Abstract
Abstract 2040
Poster Board II-17
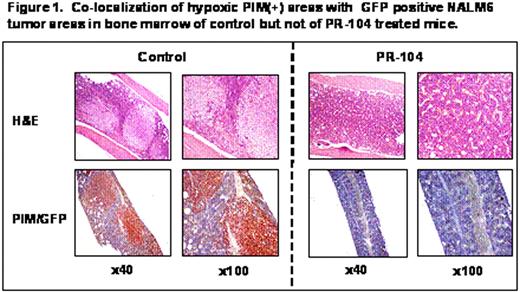
The main challenge in the treatment of acute lymphocytic leukemia (ALL) is overcoming resistance to chemotherapy. Recent studies indicate that interactions between leukemia cells and bone marrow (BM) microenvironment promote leukemia cell survival and confer resistance to drugs commonly used to treat ALL. We investigated whether hypoxia was a contributing factor in the protective role of the BM microenvironment. We found that the Hypoxia-Inducible-Factor 1a (HIF-1a; a marker normally expressed by only a few hematopoietic cells) was expressed in 68% of BM biopsies from patients with B-lineage ALL (n=53). Expression of HIF-1α detected either histochemically (n=53, p=0.023) or by Reverse Phase Protein Arrays (RPPA, n=116, p=0.0013) inversely correlated with survival of patients with newly diagnosed B-lineage ALL. Silencing of HIF-1α with siRNA, or blockade of mTOR signaling with rapamycin derivatives, reduced expression of the glucose transporter Glut-1, diminished glucose flux, decreased glycolytic rate and ATP production and sensitized leukemic cells to the pro-apoptotic effects of chemotherapeutic agents under hypoxic conditions. In line with this findings, we observed a marked expansion of hypoxic BM areas in immunodeficient mice engrafted with the ALL cell line Nalm6 or with primary ALL cells, as detected by administration of the reductive 2-nitroimidazole compound pimonidazole (PIM), which forms stable adducts in hypoxic regions. Altogether, these findings provided a rationale for examining the effects of hypoxia-activated pro-drugs or HIF-1a inhibitors to eliminate ALL progenitor cells within hypoxic niches. To this end, we tested PR-104, a hypoxia-activated dinitrobenzamide mustard currently undergoing Phase II trials in solid tumors. Under hypoxic conditions, this agent is reduced to hydroxylamine and amine metabolites that in turn induce DNA cross-links and cell death (Patterson et al., Clin Can Res 2007). In vitro, 25μM PR-104 induced hypoxia-selective cell death in Nalm6 ALL cells with 80% Annexin V-positivity at 0.1% O2, 46% at 1%O2 compared to 13% at 21%O2. The anti-leukemic efficacy of PR-104 was next examined in the in vivo leukemia models. Administration of PR-104 (250 mg/kg IP TIW for two weeks) prolonged survival of NOD/Scid/IL2Rg-KO (NOG) mice injected with cells from primary refractory FLT3-mutated AML, and decreased leukemia burden as indicated by histopathological analyses of CD45 positive cells in the BM, spleen, lung and liver. Notably, analysis of PIM distribution indicated clearance of the hypoxic leukemic niches. In NOG mice injected with leukemic cells from an infant with MLL-rearranged B-lineage ALL, PR-104 at 200 mg/kg IP on days 1, 2 and 6 resulted in a dramatic decrease in the percentage of circulating leukemic CD45+ cells on day 15 (control, 92%±6%; treated, 9%±4%; n=7 mice/group). The therapeutic effect of the drug was also tested in a Nalm6-luciferase ALL model where PR-104 administration resulted in decreased tumor burden as determined by luciferase activity and prolonged survival of the PR-104 treated as compared to control mice (p=0.006). Similar to the models of human leukemia, analysis of BM sections of control mice showed extensive areas of hypoxia (PIM-positive) in close proximity to GFP-positive leukemia cells in contrast to the treated mice in which only discrete areas of PIM positivity were detectable. Altogether, these findings strongly suggest that targeting hypoxia is feasible and may increase the sensitivity of ALL cells to chemotherapy. If successful, this approach of targeting hypoxic microenvironment, alone or in combination with other chemotherapeutic or targeted agents, may significantly impact ALL therapy and ultimately improve patient survival.
Co-localization of hypoxic PIM(+) areas with GFP positive HALMG tumor areas in bone marrow of control but not of PR-104 treated mice.
Co-localization of hypoxic PIM(+) areas with GFP positive HALMG tumor areas in bone marrow of control but not of PR-104 treated mice.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal