Abstract
In an N-nitroso-N-ethylurea (ENU) mutagenesis screen using Mpl−/− mice, we isolated a semidominant suppressor of thrombocytopenia, termed Plt6. The gene mutated in Plt6 mice encodes the transcriptional coregulator p300, and the mutation, a tyrosine to asparagine substitution at amino acid 630 (Y630N), disrupts the interaction between p300 and c-Myb. Mpl−/−p300Plt6/+ mice displayed elevated platelet counts relative to Mpl−/−p300+/+ controls, whereas mice homozygous for the Plt6 mutation produced supraphysiological levels of circulating platelets. On a wild-type genetic background, mice homozygous for the p300Plt6 mutation, or recipients of Mpl+/+p300Plt6/Plt6 bone marrow, also exhibited thrombocytosis as well as deficiencies in B-lymphoid cells. Increased platelet numbers in Plt6 mutant mice were accompanied by significant increases in megakaryocyte progenitor cells within the bone marrow and spleen with concomitantly elevated numbers of megakaryocytes. The expansion of megakaryocytopoiesis and suppression of Mpl−/− thrombocytopenia in Plt6 mutants is highly reminiscent of that observed in mice with mutations affecting the p300 partner protein c-Myb, suggesting an indispensable repressive role for the c-Myb/p300 transcriptional regulatory complex in megakaryocyte develop-ment, the inhibition of which allows substantial thrombopoietin (TPO)–independent platelet production.
Introduction
Thrombopoietin (TPO), acting through its specific receptor (Mpl), is the major cytokine regulator of steady-state platelet production. Mice lacking TPO or Mpl are profoundly thrombocytopenic due to the failure of production of sufficient megakaryocytes and their progenitors.1-3 Nevertheless, the TPO-independent mechanisms that maintain platelet levels at 10% that of normal mice in TPO- or Mpl-deficient animals are sufficient for hemostasis and are capable of transiently producing normal numbers of platelets in response to 5-fluorouracil (5-FU)–induced thrombocytopenia.4 To explore the molecular regulation of TPO-independent platelet production, we are conducting mutagenesis screens for mutations that suppress Mpl−/− thrombocytopenia. We recently reported that mutations in the transcription factor c-Myb result in supraphysiological platelet production in Mpl−/− mice.5 Previous studies have suggested that p300, a transcriptional coregulator, is an essential partner protein for c-Myb in control of megakaryocytopoiesis.6 Here we show that, like c-Myb, mutation in the gene encoding p300 results in amelioration of Mpl−/− thrombocytopenia, establishing a key role for c-Myb/p300 in preventing excessive megakaryocytopoiesis. Since modulation of c-Myb/p300 results in supraphysiological TPO-independent platelet production, this transcriptional regulatory complex may be a useful target for development of therapeutics to treat thrombocytopenia.
Methods
Plt6 mice
The founder Plt6 mouse was identified among G1 offspring of male Mpl−/− C57BL/6 mice that were treated with N-nitroso-N-ethylurea (ENU) as described.5 For mapping, affected heterozygous Plt6/+ Mpl−/− mice on a C57BL/6 background were crossed to Mpl+/− mice on a 129Sv background. Plt6/+ Mpl−/− F1 animals were then intercrossed to produce 373 mice in the F2 generation. Platelet counts were used to identify the F2 mice as +/+, Plt6/+, or Plt6/Plt6 and DNA was prepared from each mouse according to described methods.5 One hundred forty-eight simple sequence length polymorphisms (SSLPs) spaced evenly throughout the genome were amplified and analyzed, essentially as described.7 Plt6 was found to reside on chromosome 15 and the location was refined via analysis of additional markers in this region. Each of the exons and intron-exon boundaries of the p300 gene was amplified by polymerase chain reaction (PCR) and sequenced on an ABI automatic sequencer according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). The presence of the mutation exclusively in Plt6 mutants was confirmed by sequencing of the p300 gene in each of 3 Plt6/Plt6, Plt6/+, and +/+ mice. Animal experiments were performed according to protocols approved by the Melbourne Health Research Directorate or Walter and Eliza Hall Institute of Medical Research Animal Ethics Committees.
Mice carrying the Plt6 mutation on a wild-type genetic background were generated by crossing Mpl−/−p300Plt6/+, and then subsequently Mpl+/−p300Plt6/+ mice, with Mpl+/+ C57BL/6 wild-type mice followed by intercrossing Mpl+/+p300Plt6/+ mice. Bone marrow transplantation was performed by intravenous injection of 5 × 106 bone marrow cells from Ly5.2 Mpl+/+p300Plt6/Plt6 or control Ly5.2 Mpl+/+p300+/+ mice into wild-type Ly5.1 recipients. Several hours prior to transplantation, the recipient mice were irradiated with 11 Gy γ-irradiation in 2 equal doses given several hours apart from a 137Cs source (Atomic Energy, Ottawa, ON). Mice that underwent transplantation were maintained on oral antibiotic (1.1 g/L neomycin sulfate; Sigma-Aldrich, St Louis, MO) and analyzed 3 months after engraftment.
Hematology
Peripheral blood counts were determined using automated (Advia 120; Bayer, Tarrytown, NY) techniques, and clonal agar cultures of bone marrow cells or spleen cells, stimulated with 100 ng/mL murine SCF, 10 ng/mL murine IL-3, and 4 U/mL human EPO, were performed as previously described.8 Megakaryocyte counts were performed using sections of sternum and spleen following staining with hematoxylin and eosin. Colony-forming units–spleen (CFU-s's) were enumerated by intravenous injection of bone marrow cells into recipient mice that had been irradiated with 11 Gy γ-irradiation. Spleens were removed after 8 or 12 days and fixed in 60% ethanol/30% chloroform/10% acetic acid, and the numbers of macroscopic colonies were counted.
Flow cytometry
Blood, depleted of erythrocytes by lysis with 156 mM ammonium chloride (pH 7.3), and single-cell suspensions of thymus, spleen, mesenteric lymph node, and bone marrow were stained with antibodies to Ly5.1. Ly5.2, IgM, B220, CD4, or CD8 that were directly fluorochrome conjugated or biotin conjugated and visualized via streptavidin-fluorochrome conjugates (BD Pharmingen, San Diego, CA). Analyses were performed on an LSR flow cytometer (BD Biosciences, San Jose, CA) with dead cells excluded via propidium iodide staining.
Protein expression, purification, and pull-down experiments
The cDNAs encoding wild-type and Y630N murine p300 KIX domains (residues 567-667) were cloned into a modified pGEX-2T vector (GE Healthcare, Piscataway, NJ). GST and GST-KIX fusions were expressed and purified from Escherichia coli BL21-CodonPlus-RIL (Stratagene, La Jolla, CA). Cultures were grown to an optical density of 0.6 to 0.8 at 37°C before induction with 0.5 mM IPTG and incubation at 15°C for 16 hours. Harvested cells were lysed by sonication at 4°C in 200 mM NaCl, 20 mM HEPES, pH 7.5, 2 mM dithiothreitol (buffer A) supplemented with 1 mM phenylmethylsulfonyl fluoride. The lysates were clarified by centrifugation and incubated with glutathione sepharose resin (GE Healthcare) for 1 hour at 4°C, before the beads were washed extensively with buffer A.
The cDNA encoding full-length murine c-Myb was cloned into the vector, pET15b (Novagen, San Diego, CA), under the T7 promoter. 35S-labeled c-Myb was generated from the TNT in vitro transcription/translation reaction (Promega, Madison, WI) from 750 ng DNA in a 50-μL reaction volume, according to the manufacturer's instructions.
Pull-down experiments were performed to examine the capacity of GST and GST-KIX proteins to bind c-Myb. An equal volume of beads bound to 2 μg GST, GST-wild-type KIX, or GST-Y630N KIX was mixed with 5 μL 35S-labeled in vitro–transcribed/translated c-Myb in a 30-μL volume at 4°C for 40 minutes. Beads were washed 4 times with 0.5 mL buffer A supplemented with 0.5% vol/vol Nonidet P40 at 4°C, boiled with reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading dye, and resolved by SDS-PAGE. The gel was stained with Coomassie blue to visualize bait levels before drying under vacuum and phosphor screen exposure. Phosphorimages were read 16 to 48 hours later and densitometric analysis of bands was performed using the software, Image Gauge 4.0 (Fujifilm, Tokyo, Japan).
Results
In a mutagenesis screen for suppressors of Mpl−/− thrombocytopenia, the founder Plt6 mouse was identified among the first-generation offspring of an ENU-treated Mpl−/− mouse as an outlier with platelet count of 371 × 109/L, compared with 105 plus or minus 44 × 109/L in littermate mice. Approximately half the offspring of matings between affected Plt6 mice and untreated Mpl−/− mice exhibited elevated platelet counts consistent with inheritance of a suppressor of thrombocytopenia. In offspring from intercrosses between Plt6/+ parents, mice displaying platelet counts typical of Plt6/+ mice were observed along with occasional mice displaying supraphysiological platelet counts. We subsequently established that the majority of Plt6/Plt6 homozygotes die in utero or as neonates (of 71 mice weaned, ratio of Plt6/Plt6 to Plt6/+ to +/+ = 0.04:2.0:1.0) with occasional survivors exhibiting very high platelet numbers (Table 1).
Peripheral blood counts in Plt6 mutant mice
| . | Mpl−/− . | Mpl+/+ . | Transplant recipients Mpl+/+ . | ||||
|---|---|---|---|---|---|---|---|
| Plt6/Plt6,n = 5 . | Plt6/+,n = 9 . | +/+,n = 8 . | Plt6/Plt6,n = 5 . | +/+,n = 9 . | Plt6/Plt6,n = 7 . | +/+,n = 8 . | |
| Platelet count, ×109/L | 2732 ± 462* | 541 ± 194* | 208 ± 62 | 1954 ± 171* | 1066 ± 142 | 1917 ± 164* | 1081 ± 257 |
| Red cell count, ×1012/L | 10.6 ± 0.8 | 10.8 ± 0.6 | 10.0 ± 0.5 | 10.3 ± 0.2 | 10.7 ± 0.3 | 9.0 ± 0.6 | 10.0 ± 0.9 |
| Hematocrit level, % | 52.8 ± 4.9 | 52.5 ± 4.0 | 49.5 ± 2.5 | 48.1 ± 1.9 | 49.1 ± 1.2 | 49.6 ± 2.7 | 52.4 ± 4.1 |
| White cell count, ×109/L | 6.2 ± 2.7 | 6.6 ± 2.1 | 4.6 ± 1.8 | 5.4 ± 1.2* | 10.3 ± 1.4 | 6.2 ± 1.9* | 10.6 ± 2.2 |
| Neutrophil count, ×109/L | 0.48 ± 0.32 | 0.65 ± 0.44 | 0.47 ± 0.37 | 0.65 ± 0.12 | 0.89 ± 0.15 | 0.27 ± 0.20* | 0.75 ± 0.30 |
| Lymphocyte count, ×109/L | 4.8 ± 2.2 | 5.5 ± 2.0 | 3.9 ± 1.4 | 4.2 ± 1.2* | 8.7 ± 1.3 | 5.3 ± 1.6* | 9.2 ± 2.0 |
| Monocyte count, ×109/L | 0.07 ± 0.07 | 0.07 ± 0.07 | 0.05 ± 0.04 | 0.06 ± 0.03 | 0.11 ± 0.03 | 0.08 ± 0.10 | 0.15 ± 0.10 |
| Eosinophil count, ×109/L | 0.06 ± 0.04 | 0.19 ± 0.06* | 0.10 ± 0.04 | 0.08 ± 0.04* | 0.20 ± 0.04 | 0.13 ± 0.10 | 0.25 ± 0.20 |
| . | Mpl−/− . | Mpl+/+ . | Transplant recipients Mpl+/+ . | ||||
|---|---|---|---|---|---|---|---|
| Plt6/Plt6,n = 5 . | Plt6/+,n = 9 . | +/+,n = 8 . | Plt6/Plt6,n = 5 . | +/+,n = 9 . | Plt6/Plt6,n = 7 . | +/+,n = 8 . | |
| Platelet count, ×109/L | 2732 ± 462* | 541 ± 194* | 208 ± 62 | 1954 ± 171* | 1066 ± 142 | 1917 ± 164* | 1081 ± 257 |
| Red cell count, ×1012/L | 10.6 ± 0.8 | 10.8 ± 0.6 | 10.0 ± 0.5 | 10.3 ± 0.2 | 10.7 ± 0.3 | 9.0 ± 0.6 | 10.0 ± 0.9 |
| Hematocrit level, % | 52.8 ± 4.9 | 52.5 ± 4.0 | 49.5 ± 2.5 | 48.1 ± 1.9 | 49.1 ± 1.2 | 49.6 ± 2.7 | 52.4 ± 4.1 |
| White cell count, ×109/L | 6.2 ± 2.7 | 6.6 ± 2.1 | 4.6 ± 1.8 | 5.4 ± 1.2* | 10.3 ± 1.4 | 6.2 ± 1.9* | 10.6 ± 2.2 |
| Neutrophil count, ×109/L | 0.48 ± 0.32 | 0.65 ± 0.44 | 0.47 ± 0.37 | 0.65 ± 0.12 | 0.89 ± 0.15 | 0.27 ± 0.20* | 0.75 ± 0.30 |
| Lymphocyte count, ×109/L | 4.8 ± 2.2 | 5.5 ± 2.0 | 3.9 ± 1.4 | 4.2 ± 1.2* | 8.7 ± 1.3 | 5.3 ± 1.6* | 9.2 ± 2.0 |
| Monocyte count, ×109/L | 0.07 ± 0.07 | 0.07 ± 0.07 | 0.05 ± 0.04 | 0.06 ± 0.03 | 0.11 ± 0.03 | 0.08 ± 0.10 | 0.15 ± 0.10 |
| Eosinophil count, ×109/L | 0.06 ± 0.04 | 0.19 ± 0.06* | 0.10 ± 0.04 | 0.08 ± 0.04* | 0.20 ± 0.04 | 0.13 ± 0.10 | 0.25 ± 0.20 |
P value less than .05 after adjustment for multiple testing for comparison of data from Plt6/Plt6 or Plt6/+ mice with that of +/+ mice in each of the Mpl−/−, Mpl+/+, or Mpl+/+ transplant recipient groups.
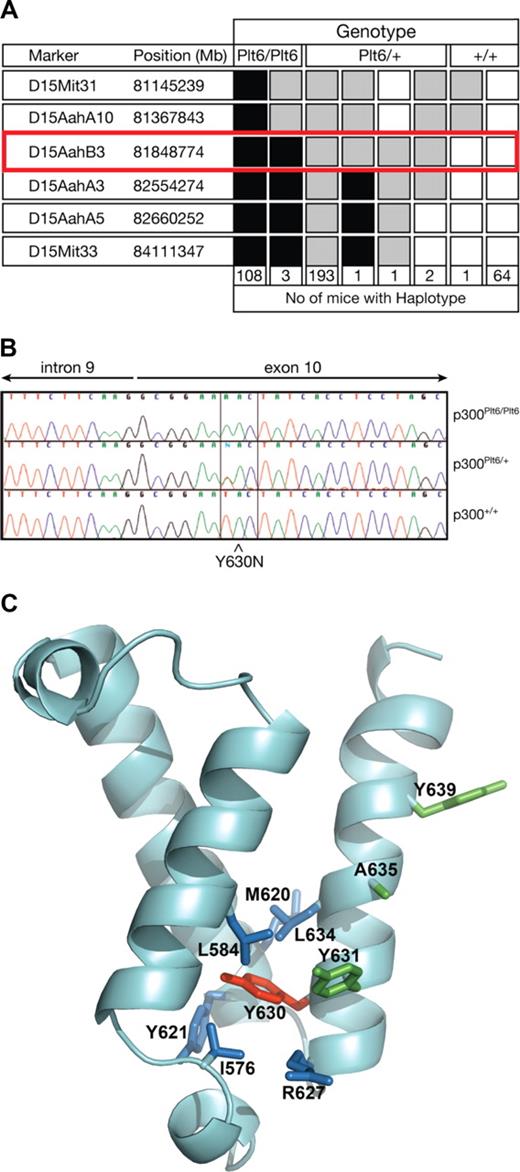
To map the chromosomal location of the Plt6 mutation, a mapping cross was established as outlined in “Plt6 mice.” The mutation was localized to a region of approximately 1.2 Mb between D15AahA10 and D15AahA3 (Figure 1A). This region of chromosome 15 included the gene encoding p300, the mutation of which has previously been associated with elevated platelet counts in wild-type mice.6 Thus, we sequenced the coding regions of p300 in DNA from Plt6/Plt6, Plt6/+, and +/+ mice and discovered a single T to A mutation resulting in substitution of tyrosine for asparagine at residue 630 within the KIX domain (Figure 1B,C). A structural model of the p300 KIX domain, prepared by homology modeling based on the solution structure of the highly homologous CBP KIX domain,10 suggested that the side chain of Y630 is a component of the KIX domain's hydrophobic core. Inspection of amino acid side chains in proximity (< 4.5 Å) to Tyr630 in this model identified several putative hydrophobic interactions that may be disrupted by the Y630N mutation (blue side chains, Figure 1C) resulting in structural distortion.
Mutation in p300 causes suppression of Mpl−/− thrombocytopenia. (A) To map the chromosomal location of Plt6, a (C57BL/6 × 129/Sv)F2 cohort was bled and phenotypically categorized as having platelet counts typical of unmutated Mpl−/−, Plt6/+, or Plt6/Plt6 mice and then genotyped using simple sequence length polymorphisms (SSLPs) spaced evenly throughout the genome. Markers found to be homozygous 129/Sv are shown in white; heterozygous, in gray; and homozygous C57BL/6, in black. The number of animals with each haplotype is shown below. Plt6 was localized between D15AahA10 and D15AahA3. (B) Sequence of PCR-amplified genomic DNA from representative Plt6/Plt6, Plt6/+, and wild-type mice showing a T to A mutation in exon 10 of Plt6 mice resulting in a Tyr to Asn substitution at amino acid 630. (C) Model of the p300 KIX domain indicating the Plt6 mutation site (red) and potentially disrupted contacts (blue), as well as residues previously mutated6 in mice (Y631, A635, and Y639; green). Modeled using FUGUE9 by homology with pdb file, 1KDX, the solution structure of mouse CREB-binding protein KIX domain.
Mutation in p300 causes suppression of Mpl−/− thrombocytopenia. (A) To map the chromosomal location of Plt6, a (C57BL/6 × 129/Sv)F2 cohort was bled and phenotypically categorized as having platelet counts typical of unmutated Mpl−/−, Plt6/+, or Plt6/Plt6 mice and then genotyped using simple sequence length polymorphisms (SSLPs) spaced evenly throughout the genome. Markers found to be homozygous 129/Sv are shown in white; heterozygous, in gray; and homozygous C57BL/6, in black. The number of animals with each haplotype is shown below. Plt6 was localized between D15AahA10 and D15AahA3. (B) Sequence of PCR-amplified genomic DNA from representative Plt6/Plt6, Plt6/+, and wild-type mice showing a T to A mutation in exon 10 of Plt6 mice resulting in a Tyr to Asn substitution at amino acid 630. (C) Model of the p300 KIX domain indicating the Plt6 mutation site (red) and potentially disrupted contacts (blue), as well as residues previously mutated6 in mice (Y631, A635, and Y639; green). Modeled using FUGUE9 by homology with pdb file, 1KDX, the solution structure of mouse CREB-binding protein KIX domain.
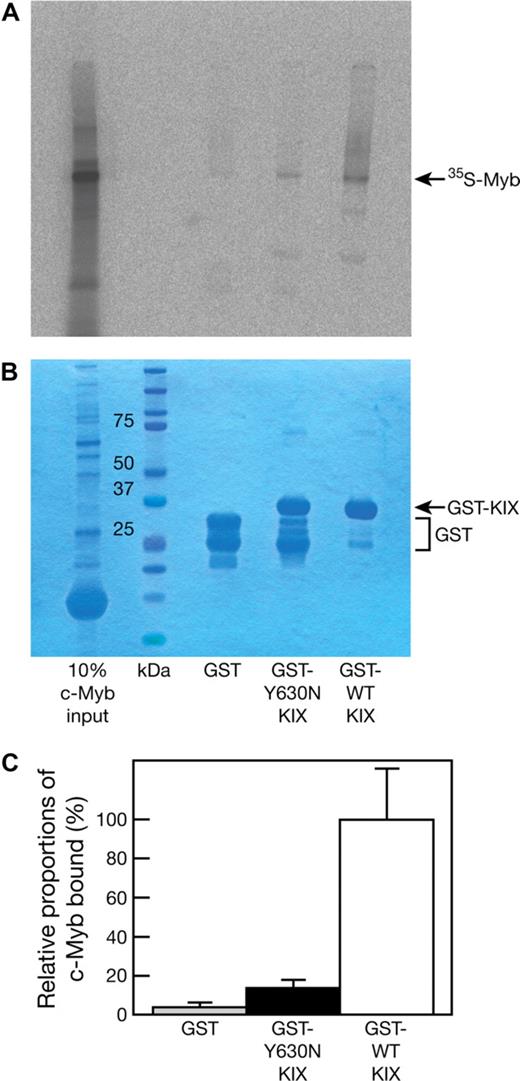
The Plt6 suppression of thrombocytopenia phenotype shows remarkable similarity to that of Mpl−/− mice with mutations in c-Myb,5 a p300 partner protein. Previous studies have established a role for the residues Y650, A654, and Y658 on the alpha3 helix of CBP, and their homologs in murine p300 (Y631, A635, and Y639, drawn in green on Figure 1C), in mediating interaction with c-Myb.6 Thus, we hypothesized that the p300Plt6 mutation may disrupt p300-c-Myb binding. To this end, GST fusions of p300 KIX domains containing the wild-type or Y630N mutant sequence were expressed and purified from E coli and their capacity to bind 35S-labeled in vitro–transcribed/translated c-Myb was examined in pull-down experiments. The GST-KIX fusion protein containing the Y630N mutation bound to c-Myb with markedly reduced affinity: on average, an approximately 7-fold reduction in the amount of c-Myb retained by GST-KIX (Y630N) relative to GST-KIX (wild-type) was observed (Figure 2). These data demonstrate that the Y630N mutation in the KIX domain of p300 severely hinders c-Myb binding, but does not completely abrogate this interaction.
The Plt6 mutation reduces p300 affinity for c-Myb. GST, GST-p300 KIX (Y630N), and GST-p300 KIX (wild type; 2 μg each) were used as baits for pull-down experiments with 35S-labeled, in vitro–transcribed/translated c-Myb. Pull-down reactions were resolved by SDS-PAGE followed by detection of bound 35S-c-Myb by autoradiography. (A) Typical autoradiogram. (B) Coomassie blue staining to determine bait levels. (C) The amount of bound 35S-c-Myb was determined by densitometric analysis of the autoradiographs of 3 independent experiments, and the 35S-c-Myb bound by GST and GST-p300 KIX (Y630N) is shown relative to the amount bound by GST-p300 KIX (wild type). Data represent the mean of 3 independent experiments with error bars corresponding to SD.
The Plt6 mutation reduces p300 affinity for c-Myb. GST, GST-p300 KIX (Y630N), and GST-p300 KIX (wild type; 2 μg each) were used as baits for pull-down experiments with 35S-labeled, in vitro–transcribed/translated c-Myb. Pull-down reactions were resolved by SDS-PAGE followed by detection of bound 35S-c-Myb by autoradiography. (A) Typical autoradiogram. (B) Coomassie blue staining to determine bait levels. (C) The amount of bound 35S-c-Myb was determined by densitometric analysis of the autoradiographs of 3 independent experiments, and the 35S-c-Myb bound by GST and GST-p300 KIX (Y630N) is shown relative to the amount bound by GST-p300 KIX (wild type). Data represent the mean of 3 independent experiments with error bars corresponding to SD.
In Mpl−/−p300Plt6/+ mice, suppression of thrombocytopenia was partial, with platelet counts elevated up to 3-fold relative to Mpl−/−p300+/+ controls and reaching levels approximately half that of Mpl+/+p300+/+ mice. Despite the absence of a functional TPO/Mpl system, thrombocytopenia was not only suppressed in Mpl−/−p300Plt6/Plt6 mice, but platelet counts reached levels up to 20-fold that of Mpl−/−p300+/+ mice and twice that of wild-type mice (Table 1). The hematocrit and numbers of red and white blood cells were unaltered in Mpl−/−p300Plt6/+ and Mpl−/−p300Plt6/Plt6 mice. To determine the effects of the p300Plt6 mutation on a wild-type genetic background, the mutant allele was also bred on a Mpl+/+ genetic background. As observed on a Mpl−/− background, the majority of Mpl+/+p300Plt6/Plt6 mice failed to survive to weaning (Plt6/Plt6 to Plt6/+ to +/+ = 0.2:2.0:1.3, n = 81). However, in Mpl+/+p300Plt6/Plt6 survivors, supraphysiological platelet counts were present (Table 1).
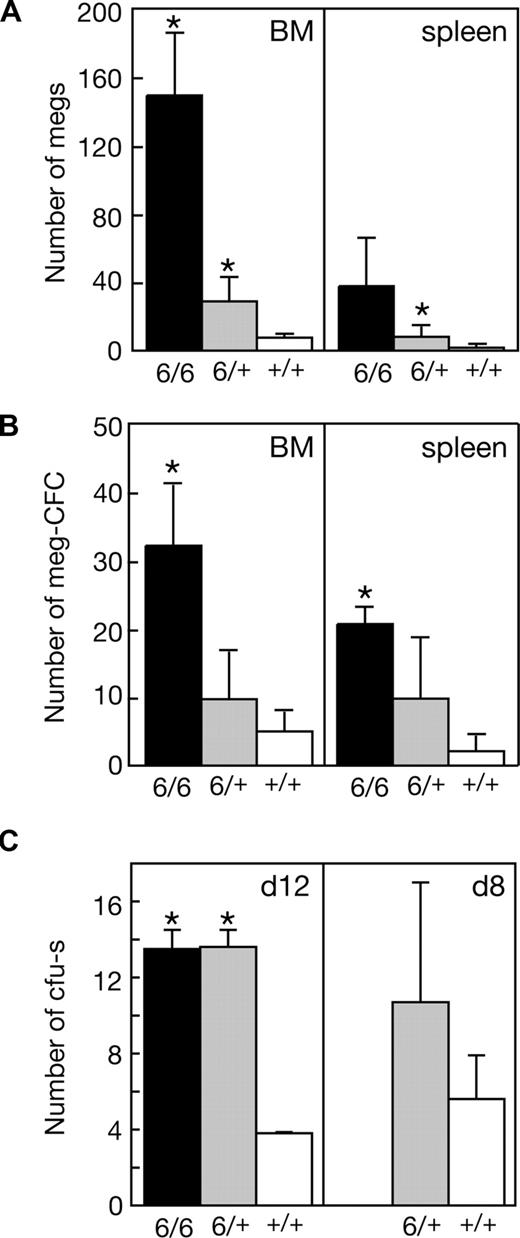
Significant increases in the numbers of megakaryocytes and megakaryocyte progenitor cells in the bone marrow and spleen accompanied the suppression of thrombocytopenia in Mpl−/−p300Plt6/+ mutants and these changes were more profound in Mpl−/−p300Plt6/Plt6 mice (Figure 3A,B). In addition to the elevated number of megakaryocyte progenitor cells, the total number of clonogenic myeloid progenitor cells was also significantly elevated in the spleens of Plt6 homozygote and heterozygote mice (Mpl−/−p300Plt6/Plt6: 25 ± 2 colonies per 105 cells [n = 3]; Mpl−/−p300Plt6/+: 11 ± 8 [n = 7]; and Mpl−/−p300+/+: 3 ± 3 [n = 9]), whereas in the bone marrow excess progenitor cells were evident in homozygotes, but not heterozygotes (Mpl−/−p300Plt6/Plt6: 68 ± 33 colonies per 2.5 × 104 cells [n = 3], Mpl−/−p300Plt6/+: 34 ± 17 [n = 7], and Mpl−/−p300+/+: 22 ± 11 [n = 9]). Consistent with a general increase in hematopoietic progenitor cells in Plt6 mutant mice, elevated numbers of CFU-s's were also apparent in the bone marrow of Mpl−/−p300Plt6/Plt6 and Mpl−/−p300Plt6/+ mice (Figure 3C).
Expanded megakaryocytopoiesis in Mpl−/−Plt6 mutants. Elevated platelet counts in Plt6 homozygous (6/6) and heterozygous (6/+) mice were accompanied by (A) increased numbers of megakaryocytes (megs), shown as number per microscopic field (×600, BM; ×200, spleen), (B) megakaryocyte colony-forming cells (meg-CFCs), shown as number per 2.5 × 104 bone marrow (BM) or 105 spleen cells, and (C) colony-forming units–spleen (CFU-s's), shown as number per 1.5 × 105 donor bone marrow cells at days 12 or 8 following transplantation. Means plus or minus SD are shown. * P value less than .05 for comparison of data from Mpl−/−Plt6/Plt6 or Mpl−/−Plt6/+ mice with that of Mpl−/−+/+ mice. n = 3 to 7 mice per genotype.
Expanded megakaryocytopoiesis in Mpl−/−Plt6 mutants. Elevated platelet counts in Plt6 homozygous (6/6) and heterozygous (6/+) mice were accompanied by (A) increased numbers of megakaryocytes (megs), shown as number per microscopic field (×600, BM; ×200, spleen), (B) megakaryocyte colony-forming cells (meg-CFCs), shown as number per 2.5 × 104 bone marrow (BM) or 105 spleen cells, and (C) colony-forming units–spleen (CFU-s's), shown as number per 1.5 × 105 donor bone marrow cells at days 12 or 8 following transplantation. Means plus or minus SD are shown. * P value less than .05 for comparison of data from Mpl−/−Plt6/Plt6 or Mpl−/−Plt6/+ mice with that of Mpl−/−+/+ mice. n = 3 to 7 mice per genotype.
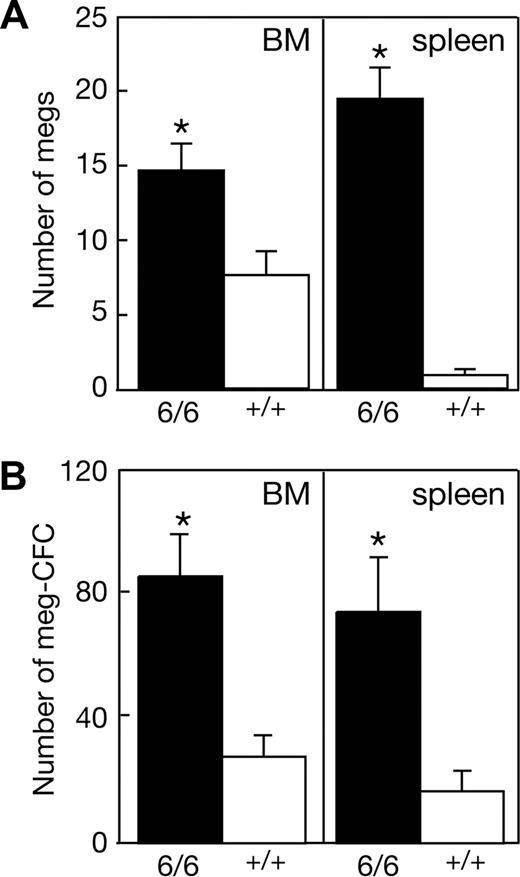
To overcome the paucity of adult Mpl+/+p300Plt6/Plt6 mice available for phenotypic analysis, cohorts of wild-type mice were irradiated and received a transplant of bone marrow cells from each of 2 Mpl+/+p300Plt6/Plt6 and 2 control Mpl+/+p300+/+ mice. Analysis of peripheral blood and hematopoietic organs, taking advantage of differences in the congenic Ly5 cell surface marker between donor and recipient mice, confirmed successful engraftment, with more than 80% contribution of donor hematopoiesis in all mice analyzed. The thrombocytosis characteristic of the donor Mpl+/+p300Plt6/Plt6 mice was present in the recipients of bone marrow of this genotype (Table 1), establishing that the Plt6 phenotype is bone marrow intrinsic. Consistent with the data from Plt6 mutants on a Mpl−/− background, the thrombocytosis in recipients of Mpl+/+p300Plt6/Plt6 marrow was accompanied by a significant increase in the numbers of megakaryocytes and megakaryocyte progenitor cells in the bone marrow and spleen (Figure 4). The total number of myeloid progenitor cells was also significantly elevated in the bone marrow (Mpl+/+p300Plt6/Plt6: 129 ± 8 colonies per 2.5 × 104 cells [n = 3]; Mpl+/+p300+/+: 93 ± 23 [n = 4]) and spleen (Mpl+/+p300Plt6/Plt6: 79 ± 18 colonies per 105 cells [n = 4]; Mpl+/+p300+/+: 19 ± 7 [n = 4]).
Expanded megakaryocytopoiesis in recipients of Mpl+/+p300Plt6/Plt6 bone marrow. Recipients of Mpl+/+p300Plt6/Plt6 (6/6) and Mpl+/+p300+/+ control (+/+) bone marrow exhibited (A) increased numbers of megakaryocytes (megs), shown as number per microscopic field (×600, BM; ×200, spleen), and (B) megakaryocyte colony-forming cells (meg-CFCs), shown as number per 2.5 × 104 bone marrow (BM) or 105 spleen cells. Means plus or minus SD are shown. * P value less than .05 for comparison of data from recipients of Mpl+/+p300Plt6/Plt6 marrow with that of Mpl+/+p300+/+ marrow recipients. n = 4 mice per genotype.
Expanded megakaryocytopoiesis in recipients of Mpl+/+p300Plt6/Plt6 bone marrow. Recipients of Mpl+/+p300Plt6/Plt6 (6/6) and Mpl+/+p300+/+ control (+/+) bone marrow exhibited (A) increased numbers of megakaryocytes (megs), shown as number per microscopic field (×600, BM; ×200, spleen), and (B) megakaryocyte colony-forming cells (meg-CFCs), shown as number per 2.5 × 104 bone marrow (BM) or 105 spleen cells. Means plus or minus SD are shown. * P value less than .05 for comparison of data from recipients of Mpl+/+p300Plt6/Plt6 marrow with that of Mpl+/+p300+/+ marrow recipients. n = 4 mice per genotype.
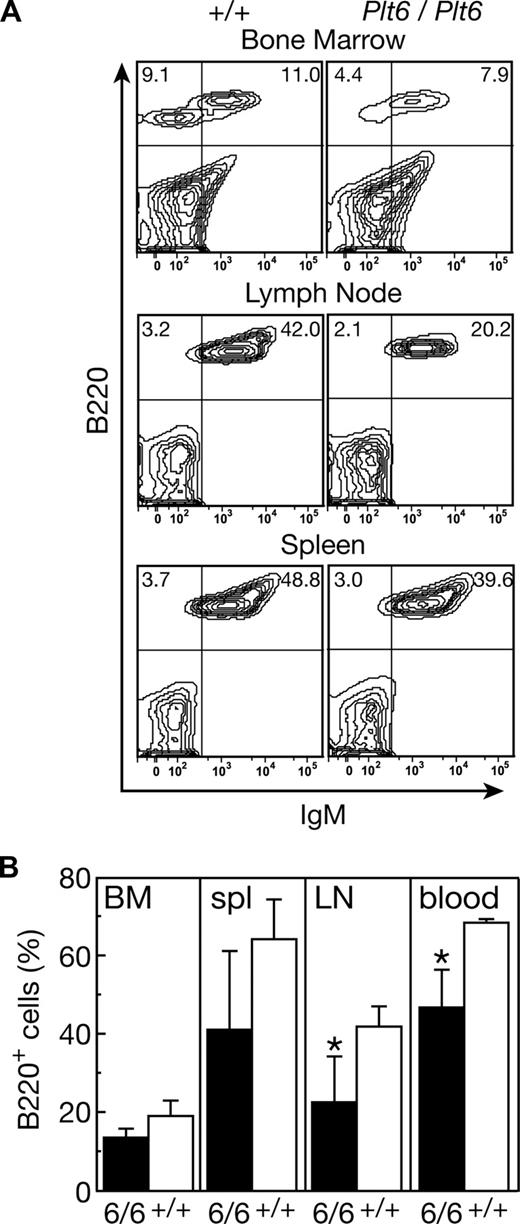
Mpl+/+p300Plt6/Plt6 mice and the recipients of Mpl+/+p300Plt6/Plt6 bone marrow also exhibited reduced numbers of circulating lymphoid cells in comparison with control Mpl+/+p300+/+ mice (Table 1). To further characterize the lymphoid compartment, flow cytometric analysis was performed on tissues from 2 Mpl+/+p300Plt6/Plt6 mice (data not shown) and the cohort of Mpl+/+p300Plt6/Plt6 transplant recipients (Figure 5). The relative numbers of T cells was not significantly altered; rather, the reduction in circulating lymphoid cells in Plt6 mutants resulted from reduced numbers of B cells. This was accompanied by reductions in B220+ B-lineage cells in the bone marrow, spleen, and lymph nodes (Figure 5). In the peripheral lymphoid tissues, reductions in IgM+ B cells were also observed. Consistent with the flow cytometry data, histologic examination of spleens revealed smaller than normal lymphoid follicles in both Mpl+/+p300Plt6/Plt6 mice examined and in the majority of the recipients of Mpl+/+p300Plt6/Plt6 bone marrow. There were no significant alterations in the relative numbers of T cells in the thymus, spleen, or lymph nodes in Mpl+/+p300Plt6/Plt6 mice or the recipients of Mpl+/+p300Plt6/Plt6 bone marrow relative to Mpl+/+p300+/+ controls, as assessed via CD4 and CD8 antibody staining (data not shown).
B lymphocyte deficit in recipients of Mpl+/+p300Plt6/Plt6 bone marrow. (A) Flow cytometry plots of bone marrow, lymph node, and spleen cells from recipients of Mpl+/+p300Plt6/Plt6 (Plt6/Plt6) and Mpl+/+p300+/+ control (+/+) bone marrow stained with antibodies to B220 and IgM showing reduced proportions of B220+ cells, including IgM+ B cells in each tissue (percentages of cells in specific quadrants is shown). (B) Percentages of B220+ cells in bone marrow (BM), spleen (spl), mesenteric lymph node (LN), and blood of recipients of Mpl+/+p300Plt6/Plt6 (6/6) and Mpl+/+p300+/+ control (+/+) bone marrow. Means plus or minus SD are shown. *P < .05 for comparison of data from recipients of Mpl+/+p300Plt6/Plt6 marrow with that of Mpl+/+p300+/+ marrow recipients. n = 4 mice per genotype.
B lymphocyte deficit in recipients of Mpl+/+p300Plt6/Plt6 bone marrow. (A) Flow cytometry plots of bone marrow, lymph node, and spleen cells from recipients of Mpl+/+p300Plt6/Plt6 (Plt6/Plt6) and Mpl+/+p300+/+ control (+/+) bone marrow stained with antibodies to B220 and IgM showing reduced proportions of B220+ cells, including IgM+ B cells in each tissue (percentages of cells in specific quadrants is shown). (B) Percentages of B220+ cells in bone marrow (BM), spleen (spl), mesenteric lymph node (LN), and blood of recipients of Mpl+/+p300Plt6/Plt6 (6/6) and Mpl+/+p300+/+ control (+/+) bone marrow. Means plus or minus SD are shown. *P < .05 for comparison of data from recipients of Mpl+/+p300Plt6/Plt6 marrow with that of Mpl+/+p300+/+ marrow recipients. n = 4 mice per genotype.
Previous analyses of mice with mutations in p300 or c-Myb have described anemia and alterations in erythroid precursor cells within hematopoietic tissues.5,6,11 Anemia was not evident in the small cohorts of p300Plt6/Plt6 mice examined, on either a Mpl+/+ or Mpl−/− genetic background, and although a trend toward reduced red blood cell number and hematocrit in the recipients of Mpl+/+p300Plt6/Plt6 bone marrow relative to control mice was observed, this was not statistically significant (Table 1). However, an increased proportion of Ter119+ cells was observed in the spleens of Mpl+/+p300Plt6/Plt6 bone marrow recipients via flow cytometry (recipients of Mpl+/+p300+/+ bone marrow: 30% ± 5% versus Mpl+/+p300Plt6/Plt6 recipients: 49% ± 7%, n = 4) and was confirmed by histologic observations of increased numbers of nucleated erythroid cells in sections of spleens from Mpl+/+p300Plt6/Plt6 bone marrow recipients compared with recipients of normal marrow (data not shown).
Histologic analysis of the major nonhematopoietic organs of mice bearing p300Plt6 mutations on either a Mpl+/+ or Mpl−/− genetic background, as well as of recipients of Mpl+/+p300Plt6/Plt6 bone marrow, revealed no consistent changes compared with respective p300+/+ controls.
Discussion
The KIX domain of p300 is a highly conserved motif implicated in binding transcriptional regulators including c-Myb.6,12 Via ENU mutagenesis, we have generated mice with a mutation in the KIX domain of p300 (p300Plt6) that significantly disrupts binding to c-Myb. Mice heterozygous for the p300Plt6 mutation exhibited amelioration of the thrombocytopenia caused by the absence of c-Mpl, the receptor for TPO, whereas homozygous Mpl−/−p300Plt6/Plt6 mice displayed supraphysiological platelet counts. The increased platelets in Plt6 mutant mice resulted from expansion of megakaryocytopoiesis, with excess levels of megakaryocytes and megakaryocyte progenitor cells in the bone marrow and spleen. The expansion of megakaryocytopoiesis and high platelet counts observed in Mpl−/−p300Plt6/Plt6 mice were highly reminiscent of the suppression of thrombocytopenia and supraphysiological platelet production in Mpl−/− mice with ENU-induced mutations in c-Myb.5,13 Thus, combined with these previous studies, our data reinforce a key role for the c-Myb/p300 transcriptional regulatory complex in restraining megakaryocytopoiesis to allow sufficient platelet production while preventing thrombocytosis.
Thrombocytosis has previously been observed in mice homozygous for a p300 KIX domain triple mutation (in mouse p300 residues Y631A, A635Q, Y639A).6 Structural studies with the p300 paralogue CBP showed that these highly conserved KIX domain residues lie along an α-helical surface that interacts with the CREB activation domain9 and that mutation of any of these amino acids disrupts CBP KIX domain interaction with CREB or c-Myb.14 Structural modeling suggests that the side chain of Y630 is a component of the KIX domain's hydrophobic core that is positioned to make several potential hydrophobic interactions, disruption of which may distort this p300 domain. Indeed, we have confirmed that the substitution of an asparagine residue in place of Y630 in the p300Plt6 protein significantly disrupts p300-c-Myb complex formation. Interestingly, although mice homozygous for the p300 KIX domain triple mutation (p300KIX/KIX mice) show anemia, thymic hypoplasia, and defective B-lymphocyte production in addition to thrombocytosis,6 these additional hematologic defects were relatively mild in homozygous p300Plt6 mutants. It is also noteworthy that the anemia and B-lymphoid deficiency typical of p300KIX/KIX mice have also been observed in mice homozygous for mutations in c-Myb.5 These observations imply that appropriate regulation of megakaryocytopoiesis, erythropoiesis, and lymphopoiesis each requires wild-type activity of the c-Myb/p300 complex. The absence of more severe hematologic anomalies other than thrombocytosis in Plt6 homozygotes may suggest that the single-residue Plt6 mutation disrupts p300 activity less profoundly than the triple p300KIX mutation. If so, the data imply that deregulation of platelet production may be more sensitive to changes in p300 activity than the erythroid or lymphoid lineages.
Homozygosity of the p300Plt6 allele resulted in substantial mortality, with fewer than 20% of the number of Mpl−/−p300Plt6/Plt6 or Mpl+/+p300Plt6/Plt6 mice expected from normal Mendelian segregation of alleles surviving to weaning. A similar degree of preweaning mortality was reported for p300KIX/KIX mice.6 The fetal and/or neonatal phenotypes of p300KIX/KIX mice have not been described, nor have we yet examined these in p300Plt6/Plt6 mutants. Thus, although it remains possible that the phenotypes described in surviving mice from both these models of p300 mutation may be milder than in mice succumbing prior to weaning, the absence of anemia or any apparent thrombotic complications arising from thrombocytosis in adult p300Plt6/Plt6 mice may imply that a nonhematologic basis for the lethality in homozygote p300 mutants might also require consideration. Indeed, mice nullizygous for p300 die at midgestation with multiple anomalies, including defective neurulation and heart development.15
Finally, our data establish that, like disruption of c-Myb activity, inhibition of p300 results in very significant production of megakaryocytes and platelets in the absence of TPO signaling. The residual platelet production in mice lacking TPO or its receptor c-Mpl can be mobilized to generate near wild-type levels of circulating platelets following cytotoxic stress,4 implying that TPO-independent mechanisms of platelet production could be exploited to enhance platelet production in thrombocytopenia. In this context, the c-Myb/p300 mutant mice not only suggest that pharmaceutical inhibition of the Myb/p300 complex may be useful in thrombocytopenia, but also provide a key resource in dissecting TPO-independent megakaryocytopoiesis, which may provide important new bases for future therapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Janelle Lochland, Jason Corbin, Sandra Mifsud, and Ladina DiRago for skilled technical assistance.
This work was supported by a program grant (461219) and fellowships (D.J.H., J.M.M., W.S.A., N.A.N.) from the Australian National Health and Medical Research Council (Canberra, Australia), fellowships from the Australian Research Council (Canberra, Australia; B.T.K.) and Cancer Council of Victoria (Melbourne, Australia; D.M.), an RO1 grant from the National Heart, Lung, and Blood Institute (Bethesda, MD; HL080019) and a collaborative research grant from MuriGen (Melbourne, Australia).
National Institutes of Health
Authorship
Contribution: M.K., J.M.M., D.J.H., and W.S.A. designed and performed experiments, analyzed data, and wrote the paper; C.A.d.G., D.M., K.T.G., A.A.H., B.T.K., and C.D.H. designed and performed experiments and collected and analyzed data; and N.A.N. analyzed data.
Conflict-of-interest disclosure: D.J.H., N.A.N., B.T.K., and W.S.A. hold shares in a company developing intellectual property derived from this study. The remaining authors declare no competing financial interests.
Correspondence: Warren S. Alexander, Cancer and Haematology Division, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, 3050, Australia; e-mail: alexandw@wehi.edu.au.
References
Author notes
*M.K. and J.M.M. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal