Abstract
Umbilical cord blood (UCB) is an attractive cell source for hematopoietic cell transplantation (HCT). Here we examine whether the combination of homeobox B4 (HOXB4) and Delta-1 ligand (DL) synergize when used together. Monkey and human UCB CD34+ cells were transduced with a HOXB4-expressing gammaretroviral vector and cultured with DL. Individual and combined effects of HOXB4 and DL were assessed by colony-forming unit assays, flow cytometry, and nonobese diabetic/severe combined immune deficienct mouse transplantation. The presence of DL yielded higher percentage of CD34+ and CD7+ cells and lower percentages of CD14+ cells than non-DL cultures. Furthermore, HOXB4 yielded higher percentages of CD34+ and CD14+ cells than non-HOXB4 cultures. Interestingly, coculture with DL-expressing OP9 cells resulted in better maintenance of HOXB4 than culture in DL-conditioned medium. Culture of HOXB4-transduced human cells in the presence of DL yielded enhanced generation of repopulating cells with higher levels of engraftment of human CD45+, CD34+, CD3+, CD20+, and CD41+ cells compared with either factor individually. Our results demonstrate enhanced generation of hematopoietic progenitors by combining HOXB4 and DL; addition of DL further enhances expansion of multipotent cells capable of repopulating lymphoid and megakaryocyte lineages, which is not observed with HOXB4 alone.
Introduction
Umbilical cord blood (UCB) stem cell transplantation provides a treatment option for patients suffering from a wide variety of hematologic and nonhematologic malignancies. Rapid accessibility and a reduced risk of graft-versus-host disease are distinct advantages in the choice of UCB as a source of stem cells for hematopoietic cell transplantations (HCTs). However, cell dose remains the major drawback of cord blood transplantation, especially in adult patients and large pediatric patients. It is well documented that total nucleated cell (TNC) dose and cluster of differentiation (CD)34 cell dose are the most important predictors of cord blood transplant success1 ; research has therefore focused on overcoming the cell dose barrier, including ex vivo expansion of cord blood cells with the goal of generating clinically meaningful cell doses. Stem cell expansion strategies that focus solely on the use of cytokines have not shown significant expansion of repopulating cells, secondary to an increased rate of differentiation resulting in a loss of primitive cells. In short, these studies have not translated into improved engraftment in clinical trials.2-4 As an alternative, most centers now use double cord blood unit transplant strategies. Although this technique has helped overcome cell dose limitations, there continues to be delayed engraftment and immune reconstitution. Therefore, even with double unit transplants, there is still a need to achieve faster engraftment and potentially better immune reconstitution to minimize infectious complications. Delaney et al have shown remarkable success using Delta-1 ligand (DL) to ex vivo expand a single cord blood unit that is infused with a second, nonmanipulated unit in a double cord blood transplant setting. Importantly, a significant reduction in the time to neutrophil engraftment was observed in patients receiving DL cultured cells,5 demonstrating the promise of manipulation of regulators of stem cell fate such as homeobox B4 (HOXB4) and the Notch signaling pathway to enhance the in vitro generation of hematopoietic repopulating cells.
We have recently shown a differential effect of HOXB4 overexpression on short- and long-term repopulating cells in vivo. Using a competitive repopulation assay in a large animal model (Macaca nemestrina), we found that HOXB4 overexpression resulted in superior engraftment over non-HOXB4 controls.6 Interestingly, HOXB4 appears to have the most dramatic effect on short-term repopulating cells, resulting in 56-fold higher short-term engraftment compared with control-transduced cells. This offers promise in the field of cord blood transplantation; HOXB4 expansion of a portion of a graft may promote short-term engraftment and provide hematopoietic rescue while awaiting engraftment of long-term repopulating cells. Furthermore, we have also demonstrated a differential effect of HOXB4 on cells from different species.7
Likewise, it has been shown that culturing cord blood cells in the presence of the Notch ligand DL has a pronounced effect on generation of CD34+ cells with nonobese diabetic (NOD)/severe combined immune deficiency (SCID) repopulating ability, and on generation of lymphoid precursors. La Motte-Mohs et al8 found that culturing human cord blood cells on DL-expressing OP9 cells leads to increased T-cell lineage commitment and sustained T-cell differentiation. Delaney et al9 showed that there is a dose-depended effect of DL on differentiation and in vivo repopulating ability of human cord blood cells cultured on an immobilized layer of DL. Recently, Hutton et al10 were able to generate a population of regulatory T cells from human cord blood by adding interleukin (IL)–2 to cord blood cells cocultured on DL-expressing OP9 cells. In addition, clinical trials conducted by Delaney et al5 have recently shown increased kinetics of neutrophil engraftment when cord blood progenitors were expanded in the presence of DL. Thus, exploitation of the Notch signaling pathway allows for manipulation of hematopoietic progenitors.
Based on the results of previous research involving HOXB4 and DL, we have hypothesized that there may be a synergistic effect when these 2 agents are used together. It is known that HOXB4 preferentially expands cells of the myeloid lineage, while DL preferentially expands cells of the lymphoid lineage. Therefore, we have studied the effects of HOXB4 in conjunction with DL on both macaque cord blood cells and human cord blood cells, to determine whether a cooperative effect exists.
Methods
Experimental design
Two different sets of studies were carried out to assess the combined effects of HOXB4 and DL on cord blood cells: in vitro studies and in vivo studies. One objective of the in vitro studies was to determine the effects of this expansion technique on macaque cord blood CD34+ cells, with the ultimate goal of developing a nonhuman primate transplantation model. Three independent experiments (using 3 separate cord blood units) showed that macaque cord blood CD34+ cells did not respond to DL in the form of an immobilized protein; thus, in vitro experiments compared DL in the form of conditioned media from DL-expressing OP9 cells and coculture on a feeder layer of DL-expressing OP9 cells, both of which had a measurable effect on macaque CD34+ cells.
In vivo NOD/SCID transplantation studies were conducted using human cord blood CD34+ cells only, both because of the ease of obtaining samples and the higher number of cells per unit compared with macaque cord blood collections. A total of 25 mice were transplanted in this study. Five mice each were randomly assigned to one of the following treatment groups: no expansion, expansion without DL, expansion with 2.5 μg/mL DL, expansion with HOXB4 and no DL, and expansion with HOXB4 and 2.5 μg/mL DL. In these studies, we bound a fusion protein consisting of the extracellular domain fused with the fragment crystallizable domain of human immunoglobulin G1 (IgG1; DLext-IgG) to the surface of tissue culture wells using the concentration 2.5 μg/mL previously shown optimal for human cord blood cells.9 We did, however, use a combination of cytokines previously optimized for growth of macaque cells that expressed HOXB4. DL exposure was in the form of 2.5 μg/mL of immobilized DL protein (empirically determined by previous studies to yield significant enhancement of repopulating ability9 ), which facilitates a higher purity, simpler, more ideal culture system than those involving conditioned media or coculture. These studies are summarized in Table 1.
Summary of conditions tested during in vitro and in vivo experiments
| . | In vitro studies . | In vivo studies . |
|---|---|---|
| Cell species | (a) Human | Human |
| (b) Macaque | ||
| Conditions compared | (a) Combination of HOXB4 + DL versus HOXB4 alone and DL alone | Combination of HOXB4 + DL versus HOXB4 alone and DL alone |
| (b) Conditioned media versus coculture | ||
| DL form | (a) Conditioned media from DL-expressing OP9 cells | 2.5 μg/mL immobilized DL protein |
| (b) Coculture on feeder layer of DL-expressing OP9 cells | ||
| Percent CD34 purity after enrichment | (a) Human: average purity of 90% (n = 3) | Human: average purity of 87% (n = 5) |
| (b) Macaque: average purity of 94% (n = 3) |
| . | In vitro studies . | In vivo studies . |
|---|---|---|
| Cell species | (a) Human | Human |
| (b) Macaque | ||
| Conditions compared | (a) Combination of HOXB4 + DL versus HOXB4 alone and DL alone | Combination of HOXB4 + DL versus HOXB4 alone and DL alone |
| (b) Conditioned media versus coculture | ||
| DL form | (a) Conditioned media from DL-expressing OP9 cells | 2.5 μg/mL immobilized DL protein |
| (b) Coculture on feeder layer of DL-expressing OP9 cells | ||
| Percent CD34 purity after enrichment | (a) Human: average purity of 90% (n = 3) | Human: average purity of 87% (n = 5) |
| (b) Macaque: average purity of 94% (n = 3) |
Cell sources
Human umbilical cord blood cells were collected from normal, full-term deliveries under an approved Fred Hutchinson Cancer Research Center Institutional Review Board protocol.
Nonhuman primate cord blood cells were obtained from pig-tailed macaques (Macaca nemestrina). All pig-tailed macaques were housed at the University of Washington National Primate Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. Experimental protocols were approved by the Institutional Animal Care and Use Committee. Approximately 1 week before delivery date, routine hysterotomy was performed through a ventral midline incision of the abdomen cranial to the pelvic rim. The uterus was exteriorized and the placental discs were located by palpation; the uterus was then incised along the longitudinal direction of the muscle between the discs. Umbilical cord blood and circulating fetal blood were collected by catheter placed into the carotid artery of the fetus, and saline was flushed through a catheter placed into the jugular vein to ensure maximal collection of cells. The fetus and placenta were then removed, the uterus was closed with absorbable sutures, and the abdomen was closed with a multilayered closure.
CD34+ enrichment
Red cells from human and macaque umbilical cord blood were lysed in ammonium chloride red cell lysis buffer. Nucleated cells were incubated for 20 minutes with the 12.8 IgM anti-CD34 antibody,11 washed, and incubated for another 20 minutes with MACS IgM microbeads (Miltenyi Biotec). CD34+ cells were enriched via magnetic column separation. Overall, samples ranged in purity from 80% to 99% CD34+ by flow cytometry, with averages for each experiment in the range of 87% to 94%. This data can be found in Table 1.
HOXB4 vector production and transduction
Construction of the HOXB4 green fluorescent protein (GFP) vector has been described previously.12 The vector was used to transiently transfect 293T-based Phoenix-RD114 packaging cells. Virus-containing medium from these cultures was used to transduce Phoenix-gibbon ape leukemia virus packaging cells as described.13 After selection of a high-titer clone, virus-containing media consisting of Dulbecco modified Eagle medium with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin was collected every 12 hours, sterile filtered, and frozen at −80°C. Titering on HT1080 cells revealed that titers were in the range of 1 × 105 to 3 × 105 infectious units per milliliter.
Transductions were carried out on fibronectin-coated, nontissue, culture-treated plates. Cells were prestimulated for 48 hours. After 48 hours, cells were exposed to virus-containing media for two 4-hour transductions (1 exposure per day for 2 consecutive days). Cells were transduced at a multiplicity of infection of 0.3 (previously determined to cause minimal toxicity).
DL conditioned media and OP9 coculture
DL-expressing OP9 cells were kindly provided by Dr J. C. Zuñiga-Pflücker (Department of Immunology, University of Toronto, Toronto, ON) .14 For conditioned media experiments, DL-expressing OP9 cells were grown to approximately 80% confluence in Alpha Minimum Essential Medium supplemented with 20% FBS and 1% penicillin/streptomycin. Media was then replaced with Iscove Modified Dulbecco Medium supplemented with 10% FBS and 1% penicillin/streptomycin. Every 48 hours, conditioned media was removed from the OP9 cells and replaced with fresh media. Conditioned media was filtered through a 0.22-μm sterile filter and immediately used as culture media for cord blood cells. Conditioned media obtained from non–DL-expressing OP9 cells was used for control experiments. For coculture experiments, DL-expressing OP9 cells at approximately 80% confluence were used as a feeder layer for direct contact coculture of cord blood cells in Iscove Modified Dulbecco Media, 10% FBS, and 1% penicillin/streptomycin. Coculture on non–DL-expressing OP9 cells was used for control experiments.
For culture of cord blood CD34+ cells (in conditioned media experiments and coculture experiments), the following growth factors were added at a concentration of 100 ng/mL: IL-3, IL-6, thrombopoietin, feline McDonough sarcoma-like tyrosine kinase receptor-3 ligand, stem cell factor, and granulocyte-colony stimulating factor. We have selected this cocktail of growth factors based on results from previous studies. Cultures were split as necessary to maintain cell concentrations in the range of 1 × 105 to 5 × 105 cells/mL.
Generation and immobilization of DL protein
Production of the construct encoding the extracellular fraction of DL fused to the fragment crystallizable domain of human IgG1, electroporation of NSO cells with this construct, and purification of DL protein from culture media were carried out in the Bernstein laboratory of Fred Hutchinson Cancer Research Center and have been detailed previously.15 Nontissue, culture-treated plates were coated with either 2.5 μg/mL of DL or 2.5 μg/mL of control human IgG, as well as 5 μg/mL of fibronectin fragment CH-296, and incubated overnight at 4°C. The following morning, plates were washed with phosphate buffered saline and blocked with 2% bovine serum albumin solution.
CFU assays
Colony forming unit (CFU) assays were carried out in 2-layer agarose in Alpha Minimum Essential Medium, supplemented with 20% FBS and 100 ng/mL erythropoietin, IL-3, IL-6, thrombopoietin, stem cell factor, granulocyte-colony stimulating factor, and granulocyte-macrophage colony stimulating factor. Plates were incubated at 37°C for 12 to 14 days; after this period, colonies of greater than 50 cells were enumerated.
Flow cytometry
Flow cytometric data were collected using a Canto I (Becton Dickinson) and analyzed using FlowJo Version 8.8.7 software (TreeStar). At least 10 000 events were collected for each sample. Samples were analyzed for expression of the GFP marker (as an indicator of HOXB4 expression), as well as for CD3, CD7, CD14, CD20, CD34, CD41, and CD45. Nontransduced cells were used as a control for the gating of HOXB4GFP positive cells, and isotype control antibodies were used as a control for gating of positive populations among antibody-labeled cells. All antibodies were purchased from Becton Dickinson.
NOD/SCID transplantation
A total of 25 NOD/SCID gamma (NSG) mice were transplanted in this study; 5 mice were transplanted per each of 5 experimental groups. After irradiation with 275 cGy total body irradiation, mice were transplanted with cells from one of the following 5 categories: (1) cryopreserved, nonexpanded cells (designated as “no expansion”); (2) cells cultured for 9 days on IgG-coated plates (designated as “0 μg/mL Delta”); (3) cells cultured on DL-coated plates (designated as “2.5 μg/mL Delta”); (4) cells transduced with HOXB4 and cultured on IgG-coated plates (designated as “HOX + 0 μg/mL Delta”); or (5) cells transduced with HOXB4 and cultured on DL-coated plates (designated as “HOX + 2.5 μg/mL Delta”). Each mouse received the progeny of 4 × 105 CD34+ cells. Thus, for mice that fall into the “no expansion” category, only 4 × 105 cells were transplanted. Mice in the other experimental groups received cells that were expanded for 9 days from the original 4 × 105 cells. Therefore, mice in groups 2 through 5 received many more cells than those received by the mice of group 1, yet these grafts were also much lower in CD34 purity due to the 9 days of ex vivo culture. Nonlethal bone marrow aspirates were obtained at weeks 4 and 8; mice were humanely killed and bone marrow was harvested at week 12.
Results
In vitro expansion of human and macaque cells
To determine the individual and combined effects of HOXB4 and DL on cord blood, cells from human and macaque units were expanded for 2 weeks under different treatment conditions. The data shown in Figure 1 represents the mean of 3 independent experiments using human cord blood cells and 3 independent experiments using macaque cord blood cells. It should be noted that 3 different controls were tested: (1) fresh, nonconditioned media, (2) conditioned media from control OP9 cells that did not express DL, and (3) coculture on control OP9 cells. There were no significant differences among these 3 controls in any of the assays carried out; therefore, for simplicity, we have used the generalized term “control” during discussion of the results. Expansion of colony forming units was determined by multiplying the percent plating efficiency by the total number of cells generated by each culture treatment.
Expansion of total nucleated cells, CD34+ cells, and CFUs under different treatment conditions over 2 weeks. Results are shown for human cord blood cells (left panel) and macaque cord blood cells (right panel). Treatment conditions include: (I) control, (II) DL-conditioned media, (III) DL coculture, (IV) HOXB4, (V) HOXB4 + DL-conditioned media, (VI) HOXB4 + DL coculture. Data shown represent the mean of 3 independent experiments. *P < .05
Expansion of total nucleated cells, CD34+ cells, and CFUs under different treatment conditions over 2 weeks. Results are shown for human cord blood cells (left panel) and macaque cord blood cells (right panel). Treatment conditions include: (I) control, (II) DL-conditioned media, (III) DL coculture, (IV) HOXB4, (V) HOXB4 + DL-conditioned media, (VI) HOXB4 + DL coculture. Data shown represent the mean of 3 independent experiments. *P < .05
Transduction with HOXB4 significantly increased human nucleated cell expansion, CD34 expansion, and CFU expansion (P < .05). The highest expansion of human nucleated cells, CD34+ cells, and CFUs was seen when HOXB4 and DL coculture were used together, resulting in a fold expansion of 1930 ± 293 nucleated cells, 757 ± 75 CD34+ cells, and 185 ± 23 CFUs over 2 weeks. Transduction with HOXB4 significantly increased macaque nucleated cell expansion (P < .05), and increased CD34 expansion and CFU expansion (although the latter 2 differences were not significant). The highest expansion of macaque nucleated cells, CD34+ cells, and CFUs was seen when HOXB4 and DL-expressing OP9 coculture were used together, resulting in a fold expansion of 1050 ± 171 nucleated cells, 105 ± 9 CD34+ cells, and 25 ± 4 CFUs over 2 weeks.
Phenotypic analysis of in vitro cultures
Flow cytometric analysis of both human and macaque cells consistently showed that CD34 expression was higher in cells transduced with HOXB4 than nontransduced cultures. Among the different DL exposures, cells exposed to DL through coculture consistently showed higher CD34 expression than cells cultured with DL conditioned media, which in turn showed higher expression than cultures with no DL exposure. Figure 2 shows CD34 expression on macaque cord blood cells after 2 weeks of culture. CD34 expression of human cells (although not shown) followed the same pattern as that of macaque cells.
Flow cytometric analysis of CD34, CD7, and CD14 expression on macaque cord blood cells following 2 weeks of culture under different conditions. Data from 1 representative experiment is shown. The highest level of CD34 expression is seen when DL coculture and HOXB4 are used together. Cells cultured in direct contact with DL-expressing OP9 cells show the highest level of CD7 expression; addition of HOXB4 does not appear to affect CD7 expression. Furthermore, cells cultured with DL conditioned media show greater inhibition of myeloid differentiation than control cultures, but not as high as cells cultured in direct contact with DL-expressing OP9 cells.
Flow cytometric analysis of CD34, CD7, and CD14 expression on macaque cord blood cells following 2 weeks of culture under different conditions. Data from 1 representative experiment is shown. The highest level of CD34 expression is seen when DL coculture and HOXB4 are used together. Cells cultured in direct contact with DL-expressing OP9 cells show the highest level of CD7 expression; addition of HOXB4 does not appear to affect CD7 expression. Furthermore, cells cultured with DL conditioned media show greater inhibition of myeloid differentiation than control cultures, but not as high as cells cultured in direct contact with DL-expressing OP9 cells.
Among the different DL exposures, DL coculture yielded the highest level of CD7 expression, followed by DL conditioned media, and lastly by cultures with no DL exposure. This trend was evident with both human cord blood cells and macaque cord blood cells. There was no obvious trend regarding CD7 expression levels of HOXB4-transduced cells versus nontransduced cells. Figure 2 shows a representative example of CD7 expression on macaque cord blood cells after 2 weeks of culture.
For both human and macaque cord blood cells, CD14 expression was higher among HOXB4-transduced populations than among nontransduced populations for each DL exposure. Among the different DL exposures, DL coculture resulted in the lowest level of CD14 expression, followed by cells cultured in DL conditioned media. Cells with no DL exposure showed the highest level of CD14 expression. Figure 2 depicts CD14 expression on macaque cord blood cells after 2 weeks; however, this trend was observed in both species.
HOXB4 expression of in vitro cultures
Interestingly, HOXB4 expression (as measured by percentage of GFP+ cells) appears to have been better maintained in cord blood cells cultured in direct contact with DL-expressing OP9 cells than in conditioned media cultures or cultures with no DL exposure. This phenomenon was seen with both human cells and macaque cells. A representative example from a culture of human cord blood cells is shown in Figure 3. The figure shows that after 2 weeks, HOXB4 expression in cultures of transduced cells with no DL exposure (Figure 3A left) and transduced cells with DL conditioned media (Figure 3A middle) was approximately 25% to 26%. However, in cells that were transduced and cocultured on DL expressing OP9 cells (Figure 3A right), approximately 42% of cells expressed HOXB4. Figure 3B confirms that this observation was not seen with conditioned media or coculture from non–DL-expressing OP9 cells. Expression of HOXB4GFP in nontransduced cultures was ≤ 1% at all time points.
Flow cytometric analysis of HOXB4GFP expression on human cord blood cells following 2 weeks of culture under different conditions. Data from 1 representative experiment is shown. (A) A significantly higher percentage of transduced cells cocultured with DL-expressing OP9 cells show higher GFP expression than cells cultured in DL conditioned media or fresh media. (B) Confirmation that this phenomenon is not observed with control conditioned media or control coculture.
Flow cytometric analysis of HOXB4GFP expression on human cord blood cells following 2 weeks of culture under different conditions. Data from 1 representative experiment is shown. (A) A significantly higher percentage of transduced cells cocultured with DL-expressing OP9 cells show higher GFP expression than cells cultured in DL conditioned media or fresh media. (B) Confirmation that this phenomenon is not observed with control conditioned media or control coculture.
Engraftment and phenotypic analysis in the NOD/SCID model
To assess the repopulating ability of expanded cells, human cord blood cells cultured for 9 days were transplanted into sublethally irradiated mice. The culture duration of 9 days was selected because prior experiments (not shown) indicated that this length of culture yielded highest expansion of CD34+ cells without excessive differentiation. Over the 9 days of ex vivo culture, cord blood cells expanded on average approximately 34-fold in the 0 μg/mL DL group, 36-fold in the 2.5 μg/mL DL group, 43-fold in the HOXB4 + 0 μg/mL DL group, and 44-fold in the HOXB4 + 2.5 μg/mL DL group. To summarize, mice receiving expanded cells were transplanted with between 14 and 18 million cells each, depending which treatment method the cells were expanded under.
Engraftment of human cells as evidenced by percent GFP+, percent human CD45+, and percent CD45+GFP+ was highest when HOXB4 and immobilized DL were used together (Figure 4A-B). Cells expanded with HOXB4 + 2.5 μg/mL DL show a several-fold higher engraftment than cells that were not expanded at all. This finding is significant and shows that the combination of HOXB4 and DL exerts a powerful effect on engraftment potential, much more so than either factor used individually.
Expression of phenotypic markers after transplantation with human cord blood cells cultured in the presence of HOXB4 and DL. In general, engraftment of human cells as evidenced by percent GFP+ (A), percent CD45+ and CD45+GFP+ (B), and percent CD34+CD45+ and CD34+GFP+ (C) is highest when HOXB4 and DL are used in conjunction. n = 5 mice per experimental treatment, *P < .05
Expression of phenotypic markers after transplantation with human cord blood cells cultured in the presence of HOXB4 and DL. In general, engraftment of human cells as evidenced by percent GFP+ (A), percent CD45+ and CD45+GFP+ (B), and percent CD34+CD45+ and CD34+GFP+ (C) is highest when HOXB4 and DL are used in conjunction. n = 5 mice per experimental treatment, *P < .05
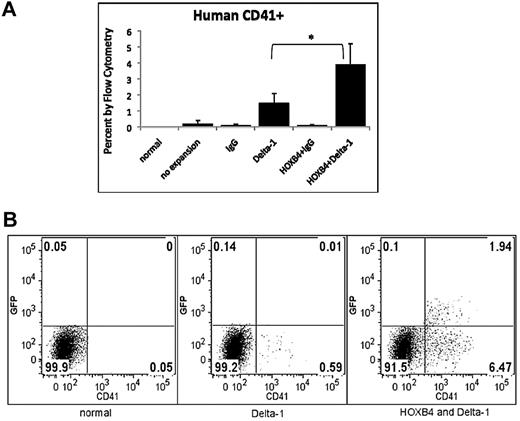
Figure 4C shows expression of human CD34+CD45+ and CD34+GFP+ on cells obtained from murine bone marrow harvests at weeks 4, 8, and 12 after transplant. In general, engraftment of human CD34+ cells was highest when HOXB4 and immobilized DL were used together. Analysis of lymphoid markers CD3 and CD20 showed that expression of these human markers was also greatest when HOXB4 and DL were used in conjunction (Figure 5A-B). In the majority of cases, addition of DL significantly (P < .05) increased differentiation into lymphoid lineages, compared with cultures with no DL exposure and only HOXB4. Figure 5C depicts the effect of different culture conditions on expression of the human myeloid marker CD14. In each case, addition of DL substantially inhibited the generation of differentiated CD14+ cells. Approximately half of the time, these differences were significant, as indicated in the figure. Interestingly, we found that DL promoted the generation of precursors that repopulate the megakaryocytic lineage (Figure 6). Human CD41+ cells were present at an average level of ≤ 0.1% in mice transplanted with cells having no DL exposure, but were present at an average level of 1.5% in mice transplanted with cells cultured with DL alone, and at an average level of 3.9% in mice transplanted with cells cultured with DL and HOXB4.
Expression of lymphoid and myeloid markers after transplantation with human cord blood cells cultured in the presence of HOXB4 and DL. In general, engraftment of human lymphoid cells (A-B) is highest when HOXB4 and DL are used in conjunction. DL has an inhibitory effect on CD14 expression (C). n = 5 mice per experimental treatment, *P < .05
Expression of lymphoid and myeloid markers after transplantation with human cord blood cells cultured in the presence of HOXB4 and DL. In general, engraftment of human lymphoid cells (A-B) is highest when HOXB4 and DL are used in conjunction. DL has an inhibitory effect on CD14 expression (C). n = 5 mice per experimental treatment, *P < .05
Generation of human CD41+ cells in NOD/SCID mice 12 weeks after transplantation with human cord blood cells cultured in the presence of HOXB4 and DL. Mice transplanted with cells cultured in the presence of DL show increased expression of CD41 megakaryocyte marker. (A) Data from 5 replications is represented as mean ± SD. Each bar represents the mean percent human CD41+ of the 5 mice within that group. *P < .05 (B) Flow cytometric data from 1 representative experiment is shown.
Generation of human CD41+ cells in NOD/SCID mice 12 weeks after transplantation with human cord blood cells cultured in the presence of HOXB4 and DL. Mice transplanted with cells cultured in the presence of DL show increased expression of CD41 megakaryocyte marker. (A) Data from 5 replications is represented as mean ± SD. Each bar represents the mean percent human CD41+ of the 5 mice within that group. *P < .05 (B) Flow cytometric data from 1 representative experiment is shown.
Discussion
Here we have studied the effects of HOXB4 in combination with DL on cord blood expansion and found a favorable effect on stem cells from 2 species: human and pigtailed macaque. Using conditions optimized for expansion of HOXB4 expressing macaque cells, we found that activation of the Notch pathway by exposure to DL in cells transduced with HOXB4: (1) yield higher expansion of TNCs and CFUs, (2) promote greater levels of engraftment in a xenotransplant model, (3) result in better maintenance of the CD34 phenotype, (4) lead to enhanced lymphoid differentiation in vivo, and (5) promote a higher degree of differentiation into the CD41 phenotype compared with the effects of either factor used alone.
These studies were performed with the purpose of developing a macaque cord blood transplant model. Thus, the conditions used were based on previous studies with HOXB4-transduced macaque cells and may not be optimal for human cells. Nonetheless, the data provided here demonstrate that DL can further enhance the expansion of HOXB4-transduced hematopoietic repopulating cells, which can be explored in preclinical transplant studies in nonhuman primates. Likewise, we understand that there are inherent risks in using retrovirally transduced cells in a clinical setting16 ; however, we stress that our studies here were initiated with the goal of developing a model, in which variables can later be altered as needed. For example, future experiments will examine safer and more clinically relevant conditions such as HOXB4 protein-mediated expansion.17
Our in vitro data consistently show that culturing cells on a feeder layer of DL-expressing OP9 cells results in greater expansion, higher CD34 and CD7 expression, and increased inhibition of CD14 differentiation, compared with culturing cells using DL conditioned media. Although worth noting, this is not particularly surprising, as several others have documented increased performance of cells when using direct cell–to–cell contact instead of conditioned media.6,18 It is well documented that the Notch signaling pathway plays a pivotal role during various stages of T-cell development,19-21 which accounts for the increased level of CD7 expression with increasing contact with the DL during in vitro experiments. It appears that the addition of HOXB4 does not have any particular effect on CD7 differentiation (Figure 2). However, it is known that HOXB4 preferentially expands cells of the myeloid lineage,17,22 while DL tends to inhibit myeloid differentiation.9 Our data suggest that the inhibitory effect of DL is more pronounced than the stimulatory effect of HOXB4, as evidenced in Figure 2.
An unexpected observation was the realization that cord blood cells of both species cultured on DL-expressing OP9 cells show better maintenance of HOXB4 (as evidenced by expression of the GFP reporter gene) than either HOXB4 control cells or HOXB4 cells grown in DL conditioned media. Cells in coculture routinely showed HOXB4GFP expression of 15 to 20 percentage points higher than noncoculture cells; mean fluorescence intensities were not significantly different. This may be because cells cultured on a feeder layer of DL-expressing OP9 cells are likely exposed not only to soluble DL, but membrane-bound DL as well, thus accounting for the differences observed. Of course, we cannot rule out that other factors, independent of DL, could also have contributed to the improved results with OP9 coculture. The increased maintenance of transgene expression is of particular value in circumstances where high gene marking is necessary, such as in transplantation studies involving genetically manipulated cells.
Translational NOD/SCID studies yielded results similar to in vitro findings. An interesting observation is that the percentage of HOXB4GFP+ cells at week 4 (Figure 4A) is higher than the percentage of CD45+GFP+ cells (Figure 4B), thus suggesting that there are approximately 1% GFP+ cells in circulation that are not CD45+. Backgating analysis on this population revealed it to be composed of lymphocyte-like cells; we suspect that these are normal hematopoietic cells that did not stain positive with the CD45 antibody, likely due to technical issues.
Figure 4 shows that engraftment was highest at week 4, and decreased with time. Although we cannot exclude the possibility that our in vitro manipulations adversely affected the long-term engraftment potential of the CD34+ cells, it is more likely that this phenomenon is due to endogenous hematopoietic competition, perhaps as a result of sublethal irradiation.23 This also accounts for the fact that the decrease in chimerism is consistent across all treatments, including nonmanipulated controls.
Furthermore, the inclination of DL to promote differentiation into the CD3+ lineage is evidenced in Figure 5A by looking at relative contributions of transduced cells to the overall CD3+ population. For example, in mice transplanted with cells expanded with HOXB4 only, approximately 1 in 10 CD3+ cells arise from HOXB4GFP+ cells. But in mice transplanted with cells expanded with HOXB4 and DL, nearly all CD3+ cells arise from HOXB4GFP+ cells. Thus, the levels of GFP expression are nonuniform among various lineages, and show distinct differentiation tendencies.
It is worth remarking on the observation that, although the percentage of HOXB4GFP+ cells in circulation in NOD/SCID mice is small (1%-3%), the contribution of human hematopoietic CD45+ cells is large (20%-40%). Although possible, it is unlikely that this effect is due to gene silencing, as we (and others) have worked with this viral vector in vitro and in vivo in various species, and gene silencing has never been observed. A more plausible explanation is that we have illustrated a noncell-autonomous effect, whereby genetically mutant HOXB4-overexpressing cells are causing other (nonmutant) cells to exhibit the mutant phenotype (which, in this case, is an increased rate of proliferation). Another interesting observation is that the most compelling evidence of DL effects in HOXB4 expressing cells emerges in vivo, rather than in vitro. These observations suggest the possibility that DL activation of Notch signaling effects engraftment by impeding differentiation of multipotent precursors capable of in vivo repopulation.
Furthermore, our data show that inclusion of DL promotes development of megakaryocytes (Figure 6). Mercher et al also noted this phenomenon; this group unexpectedly observed megakaryocyte development when culturing hematopoietic stem cells on feeder layers of DL-expressing OP9 cells.24 Here we show that megakaryocyte development in vivo is increased when cells are cultured on an immobilized layer of DL protein before transplant. Furthermore, cells cultured in the presence of both DL and HOXB4 show the greatest level of CD41 expression. Interestingly, we routinely observed that only approximately 15% to 20% of the CD41+ cells were GFP+ in each mouse receiving cells expanded with the HOXB4/DL combination. The CD41 antigen is expressed on cells during a broad period of development: from the CD34+ megakaryocyte progenitor, through maturation from megakaryocytes into proplatelets, and finally into platelets.25 It is not unusual to observe lower GFP expression in platelets than other subsets,26 providing evidence that GFP expression wanes as cells near their terminal differentiation into platelets. Thus, it is possible that a high proportion of late-stage CD41+ cells (GFP– or GFPlow) is driving down the overall GFP+ percentage in these mice. Our finding of increased megakaryocyte production with HOXB4 and DL is especially important in the field of cord blood stem cell transplantation, which is characterized by delayed platelet engraftment.27 Expansion of these cells under the influence of HOXB4 and DL before transplant may offer promise for improved platelet recovery kinetics.
We have used immobilized DL for the NOD/SCID studies to evaluate a potential synergism using an already clinically established protocol.5 However, it is important to mention that the best in vitro data were obtained when DL-expressing OP9 cells were used, and thus the delivery of protein by OP9 cells may provide some additional benefits. To our knowledge, this is the first report of the combined effects of HOXB4 and DL on cord blood stem cells. These 2 factors, individually, have shown remarkable potential for manipulating the expansion and differentiation of hematopoietic stem cells. We have now shown that these factors also collaborate to produce levels of expansion and phenotypic expression much higher than can be produced by either factor alone in nonhuman primates. These studies provide a model for further exploration of the interaction between HOXB4 and DL in transplants in the macaque model and provide a basis for further studies in human cord blood expansion.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Core Center for Excellence in Hematology (DK56465) for assistance with the NOD/SCID studies and vector productions. We also thank Drs Beverly Torok-Storb, Shelley Heimfeld, and Barry Storer for assistance in data interpretation and presentation.
This work was supported by National Institutes of Health grants R01HL08434, R01HL080245, R24HL074445, and P30DK056465. C.D. is a Damon Runyon Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI no. 35-07). I.D.B. is an American Cancer Society–F. M. Kirby Clinical Research Professor. H.-P.K is a Markey Molecular Medicine Investigator and the recipient of the Jose Carreras/E. Donnall Thomas Endowed Chair for Cancer Research.
National Institutes of Health
Authorship
Contribution: K.L.W. designed and performed research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript; C.D. contributed vital materials; R.K.H. contributed vital materials and interpreted data; I.D.B. contributed vital materials; and H.-P.K. designed research and analyzed and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hans-Peter Kiem, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, D1-100, Seattle, WA 98109-1024; e-mail: hkiem@fhcrc.org.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal