Abstract
The enucleated definitive erythrocytes of mammals are unique in the animal kingdom. The observation that yolk sac–derived primitive erythroid cells in mammals circulate as nucleated cells has led to the conjecture that they are related to the red cells of fish, amphibians, and birds that remain nucleated throughout their life span. In mice, primitive red cells express both embryonic and adult hemoglobins, whereas definitive erythroblasts accumulate only adult hemoglobins. We investigated the terminal differentiation of murine primitive red cells with use of antibodies raised to embryonic βH1-globin. Primitive erythroblasts progressively enucleate between embryonic days 12.5 and 16.5, generating mature primitive erythrocytes that are similar in size to their nucleated counterparts. These enucleated primitive erythrocytes circulate as late as 5 days after birth. The enucleation of primitive red cells in the mouse embryo has not previously been well recognized because it coincides with the emergence of exponentially expanding numbers of definitive erythrocytes from the fetal liver. Our studies establish a new paradigm in the understanding of primitive erythropoiesis and support the concept that primitive erythropoiesis in mice shares many similarities with definitive erythropoiesis of mammals.
Introduction
It was recognized more than 125 years ago that the mature red cells of adult vertebrates circulate either in nucleated or enucleated forms.1 The red cells of all birds, fish, reptiles, and amphibians retain their nucleus and contain 3 filamentous systems: an actin-spectrin–based membrane cytoskeleton, intermediate filaments that attach the cytoskeleton to the nuclear membrane, and a group of microtubules organized into a circumferential marginal band.2,3 In contrast, the red cells of mammals lose intermediate filaments and microtubules during terminal differentiation and enucleate prior to entering the bloodstream. Thus, erythrocytes of adult mammals are enucleated and contain only one filamentous system, a membrane cytoskeleton.
Nearly 100 years ago, examination of mammalian embryos revealed the presence of distinct nucleated and enucleated red cells.4 The continuous circulation of small, enucleated red cells during fetal and postnatal life was termed “definitive” erythropoiesis. Definitive erythropoiesis in the fetus is preceded by a “primitive” erythroid program that is characterized by the transient circulation of large, nucleated red cells that originate extraembryonically in the yolk sac.4,5 Because primitive erythroblasts in mammals circulate as nucleated cells and are confined to the embryo, they have been thought to share many characteristics with the nucleated red cells of nonmammalian vertebrates when compared with the enucleated definitive red cells of fetal and adult mammals.6,7
In the mouse embryo, primitive erythroid cells begin to develop in yolk sac blood islands between embryonic days 7 and 8 (E7-8).8,9 With the onset of cardiac contractions at early somite pair stages (E8.25), primitive erythroblasts enter the embryonic bloodstream10,11 where they remain until E16.5 when the primitive lineage was thought to be extinguished.12,13 Definitive erythrocytes begin to emerge from the fetal liver at E12.513,14 and rapidly become the predominant cell type in the circulation. Definitive red cells can be distinguished from their primitive counterparts by their smaller size and by their accumulation of adult, but not embryonic, hemoglobins.6,13,15 In contrast, primitive erythroblasts in the mouse are large cells that accumulate both embryonic and adult hemoglobins.15-17
More than 30 years ago, a population of enucleated red cells with the same hemoglobin content as primitive erythroblasts was described in the embryonic circulation of the mouse.14 Furthermore, large enucleated red cells have been noted in the bloodstream of mouse embryos by several investigators,14,18-20 raising the possibility that primitive erythroblasts might ultimately enucleate. Studies in the Syrian hamster and in marsupials indicate that primitive erythroblasts can enucleate,21-23 To determine whether primitive erythroblasts enucleate in the mouse embryo and to examine the transition from primitive to definitive erythropoiesis, we raised antibodies to embryonic βH1-globin and optimized the immunohistochemical identification of primitive (βH1-globin–positive) red cells. We report here that, contrary to widely held opinion, murine primitive erythroblasts enucleate and continue to circulate throughout late gestation and even into the postnatal period. Our studies support the concept that the primitive erythroid lineage in the murine embryo is truly “mammalian” in nature.
Materials and methods
Collection of embryonic peripheral blood cells
Outbred Swiss Webster mice (Taconic, Germantown, NY) were mated overnight, and vaginal plugs were checked the following morning (E0.3). At specified times during gestation, mice were killed by cervical dislocation, and the uteri were removed from the peritoneum and washed with several changes of phosphate-buffered saline (PBS). Embryos were dissected free of decidual tissues in a PB1 solution as described by Monk24 but which was modified by removing penicillin and phenol red. After removal of the placenta, individual, intact embryos were transferred to dishes containing heparinized PB1 solution (as above with 12.5 μg/mL heparin). Blood cells were collected by aspiration as they bled from severed umbilical and vitelline vessels. Postnatal mice were killed by decapitation, and peripheral blood cells were collected from jugular vessels into heparinized PB1 solution. Cytospins were prepared with 50 000 cells spun at 400 rpm for 3 minutes (Cytospin2; Thermo Shandon, Pittsburgh, PA) and stained with May-Grünwald-Giemsa (Sigma Diagnostics, St Louis, MO), and photographed with a Nikon Optiphot microscope equipped with a × 40 objective (NA 0.85) and a SPOT RT-slider digital camera (Diagnostic Instruments, Sterling Heights, MI). Images were processed in Photoshop (Adobe, San Jose, CA).
Total red cell counts in timed E12.5, E14.5, and E16.5 embryos
At least 3 individual conceptuses from each timed pregnancy were carefully dissected free of decidual tissues and transferred intact with their placentas into individual dishes of modified PB1. The yolk sac and umbilical vessels were severed at their attachment to the placenta using no. 5 watchmaker's forceps, and the embryos were immediately transferred into fresh 35-mm dishes of heparinized PB1 solution. Each embryo was allowed to exsanguinate from severed umbilical, vitelline, and jugular vessels, and all blood cells in the dish were collected and counted by hemacytometer. Data from a minimum of 3 different timed pregnancies were obtained for each time point.
Coulter analysis of peripheral blood cells
To quantify the size of the circulating red cells, samples of peripheral blood were diluted into PBS medium and analyzed on a Coulter ZM with channelyzer with preset gain of 4 and attenuation of 16 (Coulter Electronics, Essex, England).
Raising of anti–βH1-globin and anti–βmajor-globin antibodies
Peptide sequences of the murine βH1-globin and βmajor-globin proteins were compared to identify regions of divergence. Rabbit polyclonal antipeptide antibodies were generated to amino acids 71 to 84 of accession number NP_032245 (βH1-globin) and the corresponding region of β-major globin (accession no. P02088) by Biosynthesis (Lewisville, TX). Both primary antisera and Protein A–purified immunoglobulin G (IgG) fractions (CPG, Lincoln Park, NJ; prepared according to manufacturer's instructions) provided reproducible results on paraformaldehyde-fixed tissues and ethanol-fixed blood cells.
Immunohistochemical analysis
E10.5 and E12.5 mouse embryos were dissected free of maternal tissues, fixed overnight in fresh 4% buffered paraformaldehyde, embedded in paraffin, and sectioned. Immunohistochemistry was performed using standard avidin-biotin complex (ABC) protocols (Vector Laboratories, Burlingham, CA) after antigen retrieval by pretreatment of the slides for 15 minutes at 100° C in 0.1M Tris (tris(hydroxymethyl)aminomethane), pH 6.
To identify blood cells containing βH1-globin by immunohistochemistry, it was necessary to develop a protocol that optimized cell morphology, globin retention, and antigenicity, as well as cell dispersal that allowed for semiautomated morphometric analysis. Blood cells collected from timed conceptuses were centrifuged at 1000g for 5 minutes and resuspended at approximately 50 000 cells/μL in 83% modified PB1, 12.5 μg/mL heparin, 3.67% bovine serum albumin (BSA; Sigma, St Louis, MO). Diluted cells (3 μL) were smeared onto untreated glass slides using standard wedge technique. After air drying, smears were fixed with 100% ethanol for 5 minutes and stored at room temperature for up to 2 months. Following pretreatment in 3% Triton X-100 in PBS for 6 to 14 hours, immunohistochemistry was performed according to instructions included with Vector Laboratories' ABC kit with alkaline phosphatase and Vector Red as the enzyme and substrate, respectively. No specific cross-reaction was observed with preimmune sera or with preimmune IgG. For the experiment in Figure 2D, cells were incubated with DAPI (4′,6-Diamidino-2-phenylindole dihydrochloride) at 2 μg/mL in PBS for 10 minutes, after immunohistochemistry, then mounted in aqueous mounting medium. Cells were photographed in fluorescence with either a Nikon Otiphot microscope equipped with a × 4 objective (NA 0.13) (Figure 2A), × 10 objective (NA 0.50) (Figure 2B-C), × 40 objective (NA 0.85) (Figure 2G) with paired phase-contrast optics, or with a Nikon Eclipse TE 2000-S microscope equipped with a × 40 objective (NA 0.60) (Figure 2D-E) with paired Hoffman modulation contrast optics and a SPOT RT-Slider camera. Images were processed in Photoshop.
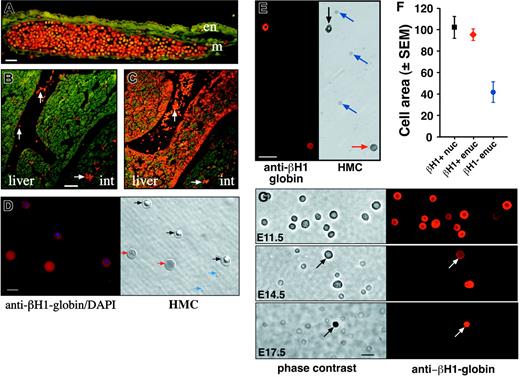
Immunohistochemistry with antiglobin antibodies. (A) E10.5 yolk sac stained with anti–βH1-globin antibody. Positive primitive erythroblasts are evident throughout a large blood vessel, whereas adjacent mesoderm-derived (m) and endoderm (en) cells are negative. The scale bar represents 25 microns. (B-C) E12.5 neighboring embryo sections stained with anti–βH1-globin (B) and anti–βmajor-globin (C) antibodies. The anti–βH1-globin antibodies decorate primitive erythroblasts in blood vessels (white arrows). Anti–βmajor-globin antibodies decorate both primitive red cells in vessels and definitive erythroblasts maturing in the liver (liver). Int indicates intestine. The scale bar represents 50 μm. (D) E13.5 peripheral blood cells stained both with anti–βH1-globin antibodies and with DAPI (left). A paired Hoffman modulation contrast image (HMC, right) shows the βH1-globin–positive nucleated cells (black arrows), βH1-globin–negative enucleated cells (blue arrows), and βH1-globin–positive enucleated cells (red arrows). The scale bar represents 10 μm. (E) E15.5 peripheral blood cells stained with anti–βH1-globin antibodies (left). A paired Hoffman modulation contrast image (HMC, right) of the same field of cells indicates a βH1-globin–positive nucleated cell (black arrow), the βH1-globin–negative enucleated cells (blue arrows), and a βH1-globin–positive enucleated cell (red arrow). The scale bar represents 25 μm. (F) Morphometric analysis of cell area of 3 populations of peripheral blood cells at E15.5 of mouse gestation. Nucleated (black square) and enucleated (red diamond) βH1-globin–positive cells are both similar in size and are significantly larger than βH1-globin–negative definitive erythrocytes (blue circle). Ordinate units are μm2. (G) Immunohistochemical analysis of βH1-globin expression in circulating blood cells from E11.5, E14.5, and E17.5 mouse embryos (right column) with paired phase contrast images (left column). At E14.5, both nucleated and enucleated (arrow) βH1-globin–positive cells are evident. All images are at the same magnification. Scale bar represents 10 μm.
Immunohistochemistry with antiglobin antibodies. (A) E10.5 yolk sac stained with anti–βH1-globin antibody. Positive primitive erythroblasts are evident throughout a large blood vessel, whereas adjacent mesoderm-derived (m) and endoderm (en) cells are negative. The scale bar represents 25 microns. (B-C) E12.5 neighboring embryo sections stained with anti–βH1-globin (B) and anti–βmajor-globin (C) antibodies. The anti–βH1-globin antibodies decorate primitive erythroblasts in blood vessels (white arrows). Anti–βmajor-globin antibodies decorate both primitive red cells in vessels and definitive erythroblasts maturing in the liver (liver). Int indicates intestine. The scale bar represents 50 μm. (D) E13.5 peripheral blood cells stained both with anti–βH1-globin antibodies and with DAPI (left). A paired Hoffman modulation contrast image (HMC, right) shows the βH1-globin–positive nucleated cells (black arrows), βH1-globin–negative enucleated cells (blue arrows), and βH1-globin–positive enucleated cells (red arrows). The scale bar represents 10 μm. (E) E15.5 peripheral blood cells stained with anti–βH1-globin antibodies (left). A paired Hoffman modulation contrast image (HMC, right) of the same field of cells indicates a βH1-globin–positive nucleated cell (black arrow), the βH1-globin–negative enucleated cells (blue arrows), and a βH1-globin–positive enucleated cell (red arrow). The scale bar represents 25 μm. (F) Morphometric analysis of cell area of 3 populations of peripheral blood cells at E15.5 of mouse gestation. Nucleated (black square) and enucleated (red diamond) βH1-globin–positive cells are both similar in size and are significantly larger than βH1-globin–negative definitive erythrocytes (blue circle). Ordinate units are μm2. (G) Immunohistochemical analysis of βH1-globin expression in circulating blood cells from E11.5, E14.5, and E17.5 mouse embryos (right column) with paired phase contrast images (left column). At E14.5, both nucleated and enucleated (arrow) βH1-globin–positive cells are evident. All images are at the same magnification. Scale bar represents 10 μm.
Morphometric analysis
To determine the areas of peripheral blood cells, smears of E15.5 blood were analyzed for βH1-globin expression and photographed using the SPOT RT-slider digital microscope camera. These images were processed in Photoshop (Adobe Systems, San Jose, CA) with Fovea Pro quantitative image analysis plug-ins (Reindeer Graphics, Asheville, NC).
Results
Changes in the distribution of red cell populations in the embryonic circulation
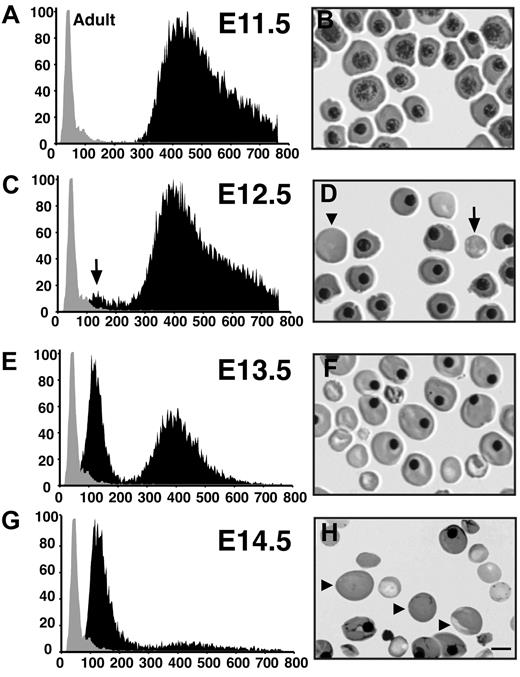
The first red cells in the mouse embryo arise in blood islands of the yolk sac and consist exclusively of large primitive erythroblasts. Examination of smears of peripheral blood cells indicates that nucleated primitive erythroblasts constitute the only circulating red cell population at E8.5, E9.5, and E10.5 (data not shown). These cells undergo progressive maturation, transitioning from proerythroblasts at E8.5 to polychromatophilic erythroblasts by E11.5 (Figure 1B and data not shown). These primitive erythroblasts are extremely large cells with volumes ranging from 300 fl to 750 fl (Figure 1A). This volume is in marked contrast to adult erythrocytes that have a mean cell volume (MCV) of approximately 70 fl (Figure 1A).
Changes in the cellular composition of peripheral blood between E11.5 and E14.5 of mouse gestation. (A,C,E,G) Cell size distribution of circulating embryonic blood cells (black area). An independent sample of adult murine red cells (gray area) is superimposed on each Coulter analysis. The x-axis represents cell volume (femtoliters); y-axis, relative cell number. (B,D,F,H) Peripheral blood cells stained with May-Grünwald-Giemsa. Some of the small and large enucleated red cells are marked with arrows and arrowheads, respectively. The scale bar represents 10 μm.
Changes in the cellular composition of peripheral blood between E11.5 and E14.5 of mouse gestation. (A,C,E,G) Cell size distribution of circulating embryonic blood cells (black area). An independent sample of adult murine red cells (gray area) is superimposed on each Coulter analysis. The x-axis represents cell volume (femtoliters); y-axis, relative cell number. (B,D,F,H) Peripheral blood cells stained with May-Grünwald-Giemsa. Some of the small and large enucleated red cells are marked with arrows and arrowheads, respectively. The scale bar represents 10 μm.
Between E11.5 and E12.5, a second population of red cells emerges in the circulation (Figure 1C, arrow). This population of cells has an MCV of 150 fl, which is intermediate in size when compared with primitive erythroblasts and adult red cells. The appearance of this second cell population coincides with the presence of smaller, enucleated red cells on peripheral smears (Figure 1D, arrow). These fetal liver–derived definitive erythrocytes rapidly become the predominant cell population between E14.5 and E15.5, as evidenced by the Coulter analysis (Figure 1E,G). Closer examination of peripheral blood smears from E14.5 embryos revealed a population of large enucleated red cells (Figure 1H, arrowheads). These large erythrocytes were evident as early as E12.5, (Figure 1D, arrowhead) and were similar in size to nucleated primitive red cells, raising the possibility that they are derived from the primitive erythroid lineage. To test this hypothesis, antibodies were raised to embryonic globin to specifically distinguish primitive red cells from other circulating blood cells.
The specificity of the anti–βH1-globin antibodies was tested by immunohistochemical analysis of sections of E10.5 and E12.5 embryos. As shown in Figure 2A, anti–βH1-globin antibodies specifically bind to circulating E10.5 primitive erythroblasts but do not bind to adjacent mesodermal (m) or endodermal (e) tissues. At E12.5, anti–βH1-globin antibodies decorate primitive red cells but do not bind to nucleated definitive erythroblasts within the liver (Figure 2B). The βH1-globin–positive cells in the fetal liver are primitive red cells localized within blood vessels. In contrast, antibodies raised to the adult βmajor-globin protein decorated both circulating primitive erythroblasts and differentiating definitive erythroblasts in the fetal liver (Figure 2C). No βH1-globin–positive cells were detected in adult peripheral blood (data not shown). These results confirm that the anti–βH1-globin antibodies recognize yolk sac–derived primitive red cells but not fetal or adult definitive erythroid cells.
Identification of enucleated primitive red cells
To determine whether primitive erythroid cells might enucleate, we established conditions for βH1-antibody staining of smeared circulating blood cells. Three different cell populations were identified when we examined peripheral blood cells from E13.5 and E15.5 fetuses (Figure 2D-E). The first population consisted of primitive erythroblasts that contain both embryonic (βH1) globin and a nucleus, as confirmed by DAPI staining (Figure 2D-E, black arrows). The second population consisted of definitive erythrocytes that lack both βH1-globin expression and a nucleus (Figure 2D-E, blue arrows). The third population consisted of cells that contain βH1-globin but lack a nucleus (Figure 2D-E, red arrows). The expression of embryonic globin by this third population of erythrocytes is consistent with their derivation from the primitive erythroid lineage.
As primitive red cells are significantly larger than definitive red cells (Figure 1), we compared the relative size of these 3 cell populations by quantifying cell areas. As shown in Figure 2F, the nucleated and enucleated βH1-globin–positive cell populations (black square and red diamond, respectively) have similar cell areas and each is significantly larger than the βH1-gobin–negative red cell population (blue circle). We thus identified a population of enucleated red cells in the bloodstream of the mouse embryo that shares both embryonic globin expression and cell size with primitive erythroblasts. These results indicate that yolk sac–derived primitive erythroblasts can enucleate and circulate as erythrocytes.
Changes in red cell population dynamics between E10.5 and E17.5 of mouse gestation
As has been noted previously25 and is evidenced by progressive changes in cell morphology (Figure 1), primitive erythroid cells differentiate in a semisynchronous manner. Our observation that primitive erythroid cells can enucleate raised the possibility that enucleation is a normal component of primitive erythropoiesis, as this lineage terminally differentiates in the bloodstream. Alternatively, these enucleated forms could be rare cells that deviate from an inherent nonmammalian character of the primitive erythroid lineage in mice. To distinguish between these alternatives, we systematically analyzed the cellular composition of peripheral blood between E11.5 and E17.5 of mouse gestation. Examples of this immunohistochemical analysis are shown in Figure 2G.
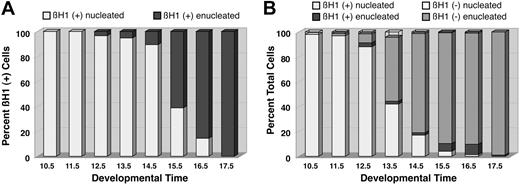
Examination of peripheral blood smears following immunohistochemistry for βH1-globin revealed that all of the primitive red cells at E10.5 and E11.5 were nucleated (Figure 2G, Figure 3A, white column). We first detected small numbers of enucleated βH1-globin–positive primitive erythrocytes at E12.5 (Figure 3A, black column). There is a progressive transition to enucleated cells that is completed by E17.5 (Figures 2G and 3A). This transition from nucleated to enucleated forms between E12.5 and E17.5 supports the concept that primitive erythroid cells terminally differentiate in the bloodstream and ultimately become enucleated primitive erythrocytes. Our immunohistochemical analysis also allowed us to examine the presence of definitive red cells in the bloodstream during the second half of mouse gestation. Enucleated definitive (βH1-globin–negative) erythrocytes were first detected at E11.5 and continue to be a minor component of the circulation at E12.5 (Figure 3B, light gray column). However, by E13.5, definitive red cells constitute more than 50% of the circulating blood cells. This transition continues so that by E17.5, 99% of the circulating cells are definitive erythrocytes (Figures 2G and 3B). We also noted a minor population of nucleated βH1-globin–negative cells (Figure 3B, clear columns) that likely comprise a diverse group of cells, including myeloid cells, definitive erythroblasts released from the fetal liver prior to enucleation, and contaminating nonhematopoietic cells. They were most prevalent at E13.5 and always constituted less than 5% of the peripheral blood cells.
Changes in the distribution of circulating red cell populations between E10.5 and E17.5 of mouse gestation. (A) Changes in the percentage of nucleated (white columns) and enucleated (black columns) βH1-globin–positive primitive red cells in the bloodstream. There is a progressive transition from nucleated to enucleated forms. (B) Changes in the distribution of βH1-globin–positive nucleated (white columns) and enucleated (black columns) cells and in βH1-globin–negative nucleated (clear columns) and enucleated (gray columns) cells. Primitive red cells become a progressively smaller component of the circulation between E12.5 and E17.5. Results in this figure are derived from the examination of 14 855 cells from more than 60 fetuses from 22 timed litters.
Changes in the distribution of circulating red cell populations between E10.5 and E17.5 of mouse gestation. (A) Changes in the percentage of nucleated (white columns) and enucleated (black columns) βH1-globin–positive primitive red cells in the bloodstream. There is a progressive transition from nucleated to enucleated forms. (B) Changes in the distribution of βH1-globin–positive nucleated (white columns) and enucleated (black columns) cells and in βH1-globin–negative nucleated (clear columns) and enucleated (gray columns) cells. Primitive red cells become a progressively smaller component of the circulation between E12.5 and E17.5. Results in this figure are derived from the examination of 14 855 cells from more than 60 fetuses from 22 timed litters.
Our analysis indicates that 2 major transitions occur between E12.5 and E17.5 of gestation. The first is progressive transition from nucleated to enucleated primitive red cells (Figure 3A). The second is a rapid transition from primitive to definitive red cells (Figure 3B). These findings suggest that the process of enucleation leads to the demise of primitive red cells. If this were the case, we would expect the total number of primitive red cells to decrease significantly as they enucleate. Alternatively, the primitive erythroid lineage could persist in the circulation but be diluted by the influx of definitive erythrocytes from the liver. To distinguish between these alternatives, we estimated the total number of primitive and definitive red cells in the embryo between E12.5, when enucleation begins, and E16.5, when more than 90% of the primitive red cells have enucleated. At E12.5, the embryo contains approximately 5 million red cells, almost all of which are primitive (Table 1). Total red cell numbers expand 30-fold over the next 4 days. This massive increase in red cell mass is due almost entirely to the entry of definitive red cells from the fetal liver into the circulation. The number of primitive red cells increases only marginally between E12.5 and E16.5 (Table 1). Because primitive red cells cease dividing by E13.5,26,27 these results indicate that the process of enucleation is not associated with loss of primitive red cells from the circulation. Rather, the disappearance of nucleated primitive red cells in the bloodstream is due both to their progressive enucleation and to their dilution by increasing numbers of definitive erythrocytes from the fetal liver.
Total number ± SEM of circulating red blood cells (RBCs) and of primitive red cells in E12.5, E14.5, and E16.5 mouse embryos
. | Total RBCs, × 106 per embryo . | Primitive RBCs, × 106 per embryo . | Nucleated primitive RBCs, % . | n . |
|---|---|---|---|---|
| E12.5 | 5.3 ± 0.9 | 4.8 ± 0.6 | 91.2 ± 4.3 | 3 |
| E14.5 | 26.3 ± 6.9 | 6.0 ± 2.2 | 22.6 ± 9.4 | 3 |
| E16.5 | 151.7 ± 28.6 | 10.8 ± 4.5 | 6.5 ± 2.0 | 4 |
. | Total RBCs, × 106 per embryo . | Primitive RBCs, × 106 per embryo . | Nucleated primitive RBCs, % . | n . |
|---|---|---|---|---|
| E12.5 | 5.3 ± 0.9 | 4.8 ± 0.6 | 91.2 ± 4.3 | 3 |
| E14.5 | 26.3 ± 6.9 | 6.0 ± 2.2 | 22.6 ± 9.4 | 3 |
| E16.5 | 151.7 ± 28.6 | 10.8 ± 4.5 | 6.5 ± 2.0 | 4 |
There are no significant changes in the numbers of primitive red cells as they enucleate between E12.5 and E16.5. n indicates the number of independent experiments.
Primitive erythrocytes continue to circulate after birth
Our results indicate that the primitive erythroid lineage is not extinguished as previously thought when nucleated red cells are no longer present in the embryonic bloodstream.12,13 To determine whether primitive red cells circulate beyond E17.5, we analyzed peripheral blood from late gestation mouse fetuses and postnatal pups for the presence of βH1-globin–positive red cells. Primitive erythrocytes were evident at E18.5, at birth (E19.5), and for several days after birth (data not shown). Even at postnatal day 5, rare βH1-positive red cells were detected in 1 of 3 mice examined. Because nucleated primitive red cells are not evident after day 16.5, these results indicate that primitive red cells can circulate as erythrocytes for several days after enucleating.
Discussion
Primitive erythroid cells are necessary for survival of the mammalian embryo because they comprise a critical component of a functional circulatory system.28 Primitive erythroid cells originate in blood islands of the yolk sac and expand exponentially in numbers between E8.5 and E10.5, when robust circulation is reflected by the high proportion of the embryo's cell mass that is red blood cells.10 As exemplified by the disruption the scl and GATA-1 genes, the lack of primitive red cells leads to fetal death in the mouse between E9.5 and 10.5.29-31 The primitive erythroid lineage continues to expand in numbers until E12.5 to 13.5 when cell division ceases. Because primitive red cells constitute the predominant cell type in the circulation through E12.5 (Figure 3B), anemia in the embryo before E13.5 reflects a disorder of the differentiation or circulation of the primitive erythroid lineage. After this time, the growing fetus's need for an increasing red cell mass is met by the exponential expansion of the definitive erythroid lineage. The 30-fold increase in red cell number between E12.5 and E16.5 is due almost entirely to the emergence of definitive erythrocytes from the fetal liver. In the complete absence of definitive red cells, as exemplified by mice lacking c-myb, the primitive erythroid lineage can sustain fetal survival until E15.5 to E16.5.32
Previous investigators have used cell size,18,33 acid elution,19,34,35 hemoglobin content,14 and antibodies generated to embryonic hemoglobins13,36 to identify primitive red cells in the rodent embryo. Because βH1-globin is restricted to the primitive erythroid lineage of the mouse, we generated antibodies to a peptide corresponding to an unique region of the βH1-globin chain and optimized an immunohistochemical protocol to identify primitive red cells. These experimental data reported here indicate that primitive erythroblasts normally enucleate during terminal differentiation. It is likely that enucleated primitive erythrocytes have not previously been well recognized in the mouse embryo because their appearance coincides with the massive entry into the bloodstream of enucleated definitive erythrocytes from the liver.
Our direct measure of cell volume with use of the Coulter counter indicates that E9.5 to E12.5 primitive erythroblasts in mice are extremely large cells with volumes ranging between 300 and 800 fl. This wide range of cell volumes likely reflects the presence of dividing cells, as evidenced by the exponential expansion in red cell numbers10 and the presence of mitotic figures at E8.5 to E11.5 (data not shown). By E13.5, when cell division has ceased, the distribution of primitive red cell size has become bell-shaped, and the mean cell volume of primitive erythroid cells is approximately 400 fl (Figure 1E). These findings are consistent with those previously reported for E13 to E15 primitive erythroblasts.37 Yolk sac–derived primitive red cells are approximately 3-fold larger than fetal liver–derived definitive erythrocytes and 5- to 6-fold larger than adult murine erythrocytes. Although the mechanisms responsible for the differences in cell size among these different red cell lineages are not known, they may relate to differential rates of cell division. Steiner38 has determined that primitive erythroblasts divide more slowly than fetal liver–derived definitive erythroblasts, allowing for increased hemoglobin accumulation during terminal maturation.
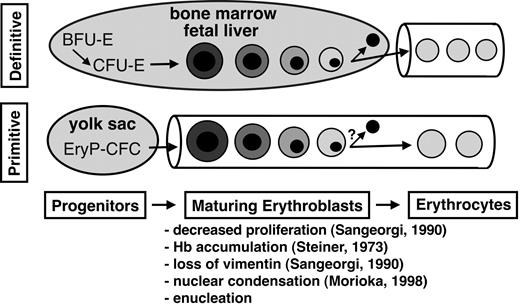
Our studies establish a new paradigm in the understanding of the primitive erythroid lineage and indicate that primitive erythropoiesis in mammals shares many processes with its definitive counterpart, including progressive phases of (1) lineage-committed progenitors, (2) erythroblast maturation with enucleation, and (3) circulation as mature erythrocytes (Figure 4). Although definitive red cells arise from committed BFU-E and CFU-E progenitors, primitive red cells arise from an unique EryP-CFC progenitor.39 We have previously identified this first phase of committed progenitors for the primitive erythroid lineage in the mouse embryo.17 EryP-CFCs arise during early gastrulation (E7.25) and rapidly expand in numbers within the yolk sac. However, this wave of primitive erythroid progenitors is transient, lasting only 48 hours before being extinguished.17,36
Primitive and definitive erythropoiesis each share 3 phases of differentiation. Committed erythroid progenitors give rise to maturing nucleated erythroblasts that ultimately generate enucleated erythrocytes. The specific erythroid progenitors are EryP-CFCs (primitive erythroid colony-forming cells), BFU-Es (erythroid burst-forming units), and CFU-Es (erythroid colony-forming units). The characteristics common to primitive and definitive erythroblast maturation in mammals are listed along with references pertaining to primitive erythropoiesis in the mouse. We propose that a major distinction between primitive and definitive erythropoiesis in mammals is that primitive red cells differentiate while circulating in the bloodstream, whereas definitive red cells enter the circulation only after completing their maturation extravascularly.
Primitive and definitive erythropoiesis each share 3 phases of differentiation. Committed erythroid progenitors give rise to maturing nucleated erythroblasts that ultimately generate enucleated erythrocytes. The specific erythroid progenitors are EryP-CFCs (primitive erythroid colony-forming cells), BFU-Es (erythroid burst-forming units), and CFU-Es (erythroid colony-forming units). The characteristics common to primitive and definitive erythroblast maturation in mammals are listed along with references pertaining to primitive erythropoiesis in the mouse. We propose that a major distinction between primitive and definitive erythropoiesis in mammals is that primitive red cells differentiate while circulating in the bloodstream, whereas definitive red cells enter the circulation only after completing their maturation extravascularly.
The second phase of erythropoiesis consists of erythroblast maturation (Figure 4). In the definitive erythroid lineage this phase is characterized by several cell divisions and a progression of morphologically identifiable forms, resulting from the accumulation of hemoglobin in the cytoplasm and condensation of the nucleus.40 With the loss of vimentin intermediate filaments, the nucleus begins to move within the cytoplasm and is eventually discarded.41 Similar events occur in primitive erythroid cells as they circulate in the embryonic bloodstream. They continue to divide26,27 and accumulate hemoglobin until E13.5.14 They mature from proerythroblasts at E8.5 to orthochromatic erythroblasts at E12.5 to E15.5 (Figure 1; and data not shown). Their nuclei progressively condense (Figure 1) and begin to move within the cell coincident with the loss of vimentin intermediate filaments.27 Here, we show that murine primitive erythroblasts, like their definitive counterparts, ultimately enucleate during terminal differentiation.
The third phase of definitive erythropoiesis in mammals is characterized by the circulation of enucleated erythrocytes (Figure 4). The maintenance of primitive red cell numbers between E12.5 and E16.5 suggests that enucleating primitive erythroblasts are not lost from the circulation. Primitive red cells are still present in the bloodstream as late as 5 days after birth, indicating that primitive erythrocytes circulate for several days after enucleating. These findings support the concept that enucleation is a normal developmental process during primitive erythroid differentiation. It is likely that this third phase of primitive erythropoiesis has not been recognized because primitive red cells are relegated to a minor component of the red cell mass by the massive influx of definitive red cells during late gestation (Figure 3B).
The mature red cells of nonmammalian vertebrates are distinguished from mammalian red cells, not only by the retention of a nucleus, but also by the presence of marginal bands and intermediate filaments. In contrast, definitive erythrocytes of almost all mammals, with the exception of the immature red cells of camels,42 lack these structural features. Although marginal bands have been detected in the primitive red cells of marsupials,23 it is not clear whether true circumferential marginal bands exist in the primitive erythroblasts of mice.43,44 Consistent with their enucleation, murine primitive erythroblasts lose intermediate filaments during their maturation in the bloodstream.27 These features further support the concept that the primitive erythroid lineage in mice shares many similarities with the definitive erythroid lineages of mammals when compared with the red cell lineages of nonmammalian vertebrates.
Although primitive and definitive erythropoiesis in mammals share many characteristics, they are distinguished by differences in transcriptional regulation (eg, c-myb), cell size, and types of hemoglobin accumulated. We conclude that another distinguishing feature of primitive erythropoiesis in mice is not the retention of a nucleus, but rather that primitive red cells mature in the bloodstream (Figure 4). This is in striking contrast to definitive erythroblasts that mature extravascularly within erythroblast islands in close association with macrophage cells and stromal elements.45,46 The mechanisms that regulate primitive erythroid maturation in the absence of stromal interactions are not currently known. The ability to obtain circulating primitive erythroblasts at progressive stages of maturation will aid in better understanding the molecular underpinnings of mammalian red blood cell maturation and the mechanisms directing enucleation.
Prepublished online as Blood First Edition Paper, March 18, 2004; DOI 10.1182/blood-2003-12-4162.
Supported by the National Institutes of Health (NIH) and the University of Rochester School of Medicine and Dentistry.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Peter Keng of the University of Rochester Cancer Center for his kind assistance with the Coulter Counter analysis and Anne Koniski for exemplary animal husbandry. We also thank members of the laboratory, as well as Bill Cohen and Rick Waugh, for helpful discussions and critical comments.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal