Abstract
Abstract 91
In normal and leukemic hemopoiesis, stem cells differentiate through intermediate progenitors into terminal cells. In human Acute Myeloid Leukemia (AML), there is uncertainty about: (i) whether there is more than one leukemic stem cell (LSC) population in any one individual patient; (ii) how homogeneous AML LSCs populations are at a molecular and cellular level and (iii) the relationship between AML LSCs and normal stem/progenitor populations. Answers to these questions will clarify the molecular pathways important in the stepwise transformation of normal HSCs/progenitors.
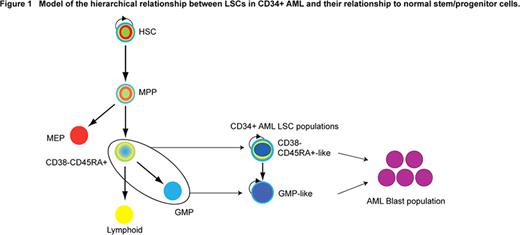
We have studied 82 primary human CD34+ AML samples (spanning a range of FAB subtypes, cytogenetic categories and FLT3 and NPM1 mutation states) and 8 age-matched control marrow samples. In ∼80% of AML cases, two expanded populations with hemopoietic progenitor immunophenotype coexist in most patients. One population is CD34+CD38-CD90-CD45RA+ (CD38-CD45RA+) and the other CD34+CD38+CD110-CD45RA+ (GMP-like). Both populations from 7/8 patients have leukemic stem cell (LSC) activity in primary and secondary xenograft assays with no LSC activity in CD34- compartment. The two CD34+ LSC populations are hierarchically ordered, with CD38-CD45RA+ LSC giving rise to CD38+CD45RA+ LSC in vivo and in vitro. Limit dilution analysis shows that CD38-CD45RA+LSCs are more potent by 8–10 fold. From 18 patients, we isolated both CD38-CD45RA+ and GMP-like LSC populations. Global mRNA expression profiles of FACS-sorted CD38-CD45RA+ and GMP-like populations from the same patient allowed comparison of the two populations within each patient (negating the effect of genetic/epigenetic changes between patients). Using a paired t-test, 748 genes were differentially expressed between CD38-CD45RA+ and GMP-like LSCs and separated the two populations in most patients in 3D PCA. This was confirmed by independent quantitative measures of difference in gene expression using a non-parametric rank product analysis with a false discovery rate of 0.01. Thus, the two AML LSC populations are molecularly distinct.
We then compared LSC profiles with those from 4 different adult marrow normal stem/progenitor cells to identify the normal stem/progenitor cell populations which the two AML LSC populations are most similar to at a molecular level. We first obtained a 2626 gene set by ANOVA, that maximally distinguished normal stem and progenitor populations. Next, the expression profiles of 22 CD38-CD45RA+ and 21 GMP-like AML LSC populations were distributed by 3D PCA using this ANOVA gene set. This showed that AML LSCs were most closely related to their normal counterpart progenitor population and not normal HSC. This data was confirmed quantitatively by a classifier analysis and hierarchical clustering. Taken together, the two LSC populations are hierarchically ordered, molecularly distinct and their gene expression profiles do not map most closely to normal HSCs but rather to their counterpart normal progenitor populations.
Finally, as global expression profiles of CD38-CD45RA+ AML LSC resemble normal CD38-CD45RA+ cells, we defined the functional potential of these normal cells. This had not been previously determined. Using colony and limiting dilution liquid culture assays, we showed that single normal CD38-CD45RA+ cells have granulocyte and macrophage (GM), lymphoid (T and B cell) but not megakaryocyte-erythroid (MK-E) potential. Furthermore, gene expression studies on 10 cells showed that CD38-CD45RA+ cells express lymphoid and GM but not Mk-E genes. Taken together, normal CD38-CD45RA+ cells are most similar to mouse lymphoid primed multi-potential progenitor cells (LMPP) cells and distinct from the recently identified human Macrophage Lymphoid progenitor (MLP) population.
In summary, for the first time, we show the co-existence of LMPP-like and GMP-like LSCs in CD34+ AML. Thus, CD34+ AML is a progenitor disease where LSCs have acquired abnormal self-renewal potential (Figure 1). Going forward, this work provides a platform for determining pathological LSCs self-renewal and tracking LSCs post treatment, both of which will impact on leukemia biology and therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal