Abstract
In the adult, platelets are derived from unipotential megakaryocyte colony-forming cells (Meg-CFCs) that arise from bipotential megakaryocyte/erythroid progenitors (MEPs). To better define the developmental origin of the megakaryocyte lineage, several aspects of megakaryopoiesis, including progenitors, maturing megakaryocytes, and circulating platelets, were examined in the murine embryo. We found that a majority of hemangioblast precursors during early gastrulation contains megakaryocyte potential. Combining progenitor assays with immunohistochemical analysis, we identified 2 waves of MEPs in the yolk sac associated with the primitive and definitive erythroid lineages. Primitive MEPs emerge at E7.25 along with megakaryocyte and primitive erythroid progenitors, indicating that primitive hematopoiesis is bilineage in nature. Subsequently, definitive MEPs expand in the yolk sac with Meg-CFCs and definitive erythroid progenitors. The first GP1bβ-positive cells in the conceptus were identified in the yolk sac at E9.5, while large, highly reticulated platelets were detected in the embryonic bloodstream beginning at E10.5. At this time, the number of megakaryocyte progenitors begins to decline in the yolk sac and expand in the fetal liver. We conclude that the megakaryocyte lineage initially originates from hemangioblast precursors during early gastrulation and is closely associated both with primitive and with definitive erythroid lineages in the yolk sac prior to the transition of hematopoiesis to intraembryonic sites.
Introduction
Hematopoiesis in the mouse embryo initiates shortly after the onset of gastrulation in the blood islands of the extraembryonic yolk sac.1 Here, the close spatial and temporal arrangement of the first hematopoietic and endothelial precursors has long suggested that both cell types originate from a common hemangioblast progenitor.2 This hypothesis has gained support from studies of embryonic stem cells cultured in vitro3-7 and more recently been confirmed by the identification of blast–colony-forming cells (BL-CFCs) in mouse embryos that possess endothelial, as well as primitive erythroid, definitive erythroid, and multiple myeloid lineage potential.8 A transient pool of primitive erythroid progenitors (EryP-CFCs) emerges between embryonic days 7.25 to 9.0 (E7.25-E9.0) and generates large, nucleated βH1-globin–expressing erythroid cells that eventually enucleate upon maturation.9-11 Subsequently, a second, overlapping wave of definitive erythroid progenitors (burst-forming units-erythroid [BFU-Es]), similar to those found later in the fetal liver and adult bone marrow, emerges in the yolk sac accompanied temporally by mast cell, granulocyte, macrophage, and multipotential high proliferative potential colony forming cells (HPP-CFCs).9,10,12,13 These findings have suggested that the definitive wave of yolk sac hematopoietic progenitors is multilineage, while the initial primitive wave is unilineage, being specifically confined to the primitive erythroid lineage.14
In the adult bone marrow, megakaryocyte progenitors (Meg-CFCs) generate large, multinucleated precursor cells that produce discoid anucleate platelets that are a critical component of hemostasis. It is not known when platelets first begin to circulate in the murine embryo. Megakaryocyte progenitors have been described in the yolk sac between E7.5 and E10.5,15,16 however, their association with primitive or definitive hematopoiesis has not been clearly established. The presence of Meg-CFCs in the yolk sac as early as E7.5 raises the possibility that megakaryocyte fate may be associated with the primitive erythroid lineage15 and specified at the level of the hemangioblast like that of the other yolk sac–derived lineages that make up the primitive and definitive waves of hematopoiesis.
The megakaryocyte lineage in the adult has been found to be closely associated with the erythroid lineage. These lineages share several growth factor receptors, including those for erythropoietin and thrombopoietin,17-19 as well as a panoply of transcription factors, including GATA-1, FOG-1, GATA-2, and NF-E2.20-25 Of importance, the megakaryocyte and erythroid lineages share a common megakaryocyte/erythroid progenitor (MEP). Initial evidence for this bipotential progenitor came from hematopoietic colonies cultured in vitro that were composed both of megakaryocyte and of erythroid cells26,27 and that were derived from single cells.28 Further evidence for the existence of bipotential MEPs came from the isolation of progenitors from adult bone marrow, the spleen of anemic mice, and fetal liver that exclusively generate both megakaryocyte and erythroid cells.29-33
Considering the close relationship of the megakaryocyte and erythroid lineages in the fetus and the adult, we investigated the early ontogeny of the megakaryocyte lineage and its specific association both with primitive and with definitive erythropoiesis in the murine embryo. Here we show that megakaryocyte potential initiates from hemangioblast precursors during gastrulation and that unique bipotential “primitive” MEPs arise during early gastrulation coincident with unipotential primitive erythroid and megakaryocyte progenitors. Subsequently, a second wave of “definitive” MEPs emerges in the yolk sac prior to the formation of the fetal liver. The presence in the bloodstream of large, highly reticulated embryonic platelets as early as E10.5 suggests that they originate from these yolk sac–derived megakaryocyte progenitors.
Materials and methods
Tissue collection
Timed pregnant ICR mice (Taconic, Germantown, NY) were killed by CO2 inhalation. Yolk sac and embryo proper tissues were isolated from staged embryos as previously described10 and dissociated in 1:30 dilution of trypsin (0.25%; Worthington, Lakewood, NJ) in PBS/EDTA for 2 to 5 minutes or treated with collagenase/dispase (Roche, Indianapolis, IN), as described,34 for more than 45 somite pair (sp) yolk sac and embryo proper. Peripheral blood and fetal liver were also isolated after 11 sp and 35 sp, respectively. Cells were resuspended in 10% plasma-derived serum (PDS; Animal Technologies, Tyler, TX) in IMDM (Invitrogen, Carlsbad, CA) and then examined for viability by exclusion of trypan blue (CellGro, Herndon, VA). Red blood cell (RBC) numbers were enumerated by benzidine staining (Sigma-Aldrich, St Louis, MO) for each tissue beyond 4 sp as previously described.35 All animal experiments were approved by the University of Rochester Committee on Animal Resources.
Hemangioblast assay
The hemangioblast assay was performed as previously described.8 Dissociated cells from staged embryos were filtered through a 35-μm nylon mesh (BD Biosciences, San Jose, CA) and plated into 65% methylcellulose (Invitrogen) in IMDM,36 2 mM glutamine (Gibco-Brl, Gaithersburg, MD), 4 × 10−4 M monothialglycerol (MTG; Sigma-Aldrich), 10% PDS, 300 μg/mL transferrin (Sigma-Aldrich), 1 ng/mL LIF (Chemicon International, Temecula, CA), 5 ng/mL VEGF, 50 ng/mL insulin-like growth factor, 5 ng/mL IL-6, 10 ng/mL TPO6 (all purchased from Peprotech, Rocky Hill, NJ), and 25% D4T conditioned media (from D4T cells) for 4 days. Individual hemangioblast colonies were replated in liquid expansion media37 (10% PDS, 10% horse serum [Invitrogen], 2 U/mL EPO [Amgen, West Greenwich, RI], 5 ng/mL VEGF, 10 ng/mL IGF-1, 10 ng/mL bFGF, 100 ng/mL SCF, 1 ng/mL IL-3 [all purchased from Peprotech], 2 mM glutamine, and 4.5 × 10−4 M MTG) and expanded for 2 to 3 days. Nonadherent cells were assayed for hematopoietic progenitor activity in collagen cultures (as described in “Collagen culture”). The remaining adherent cell populations were cultured for an additional 7 days in expansion media, and then fixed in 4% paraformaldehyde. All cultures were maintained at 37°C in 5% CO2.
RT-PCR
cDNA was prepared from DNase-treated RNA extracted from individual primitive erythroid colonies according to the manufacturers' instructions (SuperScript III First-Strand [Invitrogen]; RNase-Free DNase Set [Qiagen, Valencia, CA]; PicoPure RNA Isolation Kit [Arcturus Bioscience, Mountain View, CA]). Reverse-transcription–polymerase chain reaction (RT-PCR) was performed according to the manufacturer's instructions using Advantage PCR Kit & Polymerase mix (Clontech, Mountain View, CA) using primers designed for βH1-globin, β1-globin, acetylcholinesterase, von Willebrand factor, and granulocyte–colony-stimulating factor receptor (G-CSFR). Primer sequences are provided in Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article.
Collagen culture
Dissociated cells were suspended in Megacult substitute and 30% collagen (StemCell Technologies, Vancouver, BC, Canada) as described,36 with 10 ng/mL IL-3, 20 ng/mL IL-6, 100 ng/mL SCF, 3 ng/mL GM-CSF (all from Peprotech) and 2 U/mL EPO or with 10 ng/mL IL-3, 20 ng/mL IL-6, 50 ng/mL TPO, and 50 ng/mL IL-11 (Peprotech) and plated on chambered glass slides (Nalge Nunc, Rochester, NY). Cultures were maintained at 37°C in 5% CO2 for 7 or 10 days and then terminated by wicking-off excess liquid with polypropylene spacers (P/N 66548; Pall Life Sciences, East Hill, NY) and fixing in cold acetone for 5 to 10 minutes. Acetylcholinesterase staining was performed as previously described.36
Tissue sections
Embryos were embedded in Tissue Tek Cryomolds and OCT embedding medium (Sakura, Zoeterwoude, the Netherlands), snap-frozen, and sectioned 10-μm thick on a Microm HM 550 OMVP cryostat (Richard-Allan Scientific, Kalamazoo, MI) at −10°C.
Immunohistochemistry
Antibodies were diluted in blocking buffer (1.5% goat serum/PBS; Vector Laboratories, Burlingame, CA), unless specified otherwise. All serum, secondary antibodies, ABC Kit, Vector Red, Nickel DAB, and Vectashield were purchased from Vector Laboratories. Cultures were stained using a double-antigen labeling protocol for alkaline phosphatase–modified antigens (ABC Kit; Vector Laboratories38 ). In brief, fixed collagen cultures were permeated for 1 hour in 3% Triton-X 100 (Sigma-Aldrich) or directly blocked. First, erythroid cells were labeled with βH1-globin (1:25011) and goat anti–rabbit biotin (1:250) or Ter119 (1:200; BD Pharmingen, Franklin Lakes, NJ) and goat anti–rat biotin (1:1500), and developed in nitroblue tetrazolium/BCIP (NBT/BCIP; Roche) after ABC amplification according to the manufacturer's instructions (Vector Laboratories). Second, megakaryocyte cells were labeled with GP1bβ (1:600; Emfret Analytics, Würzburg, Germany) or CD41 (1:200; BD Pharmingen) and goat antirat biotin modified by ABC and developed in Vector Red. All antibody incubations were performed for 30 minutes at room temperature. Cultures were fixed in 95% ethanol and rehydrated in ddH2O, then mounted. Sections were brought to room temperature, fixed in cold acetone, rehydrated in 1x Tris-buffered saline, and blocked in acetic acid, then 10% goat and 2.5% mouse serum. GP1bβ and goat antirat alkaline phosphatase antibodies (1:500) were diluted in 5% goat serum, incubated for 60 minutes and 30 minutes, respectively, and developed in BCIP/NBT. Paraformaldehyde-fixed cells from hemangioblast cultures were blocked in 0.1% Fraction V BSA (Sigma-Aldrich) in Triton-X, incubated with anti-PECAM (1:10, BD Pharmingen) and goat antirat HRP (1:500) for 1 hour each, developed with Nickel DAB, and then fixed in 4% paraformaldehyde. Control experiments following removal of all antibodies, removal of first and second primary and first and second secondary antibodies, were performed.
Size measurement of circulating platelets
Peripheral blood from E10.5 to E15.5 fetuses was collected in PB-1 containing 12.5 μg/mL heparin and centrifuged at 700g for 5 minutes to create a platelet-rich supernatant. Platelets were diluted 10-fold in 5% fetal calf serum (Sigma-Aldrich) in PBS, stained with anti–GPV-FITC (1:200; Emfret Analytics) for 20 minutes, washed, suspended in 1% Pierce Surfasil Silconizing Fluid (Pierce Chemical, Rockford, IL) in acetone, mounted in a silicon-coated cell chamber on the stage of a Nikon Diaphot Inverted Microscope with a 60 × objective (NA 0.85; Nikon, Melville, NY), and recorded on an MTI CCD72S video camera (Sony, New York, NY). Platelet diameters were measured using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image/).
Transmission electron microscopy
Platelet-rich fractions were fixed in 0.1 M phosphate-buffered 2% glutaraldehyde overnight at 4°C, washed in buffer, postfixed in 0.1 M phosphate-buffered 1% osmium tetroxide for 30 minutes, washed, and trapped in 2% agarose. The pellets were dehydrated through a graded series of ethanol to 100%, transitioned into propylene oxide, and infiltrated with a mixture of epon/araldite epoxy resin, embedded, and polymerized for 2 days at 70°C. Thin sections (70 nm) were placed onto 200 mesh copper/rhodium grids and stained with aqueous uranyl acetate and lead citrate. The grids were examined using a Hitachi 7100 transmission electron microscope (Tokyo, Japan) at accelerating voltage of 75 kv. Digital images were captured using a MegaView III camera and AnalySIS software (Soft-Imaging System, Lakewood, CO).
Flow cytometric analysis of circulating platelets
Adult blood was collected in a heparinized 1-mL syringe following cardiac puncture of mice that had been killed. Fetal and neonatal peripheral blood was collected as previously described.11 Blood was diluted in 2 to 3 volumes Tyrode buffer, and 1 volume PBS plus 1 μg/mL thiazole orange (Sigma-Aldrich), 2.5 μg/mL Hoechst 33342 (Molecular Probes, Eugene, OR), 1 μL each of anti–CD41-PE (BD Pharmingen) and anti–Ter119-APC (eBioscience, San Diego, CA) and incubated for 20 minutes at 37°C. Cells were diluted 2-fold in PBS and immediately analyzed on a BD LSR-II flow cytometer (BD Biosciences). Samples were compensated and compared against unstained controls. Platelet gates were selected as shown and described in Figure 6. Reticulocytes in stained adult blood were used for setting the threshold of the thiazole orange signal. Flow cytometric data were analyzed using FlowJo software, version 6.1.1 (Tree Star, Stanford University, CA).
RNase treatment of platelets
RNase treatment of platelets was performed as described by Matic et al.39 Fetal blood was centrifuged for 10 minutes at 80g, and the platelet-rich supernatant was diluted 1:10 in 1% paraformaldehyde and fixed for 30 minutes at room temperature. Following centrifugation (10 minutes at 1200g), cells were resuspended in PBS and diluted 1:10 in permeablization buffer (10 mM Tris, pH 7.5, 20 mM NaCl, 1 mM EDTA, 50% glycerol, 0.1% Triton-X; Sigma-Aldrich) with and without RNase (10 μg/mL final concentration). Ter119-APC and CD41-PE were added and platelets were incubated for 15 minutes, then diluted in one volume thiazole orange (1 μg/mL final concentration) and incubated for an additional 15 minutes. Cells were diluted 2-fold in PBS and immediately analyzed by flow cytometry. The mean thiazole orange signal of Ter119-APC–negative, CD41-PE–positive platelet fractions was determined using FlowJo software.
Photography
Immunostained colonies and tissue sections were viewed with an Eclipse TE 2000-S microscope (Nikon 10 ×, 20 ×, or 40 × objectives with NA 0.30, 0.40, and 0.60, respectively) and an Optiphot microscope (Nikon 10 ×, 20 ×, or 40 × objectives with NA 0.5, 0.75, and 0.85, respectively), respectively, and photographed with a SPOT RT-slider digital camera (Diagnostic Instruments, Sterling Heights, MI). Images were processed with lens defect correction and brightness/contrast optimization in Photoshop (Adobe, San Jose, CA) with Fovea Pro plug-in (Reindeer Graphics, Ashville, NC).
Results
The megakaryocyte lineage arises from hemangioblast precursors
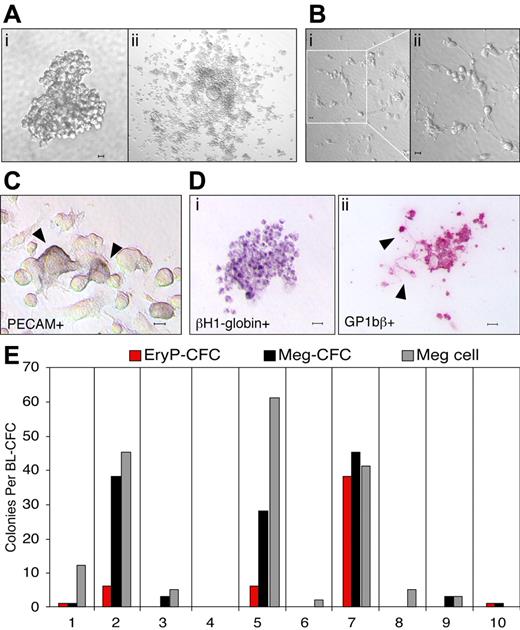
It has recently been shown that primitive and definitive erythroid as well as multiple myeloid lineages originate from hemangioblast precursors that emerge in the primitive streak of the mouse during gastrulation.8 We asked whether hemangioblast precursors also contain megakaryocyte potential. Cells from mid primitive streak through mid neural plate mouse embryos were plated into semisolid media, and a total of 66 colonies with BL-CFC morphology (Figure 1Ai) from 49 embryos was replated into expansion media. Thirty-nine of these replated colonies expanded to generate round, nonadherent cells overlaying flat, PECAM-positive adherent cells (Figure 1Aii,C). Of interest, proplatelet formation40,41 was detected in 29% of the 39 colonies that expanded in culture, indicating that megakaryocyte maturation was proceeding rapidly in these cultures (Figure 1B). Following expansion, nonadherent cells were plated into collagen to assay for hematopoietic progenitor potential. The resulting colonies were stained with βH1-globin and GP1bβ antibodies to identify primitive erythroid and megakaryocyte cells, respectively (Figure 1D). GP1bβ labeled both cells and proplatelet extensions (Figure 1Dii), consistent with its known expression in megakaryocytes and platelets.42 As found previously,8 there was marked heterogeneity in progenitor composition derived from the expanded hemangioblast cultures (Figure 1E). Meg-CFCs were detected in 64% and EryP-CFCs in 16% of the 39 expanded blast-CFC colonies recultured into collagen. Individual GP1bβ-positive cells were also noted in 86% of the collagen cultures. These results indicate that a large proportion of hemangioblast precursors in the gastrulating mouse embryo contains megakaryocyte potential.
Megakaryocyte potential is derived from hemangioblast precursors. (A) Hemangioblast-derived colony from E7.5 mouse embryo (i). Adherent and nonadherent cells from a hemangioblast-derived colony after 3 days in liquid expansion media (ii). (B) Proplatelet formation of cells differentiated from a hemangioblast-derived colony after 3 days in liquid media (i-ii). (C) PECAM-positive cells (arrowheads) in the adherent cell population after 10 days in liquid expansion media. (D) Primitive erythroid and megakaryocyte colony formation from expanded, nonadherent cells placed into progenitor assays. An EryP-CFC–derived colony contains cells positive for βH1-globin (i) and a Meg-CFC–derived colony contains cells and proplatelet extensions (arrowheads) positive for GP1bβ (ii). (E) Hematopoietic potential of 10 individual hemangioblast colonies expanded in liquid culture. The number of EryP-CFCs, Meg-CFCs, and megakaryocyte cells (meg cells) is shown. Scale bars represent 10 μm.
Megakaryocyte potential is derived from hemangioblast precursors. (A) Hemangioblast-derived colony from E7.5 mouse embryo (i). Adherent and nonadherent cells from a hemangioblast-derived colony after 3 days in liquid expansion media (ii). (B) Proplatelet formation of cells differentiated from a hemangioblast-derived colony after 3 days in liquid media (i-ii). (C) PECAM-positive cells (arrowheads) in the adherent cell population after 10 days in liquid expansion media. (D) Primitive erythroid and megakaryocyte colony formation from expanded, nonadherent cells placed into progenitor assays. An EryP-CFC–derived colony contains cells positive for βH1-globin (i) and a Meg-CFC–derived colony contains cells and proplatelet extensions (arrowheads) positive for GP1bβ (ii). (E) Hematopoietic potential of 10 individual hemangioblast colonies expanded in liquid culture. The number of EryP-CFCs, Meg-CFCs, and megakaryocyte cells (meg cells) is shown. Scale bars represent 10 μm.
Megakaryocyte progenitors originate in the yolk sac
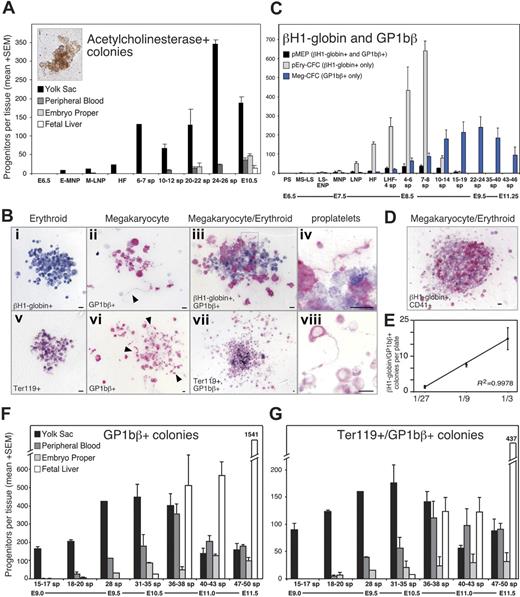
We next asked whether the megakaryocyte potential found in hemangioblasts is realized in the embryo by examining the temporal and spatial emergence of megakaryocyte progenitors in staged mouse embryos. Colonies derived from murine Meg-CFCs were identified by staining with acetylcholinesterase (Figure 2A).43 No megakaryocyte progenitors were identified at early primitive streak stages (E6.5; Figure 2A), consistent with the lack of commitment of mesoderm cells to hematopoietic fates at the initiation of gastrulation.10 Megakaryocyte progenitors were detected at low but reproducible numbers by early to mid neural plate stages. Over the next 48 hours, Meg-CFCs increased exclusively within the yolk sac. After the onset of circulation, low numbers of Meg-CFCs were found in the embryo proper and the bloodstream. By E10.5, the number of megakaryocyte progenitors had decreased in the yolk sac and increased in the fetal liver.
Meg-CFCs and 2 novel bipotential MEPs emerge during embryonic hematopoiesis. (A) Number (+SEM) of acetylcholinesterase-positive (megakaryocyte) colonies grown from individual embryonic tissues by stage. The inset (i) shows an acetylcholinesterase-positive colony. (B) Immunohistochemical staining of erythroid and megakaryocyte progenitor–derived colonies. (Top) Primitive erythroid (i), megakaryocyte (ii), and bipotential primitive MEP (iii) colonies from neural plate–stage cultures stained with primitive erythroid-specific βH1-globin (blue) and megakaryocyte-specific GP1bβ (pink) antibodies. (Bottom) Erythroid (v), megakaryocyte (vi), and bipotential definitive MEP (vii) colonies from E9.5 to E10.5 yolk sac stained with panerythroid Ter119 (blue) and with GP1bβ (pink) antibodies. Boxed areas in panels iii and vii highlight proplatelet formation at 100 × shown in panels iv and viii, respectively. Arrowheads indicate proplatelet formation in panels ii and vi. Scale bars represent 10 μm. (C) Average number (+SEM) of primitive erythroid, megakaryocyte, and bipotential primitive MEP colonies from plated whole embryos (PS-LNP) and yolk sac (HF-46 sp) as determined by immunohistochemistry. (D) Primitive MEP–derived colony derived from a neural plate–staged embryo contains cells positive for βH1-globin and CD41. (E) Number (+SEM) of βH1-globin/GP1bβ-double positive colonies from a dilution series of 4 to 5 sp yolk sacs, in triplicate. (F) Spatial and temporal distribution of Meg-CFCs as determined by GP1bβ-stained colonies per tissue. (G) Spatial and temporal distribution of definitive MEPs, as determined by Ter119/GP1bβ double-positive colonies per tissue. sp indicates somite pair; PS, preprimitive streak; MS, mid primitive streak; LS, late primitive streak; ENP, early neural plate; MNP, mid neural plate; LNP, late neural plate; HF, head fold; and LHF, late head fold. Approximate embryonic day (E) is provided below the developmental stages.
Meg-CFCs and 2 novel bipotential MEPs emerge during embryonic hematopoiesis. (A) Number (+SEM) of acetylcholinesterase-positive (megakaryocyte) colonies grown from individual embryonic tissues by stage. The inset (i) shows an acetylcholinesterase-positive colony. (B) Immunohistochemical staining of erythroid and megakaryocyte progenitor–derived colonies. (Top) Primitive erythroid (i), megakaryocyte (ii), and bipotential primitive MEP (iii) colonies from neural plate–stage cultures stained with primitive erythroid-specific βH1-globin (blue) and megakaryocyte-specific GP1bβ (pink) antibodies. (Bottom) Erythroid (v), megakaryocyte (vi), and bipotential definitive MEP (vii) colonies from E9.5 to E10.5 yolk sac stained with panerythroid Ter119 (blue) and with GP1bβ (pink) antibodies. Boxed areas in panels iii and vii highlight proplatelet formation at 100 × shown in panels iv and viii, respectively. Arrowheads indicate proplatelet formation in panels ii and vi. Scale bars represent 10 μm. (C) Average number (+SEM) of primitive erythroid, megakaryocyte, and bipotential primitive MEP colonies from plated whole embryos (PS-LNP) and yolk sac (HF-46 sp) as determined by immunohistochemistry. (D) Primitive MEP–derived colony derived from a neural plate–staged embryo contains cells positive for βH1-globin and CD41. (E) Number (+SEM) of βH1-globin/GP1bβ-double positive colonies from a dilution series of 4 to 5 sp yolk sacs, in triplicate. (F) Spatial and temporal distribution of Meg-CFCs as determined by GP1bβ-stained colonies per tissue. (G) Spatial and temporal distribution of definitive MEPs, as determined by Ter119/GP1bβ double-positive colonies per tissue. sp indicates somite pair; PS, preprimitive streak; MS, mid primitive streak; LS, late primitive streak; ENP, early neural plate; MNP, mid neural plate; LNP, late neural plate; HF, head fold; and LHF, late head fold. Approximate embryonic day (E) is provided below the developmental stages.
The emergence of Meg-CFCs at neural plate stages (E7.5) and their expansion in the yolk sac of 20 to 30 sp embryos (E9.5) indicated that the megakaryocyte lineage overlaps temporally and spatially both with primitive and with definitive erythropoiesis.10 To better understand the relationship of the megakaryocyte and erythroid lineages during early embryogenesis, we combined progenitor assays in collagen with immunohistochemistry to identify colonies containing erythroid (βH1-globin–positive or Ter119-positive) and megakaryocyte (GP1bβ-positive) cells. Anti–βH1-globin antibodies label primitive, but not definitive, erythroid cells.11 Examples of erythroid and megakaryocyte colonies derived from mouse embryos are shown in Figure 2Bi-ii,v-vi.
Meg-CFCs were first detected along with EryP-CFCs in mid to late primitive streak–stage embryos (E7.25). Both Meg-CFCs and EryP-CFCs expanded in numbers in the yolk sac and peaked at 7 to 8 sp stages (E8.25; Figure 2C). The subsequent precipitous drop in primitive erythroid progenitors was associated with a near doubling of Meg-CFCs between 10 to 14 sp (Figure 2C). Meg-CFCs continued to increase in the yolk sac and peaked between 22 to 24 sp (E9.5), and decreased to low levels by 46 sp. This peak of Meg-CFCs in the yolk sac coincides temporally with that of BFU-Es, Mac-CFCs, GM-CFCs, and Mast-CFCs in the yolk sac.10 The data generated from acetylcholinesterase staining and GP1bβ immunohistochemistry were similar (Figure 2A,C) and indicate that megakaryopoiesis in the yolk sac coincides temporally and spatially with the primitive and definitive waves of erythropoiesis. These results raised the possibility that the megakaryocyte and erythroid lineages in the early embryo share a common progenitor.
Bipotential megakaryocyte/erythroid progenitors (MEPs) are associated with primitive hematopoiesis
During the immunohistochemical analysis of megakaryocyte and erythroid colonies, we identified colonies containing both primitive erythroid (βH1-globin–positive) and megakaryocyte (GP1bβ-positive) cells (Figure 2Biii). The cellular composition of these colonies was consistent with their origin from megakaryocyte/primitive erythroid progenitors (primitive MEPs), previously undescribed bipotential progenitors in the mouse embryo. Colonies generated by primitive MEPs varied in size between 10 and 50 cells and generally contained several-fold more erythroid than megakaryocyte cells. The megakaryocyte cells were typically smaller than 15 μm in diameter and contained only 1 to 2 nuclei. Primitive MEP–derived colonies matured after 9 days of culture, consistent with the notion that primitive MEPs were more immature than unipotential Meg-CFCs and EryP-CFCs, which mature in 5 to 7 days of in vitro culture. Megakaryocyte maturation within the colony was evident by the production of proplatelets (Figure 2Biv). To determine if primitive MEPs behave as clonal cells, we scored the number of βH1-globin/GP1bβ-positive colonies in serially diluted cells from 4 to 6 sp (Figure 2E) and 10 to 14 sp yolk sacs (not shown). At both stages, a linear relationship existed between cells plated and double-positive colonies scored, suggesting βH1-globin/GP1bβ-positive colonies arise from single cells.
Consistent with the existence of bipotential pMEPs, we detected dual positive βH1-globin/CD41 colonies in neural plate–staged embryos (Figure 2D) at a frequency similar to βH1-globin/Gp1bβ-positive colonies. Furthermore, gene expression studies of individual primitive erythroid colonies revealed that 37 of 48 colonies contained not only the expected βH1- and β1-globin transcripts but also transcripts associated with megakaryopoiesis (acetylcholinesterase and/or von Willebrand factor). None of the colonies contained detectable transcripts for the granulocyte marker G-CSFR (data not shown). These data provide further evidence that primitive MEPs emerge during gastrulation in the yolk sac of mouse embryos.
We examined the spatial and temporal kinetics of primitive MEPs during mouse ontogeny and found that they first emerged in mid primitive streak embryos along with unipotential megakaryocyte and primitive erythroid progenitors (Figure 2C). Primitive MEPs expanded in numbers along with Meg-CFCs and EryP-CFCs, and peaked at 4 to 6 sp stages. Like EryP-CFCs, primitive MEPs declined to low levels by 15 to 19 sp (Figure 2C) and were always confined spatially to the yolk sac (data not shown). Our data indicate that primitive hematopoiesis is not confined to the primitive erythroid lineage but is bilineage in nature.
Bipotential MEPs are associated with definitive hematopoiesis during the transition from yolk sac to fetal liver
The association of primitive MEPs with the transient wave of primitive erythroid progenitors prompted us to explore the relationship of the megakaryocyte and erythroid lineages as BFU-Es subsequently emerge in the yolk sac between E8.25 and E10.5.10 Unlike the restricted expression of embryonic globins to primitive erythroid cells, there are no currently known genes specifically expressed in definitive erythroid cells. Therefore, we used the panerythroid Ter119 antibody to continue tracking erythroid progenitors during later stages of development (Figure 2Bv,vii). We analyzed the time frame after primitive erythroid progenitors, that is, decreased to low levels after 15-19 sp (E9.0). At this stage, there were 13-fold more Ter119/GP1bβ-positive (Figure 2Bvii) than βH1-globin/GP1bβ-positive colonies in the yolk sac (compare MEPs Figure 2C with 2G). Using this assay system, we tracked the spatial and temporal kinetics of Meg-CFCs and “definitive” MEPs in staged embryos between 15 sp (E9.0) and 50 sp (E11.5) as shown in Figure 2F and 2G, respectively.
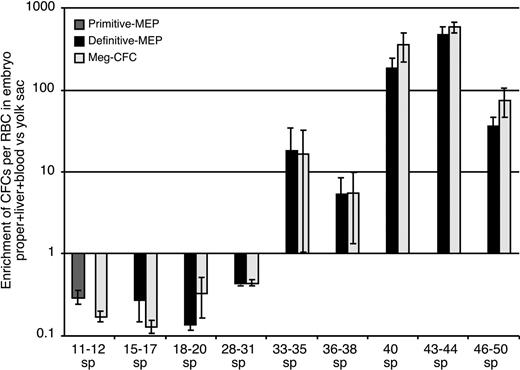
Initially (15-17 sp), both Meg-CFCs and MEPs were localized exclusively within the yolk sac. As development proceeded, the numbers of both of these progenitors expanded in the yolk sac and were found in increasing numbers in the circulation. At 31 to 35 sp, Meg-CFCs and definitive MEPs peak in the yolk sac. Small numbers of Meg-CFCs and definitive MEPs were also detected within the liver at this developmental stage (Figure 2F-G). By E11.5, the liver had become the predominant site of megakaryocyte progenitor expansion as the number of Meg-CFCs and definitive MEPs decreased in the yolk sac. These data suggest that yolk sac–derived megakaryocyte progenitors are among the first hematopoietic elements to engraft the newly forming liver rudiment. To determine whether definitive MEPs and Meg-CFCs preferentially associate with the yolk sac or intraembryonic sites or, alternatively, if they circulate freely throughout the bloodstream, we compared the number of progenitors to the number of circulating (benzidine-positive) erythroid cells in intraembryonic and extraembryonic regions. The ratio of intraembryonic progenitors/RBCs to extraembryonic progenitors/RBCs would equal 1 if they freely circulated between tissues.35 As shown in Figure 3, megakaryocyte progenitors were preferentially associated with the yolk sac until 31 sp, after which they became preferentially associated with the embryo proper. The timing of this transition coincides with the development of the liver as a hematopoietic organ44 and suggests that yolk sac–derived megakaryocyte progenitors seed the nascent fetal liver. The subsequent expansion of Meg-CFCs in the fetal liver likely reflects a contribution of Meg-CFCs from AGM-derived hematopoietic stem cells that are evident in the liver beginning at E11.10,45,46 We found that the liver contained the majority of Meg-CFCs and definitive MEPs within intraembryonic regions (Figure 2F-G).
Enrichment of progenitors in extraembryonic (yolk sac) versus intraembryonic regions. Numbers of primitive MEP, definitive MEP, or unipotential Meg-CFC hematopoietic progenitors (as determined by immunohistochemistry) and erythroblasts in all intraembryonic tissues versus yolk sac. Fold enrichment was calculated as the ratio of intraembryonic progenitors to erythroblasts divided by the ratio of yolk sac progenitors to erythroblasts.35 Tissues from individual experiments were grouped and averaged by stage (± SEM).
Enrichment of progenitors in extraembryonic (yolk sac) versus intraembryonic regions. Numbers of primitive MEP, definitive MEP, or unipotential Meg-CFC hematopoietic progenitors (as determined by immunohistochemistry) and erythroblasts in all intraembryonic tissues versus yolk sac. Fold enrichment was calculated as the ratio of intraembryonic progenitors to erythroblasts divided by the ratio of yolk sac progenitors to erythroblasts.35 Tissues from individual experiments were grouped and averaged by stage (± SEM).
Megakaryocytes are first detected in the yolk sac
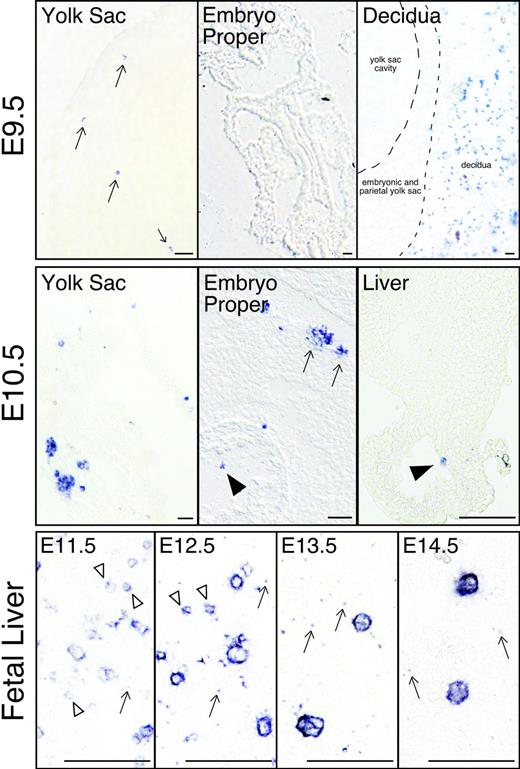
It is well established that primitive erythroid progenitors found in the yolk sac beginning at E7.25 rapidly generate morphologically identifiable erythroid precursors by E8.5.11 Since megakaryocyte progenitors are first detected at the same time and place as EryP-CFCs, we asked when and where megakaryocyte precursors are first found in the mouse embryo. Rather than using morphologic criteria,47 we relied upon GP1bβ expression to identify megakaryocytes.42 No GP1bβ-positive cells were evident in the mouse conceptus at E8.5 (data not shown). Rare GP1bβ-positive cells were first detected at E9.5 exclusively in the yolk sac (Figure 4; E9.5 yolk sac). The embryo proper did not contain positive cells, while the surrounding maternal decidual tissues contained abundant numbers of GP1bβ-positive platelets (Figure 4; E9.5 embryo proper and decidua). By E10.5, GP1bβ-positive cells were frequently identified in the yolk sac in 2- to 6-cell clusters (Figure 4; E10.5 yolk sac). Megakaryocytes were also found in intraembryonic vessels and chambers of the developing heart. At E10.5, low numbers of GP1bβ-positive cells were detected in the liver, consistent with the findings of Matsumura and Sasaki.48 By E11.5, GP1bβ-positive cells of varying sizes were frequently detected in the fetal liver (Figure 4; fetal liver). As development proceeds, the GP1bβ-positive cells in the liver were less frequent but larger in size. Overall, these findings indicate that megakaryocyte precursors first appear in the yolk sac approximately 48 hours after the initial appearance of Meg-CFCs and subsequently are found both in the circulation and in the fetal liver.
Localization of megakaryocytes (GP1bβ-positive cells) in mouse embryos. Individual megakaryocytes were first identified in the yolk sac of E9.5 conceptuses (arrows, top row, left panel). No GP1bβ-positive cells were detected in the E9.5 embryo proper (top row, middle panel). Maternally derived GP1bβ-positive platelets were detected in the surrounding E9.5 decidua (top, right panel). At E10.5 (middle row), clusters of GP1bβ-positive cells were evident in the yolk sac and intraembryonic megakaryocytes were seen in the circulation (arrowhead), associated with the wall of the aorta (arrows), and in the fetal liver (arrowhead). GP1bβ antibodies label both small (open arrowhead) and large cells in the E11.5 to E12.5 fetal liver, and predominantly large cells in the E13.5 to E14.5 fetal liver (lower row). Platelets are seen in E11.5 to 14.5 livers (arrows). Scale bars represent 50 μm.
Localization of megakaryocytes (GP1bβ-positive cells) in mouse embryos. Individual megakaryocytes were first identified in the yolk sac of E9.5 conceptuses (arrows, top row, left panel). No GP1bβ-positive cells were detected in the E9.5 embryo proper (top row, middle panel). Maternally derived GP1bβ-positive platelets were detected in the surrounding E9.5 decidua (top, right panel). At E10.5 (middle row), clusters of GP1bβ-positive cells were evident in the yolk sac and intraembryonic megakaryocytes were seen in the circulation (arrowhead), associated with the wall of the aorta (arrows), and in the fetal liver (arrowhead). GP1bβ antibodies label both small (open arrowhead) and large cells in the E11.5 to E12.5 fetal liver, and predominantly large cells in the E13.5 to E14.5 fetal liver (lower row). Platelets are seen in E11.5 to 14.5 livers (arrows). Scale bars represent 50 μm.
Platelets circulate in the embryonic vasculature as early as E10.5
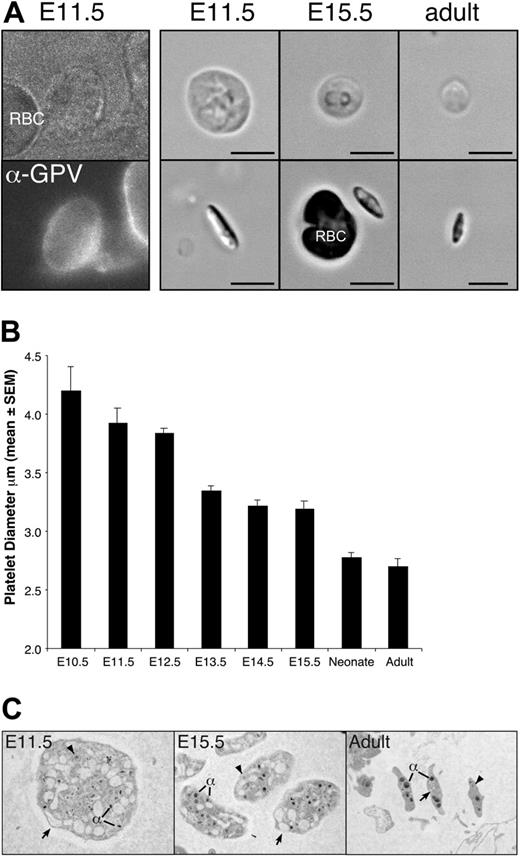
The presence of megakaryocyte cells in the yolk sac at E9.5 suggested that platelet production could potentially start soon afterward. Platelets have been identified in the bloodstream of murine fetuses as early as E12.5,49-51 however, it is not known when platelets first begin to circulate in the mouse. To determine the onset of thrombopoiesis, we analyzed peripheral blood from E10.5 to E15.5 fetuses for the presence of platelets (Figure 5A). Small numbers of large, GPV-positive platelets were first detected in pooled blood from E10.5 embryos in 2 of 5 litters examined. By E11.5, all individual fetuses contained circulating platelets, indicating that platelets are released into the bloodstream between E10.5 and E11.5. Like adult platelets, fetal platelets were anucleate, discoid, and biconvex in shape (Figure 5A-C). Fetal platelets were extremely large with a diameter 1.6-fold larger than adult platelets (Figure 5A-B). The average platelet diameter decreased during embryogenesis reaching a platelet diameter similar to adult platelets by birth (Figure 5B). A comparison of the ultrastructure of E11.5, E15.5, and adult platelets by transmission electron microscopy revealed the presence of alpha granules and dense granules in fetal and adult platelets, though the alpha granules of E11.5 platelets were smaller than those found in E15.5 and adult platelets (Figure 5E). The most notable feature of E11.5 platelets was the larger open canalicular system than seen in adult platelets.
Large platelets circulate in the embryonic and fetal vasculature. (A) Platelet-enriched blood was stained with anti–GPV-FITC antibodies (E11.5 shown, left panel), which specifically label platelets but not red blood cells. Shown to the right are examples of platelets from E11.5, E15.5, and adult peripheral blood, depicting the broad face (top) and narrow side (bottom). Regardless of cell diameter, all platelets were narrow and biconvex. Scale bar equals 5 μm. (B) Mean platelet diameter (± SEM) in E11.5 to E15.5 fetuses, in neonates, and in adult mice. A minimum of 100 platelets was measured per peripheral blood sample and at least 3 independent samples were studied, except for E10.5, of which 2 pooled blood samples were examined and a total of 11 platelets was measured. (C) Transmission electron microscopic images of E11.5, E15.5, and adult platelets. All images taken at × 10 000. Open canalicular system (arrows), dense granules (arrowheads), and alpha granules (α) are evident.
Large platelets circulate in the embryonic and fetal vasculature. (A) Platelet-enriched blood was stained with anti–GPV-FITC antibodies (E11.5 shown, left panel), which specifically label platelets but not red blood cells. Shown to the right are examples of platelets from E11.5, E15.5, and adult peripheral blood, depicting the broad face (top) and narrow side (bottom). Regardless of cell diameter, all platelets were narrow and biconvex. Scale bar equals 5 μm. (B) Mean platelet diameter (± SEM) in E11.5 to E15.5 fetuses, in neonates, and in adult mice. A minimum of 100 platelets was measured per peripheral blood sample and at least 3 independent samples were studied, except for E10.5, of which 2 pooled blood samples were examined and a total of 11 platelets was measured. (C) Transmission electron microscopic images of E11.5, E15.5, and adult platelets. All images taken at × 10 000. Open canalicular system (arrows), dense granules (arrowheads), and alpha granules (α) are evident.
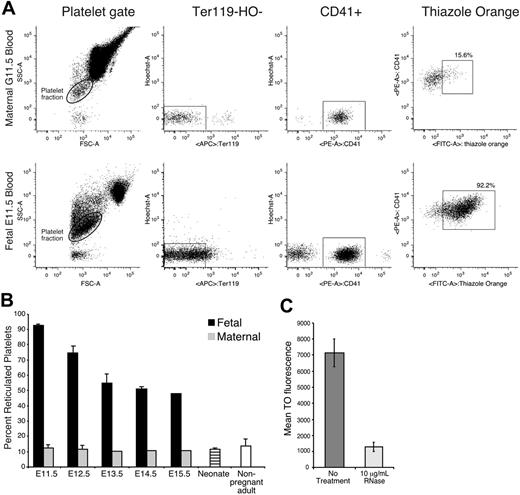
Similar to reticulocytes in the erythroid lineage, newly formed platelets contain RNA and can be identified using the nucleotide dye thiazole orange.52-54 Approximately 10% to 15% of maternal platelets were reticulated (Figure 6A-B). In contrast, more than 90% of platelets at E11.5 were reticulated, consistent with their recent release from megakaryocyte precursors at the onset of thrombopoiesis in the fetus. These fetal platelets stained with thiazole orange much more highly than adult platelets (Figure 6A). Treatment of fetal platelets with RNase decreased the thiazole orange signal by 81% (Figure 6C), indicating that the majority of the thiazole orange signal was due to RNA.55 The percentage of reticulated platelets at E14.5 to E15.5 remained elevated at approximately 50%. This likely reflects the increased production of platelets needed to keep up with a continually expanding vascular volume. However, by the end of gestation, reticulated platelet levels approached those seen in adults (Figure 6B).
Fetal platelets are highly reticulated. (A) Flow cytometric analysis of adult and fetal blood. Platelet fractions were identified by forward and side scatter parameters (left panels), lack of Ter119 and Hoechst staining (middle panels), and CD41 staining (right panels). Thiazole orange–positive platelets were gated as described in “Materials and methods.” (B) The mean percentage (+SEM) of reticulated platelets in E11.5 to E15.5 fetuses (▪), in maternal blood (⊡), in neonates (), and in nonpregnant adult mice (□). Fetal blood samples were analyzed from 3 littermates (E11.5, E14.5, and neonate), 3 fetuses from 3 separate experiments (E12.5), 4 embryos from 2 separate experiments (E13.5), or taken from a single fetus (E15.5). Adult blood was analyzed from a single sample at gestational days 13.5 to 15.5, in triplicate at gestational days 11.5 to 12.5, and from 5 independent nonpregnant adult samples. (C) Mean fluorescence (± SEM) of thiazole orange in untreated platelets and platelets treated with 10 μg/mL RNase A.
Fetal platelets are highly reticulated. (A) Flow cytometric analysis of adult and fetal blood. Platelet fractions were identified by forward and side scatter parameters (left panels), lack of Ter119 and Hoechst staining (middle panels), and CD41 staining (right panels). Thiazole orange–positive platelets were gated as described in “Materials and methods.” (B) The mean percentage (+SEM) of reticulated platelets in E11.5 to E15.5 fetuses (▪), in maternal blood (⊡), in neonates (), and in nonpregnant adult mice (□). Fetal blood samples were analyzed from 3 littermates (E11.5, E14.5, and neonate), 3 fetuses from 3 separate experiments (E12.5), 4 embryos from 2 separate experiments (E13.5), or taken from a single fetus (E15.5). Adult blood was analyzed from a single sample at gestational days 13.5 to 15.5, in triplicate at gestational days 11.5 to 12.5, and from 5 independent nonpregnant adult samples. (C) Mean fluorescence (± SEM) of thiazole orange in untreated platelets and platelets treated with 10 μg/mL RNase A.
Discussion
Hematopoietic potential in the mouse embryo originates from hemangioblast precursors that can generate primitive erythroid, definitive erythroid, and multiple myeloid lineages when assayed in vitro. Here, we show that the majority of hemangioblast precursors also contains megakaryocyte potential, indicating this lineage is also an integral component of embryonic hematopoiesis. Furthermore, we found that the emergence of megakaryocyte progenitors in the yolk sac overlaps spatially and temporally with the appearance of the primitive and definitive erythroid lineages.9,10 The specific association of the megakaryocyte and the primitive erythroid lineages through the identification of bipotential megakaryocyte/primitive erythroid progenitors indicates that primitive hematopoiesis in the yolk sac is at least bilineage in nature. The early emergence of macrophage progenitors along with primitive erythroid progenitors10 suggests that primitive hematopoiesis may also contain a limited myeloid component as has been postulated in the zebrafish embryo.56 A subsequent increase in the number of megakaryocyte progenitors in the yolk sac is associated with the definitive erythroid lineage through a common bipotential progenitor as occurs in the adult. Thus, the early ontogeny of the megakaryocyte lineage is characterized not only by its early and rapid emergence during gastrulation but also by its close association both with primitive and with definitive erythropoiesis in the yolk sac.
Children with Down syndrome are at increased risk of developing a transient myeloproliferative disease (TMD) as neonates and acute megakaryocytic leukemia as young children (reviewed in Gurbuxani et al57 and Massey et al58 ). These disorders have recently been shown to be associated with a truncated form of the GATA-1 transcription factor (Gata-1s).59,60 Forced expression of truncated Gata1 in the mouse embryo induces the massive proliferation of megakaryocyte cells.61 Of interest, this occurs primarily in the E9.5 yolk sac and early (E12.5) fetal liver, suggesting that the target cells of GATA-1's action are the yolk sac–derived megakaryocyte progenitors described here.
We characterized the onset of thrombopoiesis in the mouse embryo. The appearance of circulating platelets at E10.5 indicates that platelet production has already begun at the time adult-repopulating hematopoietic stem cells first emerge in the AGM region.46 This finding, along with the initial appearance of megakaryocyte progenitors and megakaryocyte cells in the yolk sac at E7.5 and E9.5, respectively, supports the concept that these first embryonic platelets are ultimately derived from the yolk sac. Compared with adult platelets, embryonic platelets are distinguished by their large size, massive canalicular system, and small alpha granules. Of interest, large, hypogranular platelets have been described in the human yolk sac62,63 during a similar developmental window as when we first detected platelets in the mouse embryo.
The large diameter of embryonic platelets may be related to their release from younger megakaryocyte precursors. Xu et al64 have shown that megakaryocytes cultured in vitro from unstaged E8.5 mouse embryos have a lower ploidy when compared with those derived from the adult bone marrow. These data are consistent with our observations that smaller GP1bβ-positive megakaryocytes are present in early but not later fetal liver (Figure 4). In adults with immune thrombocytopenic purpura, large platelet size is positively correlated with an increased proportion of immature megakaryocytes with lower ploidy in the marrow.65 This suggests that embryonic megakaryopoiesis and adult stress-megakaryopoiesis share some common features. Like erythroid cells, newly released platelets contain RNA and can be identified by staining with thiazole orange.52-54 Nearly all of the circulating platelets at E11.5 are reticulated, consistent with their recent synthesis, while non-RNA components of the platelet can also stain with thiazole orange.55 We found that the majority of the thiazole orange signal in fetal platelets was lost following RNase treatment. Thus, the persistence of high percentages of reticulated platelets through E15.5 suggests that active thrombopoiesis continues in the developing fetus. Similarly in humans, the levels of reticulated platelets are higher in preterm than term infants.66 The decrease in platelet size during ontogeny could be related in part to a decrease in platelet size as they age in the circulation.67 In support of this, we found that reticulated platelets at any given gestational age displayed higher forward and side scatter when compared with cocirculating nonreticulated platelets (data not shown).
In the mouse, primitive erythroid cells are 3 times larger than fetal definitive red cells, which are twice as large as adult red cells.11,68 It is intriguing to speculate that the large platelets seen between E10.5 and E12.5 (Figure 5A) might be derived from primitive Meg-CFCs, while the intermediate-sized platelets seen at E13.5 to E15.5 might be derived from definitive fetal Meg-CFCs. However, unlike differential globin expression in erythropoiesis,68 it is currently not possible to distinguish the products of primitive and definitive megakaryopoiesis given the lack of differentiating markers. That both primitive and definitive Meg-CFCs can produce platelets is suggested by the appearance of 2 waves of platelet production when embryonic stem cells are differentiated in vitro.69
The rapid initiation of thrombopoiesis between E10.5 and E11.5 suggests that platelets may be required during gestation. Mice lacking functional Runx1 lack identifiable platelets and succumb to lethal hemorrhage by midgestation.49,50 NF-E2–null mice produce low levels of abnormal platelets and remain viable until birth, when the majority of animals dies of hemorrhage after vaginal, but not cesarean, delivery.25,70 Furthermore, genetic ablation of thrombopoietin or its receptor causes severe thrombocytopenia, yet mice survive,71,72 indicating that low platelet numbers are sufficient for survival through gestation and birth. These data suggest that platelets are required during embryogenesis, possibly to meet the demands placed on the developing vascular system and to survive the birthing process. Of interest, adult platelets contain angiogenic factors such as VEGF73,74 and can promote endothelial migration and tube formation in vitro.75,76 Whether platelets serve such roles during development remains to be determined.
Authorship
Contribution: J.T. performed experimental and intellectual design, animal husbandry, in vitro assays, tissue collection, flow cytometry, RT-PCR, and data analysis, and contributed to paper and figure preparation; A.K. performed experimental and intellectual design, acetylcholinesterase assays, data analysis, and technical development of in vitro culture conditions; K.E.M. performed flow cytometry, data analysis, intellectual design, and paper preparation; R.E. performed technical development, and processing and immunostaining of tissue sections; R.V. performed data collection and analysis of fetal platelet diameters; K.K.L.M.-B. performed transmission electron microscopy of platelets; R.W. designed analytical tools for platelet diameter measurements; J.P. served as the primary investigator implementing experimental and intellectual design, performed tissue collection, and contributed to paper preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James Palis, Department of Pediatrics, University of Rochester Medical Center, Box 703, 601 Elmwood Ave, Rochester, NY 14642; e-mail: james_palis@urmc.rochester.edu.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by funding from the National Institutes of Health.
We thank Gordon Keller for kindly providing us with D4T cells, Christine Gorman-Zanghi with assistance in setting up the megakaryocyte colony assay in collagen, Gayle Callis for her advice on processing sectioned tissues, Timothy Bushnell for training and expertise with flow cytometry, Jeff Malik for designing primers, Paul Kingsley for technical assistance with blood collection, and Ric Bauserman for guidance with platelet microscopy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal