CAFC day 28 pool.30 Thus, even if all of them migrated to the marrow, they would have an inconsequential effect on marrow numbers.
Two hypotheses can be proposed which may explain this expansion of stem cell numbers. First, this finding may be related to results obtained by Rosendaal et al,38 leading them to formulate the “generation-age hypothesis.” According to this hypothesis, cells “count” their divisions, and hematopoietic cell production would be regulated so that those stem cells which have undergone many divisions are the ones most likely to differentiate. This would result in a relative accumulation of cells that have undergone few divisions (ie, more primitive cells) during aging. In fact, this is what we have observed in the present study, but our results extend the notion. We found that the increase in stem cell number was not only relative, but also absolute, suggesting that the self-renewal probability P of stem cells is higher than .5. Thus, a corollary or alternative hypothesis for the age-related increase in stem cell number may be that due to the reduction of cycling activity, and the continued need for the production of normal numbers of mature blood cells, the number of primitive cells is necessarily increased. In other words, in aged mice more, slowly dividing, stem cells are producing an equal number of blood cells as are more actively cycling, but fewer, stem cells in young mice.
Lastly the observation that a negative correlation was observed beween maximal mouse lifespan and stem cell proliferation in young mice is intriguing. This finding parallels several observations made with other renewing tissues. It has been shown that there is a relationship between the proliferative potential of fibroblasts and both the age of the donor,3,4 and the maximal lifespan of the donor species.5 More recently it has been demonstrated that an inverse relationship exists between telomere length in fibroblasts6 but also in hematopoietic cells,7 and age of the donor. Our data clearly show a relationship between primitive cell proliferation and maximal lifespan, but only in young mice. Therefore, it is tempting to speculate that genetic differences in telomere shortening cause, or are at least associated with, the strain-dependent effects that we describe. If all mice, regardless of strain, were born with an equal telomere length, strains with highly cycling cells (which appear to be the short-lived ones) would encounter earlier in life a critical minimum telomere length associated with senescence. This may explain why C3H/He and CBA/J mice particularly demonstrate the most pronounced effect of age on stem cell cycling.
However, other data make it likely that the story may be more complicated. First, intrinsic telomere length in mice of different strains seems to differ considerably; cells of C57BL/6 mice having longer telomeres than either DBA/2 and CBA/J.39 Moreover, inter-species comparison has shown that telomere length in mice is far longer than in humans, making it difficult to understand the relationship of overall telomere length and longevity.39 Second, telomerase, the enzyme capable of elongating telomeres seems to be abundant in murine tissues,40 whereas it is virtually absent in normal somatic human cells, although it can be detected at low levels in normal leukocytes.41 Finally, telomere length may be increased independently of telomerase.42 Thus, although it is tempting to speculate about differences with respect to telomere dynamics to explain our current data and aging of cell populations in general, much more needs to be known of the involvement of telomere length in cell proliferation. Nevertheless, we show here that genetic factors are likely to regulate the proliferative behavior of primitive hematopoietic cells and it is the identification of this gene(s) that ultimately will lead to a unifying explanation of our findings.
ACKNOWLEDGMENT
The FBMD-1 cell line was kindly provided by Dr R. Ploemacher.
Supported in part by the Department of Internal Medicine, University of Kentucky Hospital, the Markey Cancer Center and the Jan Cornelis de Cock Stichting. G.d.H. is a recipient of a “TALENT”-stipend, awarded by the Netherlands Organization for Scientific Research (NWO).
Address reprint requests to Gerald de Haan, PhD, University of Kentucky Hospital, Markey Cancer Center, Division of Hematology/Oncology, 800 Rose St, Lexington, KY 40536-0093.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal