Abstract
Stromal-derived factor 1 (SDF-1) is a -CXC- chemokine that plays a critical role in embryonic and adult hematopoiesis, and its specific receptor, CXCR4, has been implicated in stem cell homing. In this study, it is shown that the addition of SDF-1 to long-term cultures (LTCs) of normal human marrow can selectively, reversibly, and specifically block the S-phase entry of primitive quiescent erythroid and granulopoietic colony-forming cells (CFCs) present in the adherent layer. Conversely, addition of anti–SDF-1 antibody or SDF-1(G2), a specific CXCR4 antagonist, to preactivated human LTCs prevented both types of primitive CFCs from re-entering a quiescent state, demonstrating that endogenous SDF-1 contributes to the control of primitive CFC proliferation in the LTC system. Interestingly, SDF-1 failed to arrest the proliferation of primitive chronic myeloid leukemia CFCs in the adherent layer of LTCs containing normal marrow stromal cells. In vivo, injection of SDF-1 arrested the cycling of normal human LTC-initiating cells as well as primitive CFCs in the marrow of nonobese diabetic/severe combined immunodeficient mice engrafted with human cord blood cells. Conversely, injection of the antagonist, SDF-1(G2), reactivated the cycling of quiescent primitive human CFCs present in the marrow of mice engrafted with human marrow cells. These studies are the first to demonstrate a potential physiological role of SDF-1 in regulating the cell-cycle status of primitive hematopoietic cells and suggest that the deregulated cycling activity of primitive chronic myeloid leukemia (CML) cells is due to the BCR-ABL–mediated disruption of a pathway shared by multiple chemokine receptors.
Introduction
The exogenous control of blood cell output in vivo is a highly complex process involving multiple positive and negative regulators. Many of these are cytokines with activities that are specific to particular lineages and stages of differentiation in the hematopoietic cell hierarchy. Together, the intracellular signaling mechanisms activated by these cytokines determine the proportion of cells in each compartment that remain viable, that proliferate, and that initiate and complete the various programs of hematopoietic differentiation. In the healthy adult, a majority of the most primitive hematopoietic cells are, at any given moment, quiescent.1-7 However, over time, all slowly and randomly enter the cell cycle and proliferate.3,8,9 In successively later compartments, the proportion of dividing cells increases progressively, as shown by studies of distinct subpopulations of in vitro and in vivo colony-forming cells (CFCs) and their precursors detected as long-term culture–initiating cells (LTC-ICs). The LTC system has been particularly useful for allowing the identification of particular cytokines that can either stimulate or inhibit the cell-cycle progression of primitive hematopoietic cells in a stage-specific fashion. In such cultures, the high proliferative potential (HPP)–CFCs in the adherent layer are maintained in a quiescent state unless the cultures are appropriately stimulated. In contrast, the low proliferative potential (LPP)–CFCs cycle continuously, thus mimicking the situation in normal adult marrow. Thus, each week when fresh medium containing horse serum is added to such LTCs, the prevailing endogenous negative signals active on these HPP-CFCs in the adherent layer are overcome; this results in a transient but pronounced activation of these cells into cycle that peaks 2 to 3 days later.10,11 In previous studies, we have identified a variety of cytokines whose addition to LTCs can have an effect similar to that of adding fresh horse serum, either because these cytokines can stimulate HPP-CFC proliferation directly or because they induce the secondary production of such factors.11-14Coincident addition of cytokines that block HPP-CFC activation in this system has similarly allowed various specific inhibitors of HPP-CFC mitogenesis to be identified. These include transforming growth factor β (TGF-β)11 and 2 members of the chemokine family: monocyte chemoattractant protein-1 (MCP-1)15 and macrophage inhibitory protein 1α (MIP-1α).16Furthermore, the addition of specific antibodies or antagonists of these inhibitors to LTCs 2 to 3 days after a half-medium change (when the activating stimuli begin to decline and inhibitors produced within the cultures begin to regain dominance) has shown that both TGF-β and MCP-1 (but not MIP-1α) are endogenously active in this system and that both are required to arrest the cycling of the primitive progenitors in the adherent layer. Thus, when either of these endogenous inhibitors is inactivated, the HPP-CFCs in the adherent layer fail to return to a quiescent state another 4 to 5 days later.11,15 Interestingly, the autonomously proliferating neoplastic (Ph+) HPP-CFCs produced from primitive chronic myeloid leukemia (CML) progenitors17 are insensitive to the cytostatic activity of either MIP-1α or MCP-1 in the LTC system,15,16 in spite of a normal responsiveness to TGF-β.18
More recently, we have examined the proliferative activity of primitive normal human progenitors in engrafted NOD/LtSz-scid/scid (NOD/SCID) mice and have used this model to test the ability of various cytokines to stimulate or inhibit the turnover of different normal human progenitor subsets in vivo.19-21 Up to 6 weeks after transplantation, all of the human CFCs and LTC-ICs present in the marrow of NOD/SCID mice that received human adult marrow or cord blood cell transplants proliferate rapidly. This situation persists for many months in the cord blood–engrafted mice. However, after 10 weeks, further proliferation of the human CFCs is markedly downregulated in human marrow–engrafted mice, even though the LTC-ICs continue to divide. These differences in the cycling behavior of human marrow–derived CFCs and LTC-ICs in this xenotransplant model suggest that LTC-ICs and CFCs are sensitive to different proliferation-control mechanisms. Subsequent studies showed this to be the case. Injections of TGF-β, MCP-1, and MIP-1α were all able to arrest the endogenously stimulated cycling of human CFCs and LTC-ICs in NOD/SCID mice that received transplants, but with different target cell specificities and TGF-β was the only cytokine that could inhibit the proliferation of human LTC-ICs.21
As a follow-up to these studies, we surveyed another 3 chemokines for their ability to selectively inhibit the cell-cycle progression of normal human HPP-CFCs in the LTC system. The 3 chemokines selected were stromal-derived factor 1 (SDF-1), hemofiltrate cc chemokine (HCC-1), and I-309. SDF-1 was chosen in part because its receptor, CXCR4, has been found on most types of primitive human hematopoietic cells.22-24 SDF-1 has also been found to be a potent inducer of primitive human hematopoietic cell migration in vitro and has been implicated in human stem cell homing in vivo.24-28 Evidence that SDF-1 can mobilize murine stem cells in vivo was also recently reported.29 However, the potential of this chemokine to regulate the proliferative status of primitive hematopoietic progenitors has not been previously investigated. HCC-1 was of interest because it is structurally related to MIP-1α and acts via receptors that also recognize MIP-1α.30 I-309 is another recently described chemokine31 whose action on hematopoietic cells has not been previously reported. On the basis of the positive results obtained in our initial studies, a series of additional experiments were then undertaken: (1) to investigate the sensitivity of primitive CML progenitors to SDF-1; (2) to determine whether this chemokine might also be active as an inhibitor in vivo in human cell engrafted–NOD/SCID mice; and (3) to explore its potential endogenous role as an inhibitor with the use of a specific anti–SDF-1 antibody and a specific SDF-1 antagonist we have recently developed.32
Materials and methods
Cytokines
Human TGF-β1, HCC-1, I-309, a neutralizing goat anti–SDF-1 antibody (15 to 30 μg/mL able to neutralize 100 ng/mL human SDF-1), and control goat immunoglobulin (Ig)–G were purchased from R&D Systems (Minneapolis, MN). SDF-1 and the antagonist, SDF-1(G2), were synthesized by stepwise solid-phase methods and purified as previously described.33 Purity of the products was assessed by ion-exchange high-pressure liquid chromatography and mass spectrometry. SDF-1(G2) is a variant of SDF-1 in which a glycine residue is substituted for the proline normally at position 2. This results in a complete loss of activity although the receptor-binding affinity of SDF-1(G2) is only approximately 3-fold less than that of SDF-1, thus making it an effective antagonist.32
Human cells
Cord blood was obtained with informed consent from the mothers of healthy, full-term infants. Normal adult human bone marrow cells were obtained as cadaveric marrow samples from the Northwest Tissue Center (Seattle, WA) or as leftover aspirate material from consenting donors of allogeneic marrow transplants harvested at our center. Peripheral blood cells were obtained from 7 patients with Ph+/BCR-ABL+ CML in chronic phase but with high white blood cell (WBC) counts (> 50 × 109/L [50 000/μL]) as discard material from samples taken for diagnostic or follow-up purposes. For experiments with normal cord blood or adult marrow, low-density (< 1.077 g/mL) cells were used either as fresh or as previously cryopreserved material. Low-density CML cells were further depleted of lineage-marker–positive cells34 and were stored frozen until required.
In vitro cytokine experiments
Four to 8 × 106 washed normal human marrow or CML blood cells were suspended in 2.5 mL myeloid LTC medium (MyeloCult; StemCell Technologies, Vancouver, BC, Canada) supplemented with 10−6 M freshly dissolved hydrocortisone (Sigma Chemicals, St Louis, MO); the cells were then placed into 35-mm tissue-culture dishes containing pre-established, irradiated adherent cell layers of normal human marrow as previously described.16 After an initial 7- to 10-day period of incubation at 33°C, half of the medium was replaced with fresh complete LTC medium and other agents, either simultaneously (cytokines) or 2 to 3 days later (antibodies or the SDF-1 antagonist). At the appropriate time, the nonadherent cells were removed and the adherent cells harvested enzymatically for assessment of the numbers and cycling activity of the CFCs present as described below.10
Enzyme-linked immunosorbent assay measurements of SDF-1 concentrations in LTC supernatants
Cells were removed from media harvested from LTCs that were being analyzed for progenitor cycling status, and the supernatants were stored frozen. SDF-1 concentrations were simultaneously determined on the thawed supernatants by means of a commercial enzyme-linked immunosorbent assay (ELISA) kit (Quantikine; R&D Systems) according to the manufacturer's directions.
Animals
NOD/SCID mice were bred and maintained in the animal facility of the British Columbia Cancer Research Centre (Vancouver, BC, Canada) from mice originally obtained from the Jackson Laboratories (Bar Harbor, ME). All animals were kept in microisolator units and provided with sterilized food and water. Mice were irradiated (350 cGy of137Cs γ-rays) at 6 to 8 weeks of age and then injected intravenously with human cells suspended in phosphate-buffered saline (PBS) (107 or 2 × 107 low-density, trypan blue–excluding cord blood or adult marrow cells per recipient mouse, respectively). Thereafter, the drinking water was acidified (pH = 3) and supplemented with 100 mg/L ciprofloxacin (Bayer, Leverkusen, Germany). At 6 to 8 weeks after transplantation, cord blood–engrafted mice were given 2 intraperitoneal injections, 24 hours apart, of 10 μg SDF-1 diluted in PBS (or an equal volume of PBS as a control). Similarly, bone marrow–engrafted mice were each injected twice with 10 μg SDF-1(G2), but not until 10 to 12 weeks after transplantation. At 1 day after the second injection, the mice were killed, and the marrow cells then removed from the 4 hind leg bones. Cell counts, phenotyping, and 3H-thymidine suicide assays were performed individually on the cells obtained from each mouse.
Flow cytometry
Cells removed from the mice were counted, subjected to red cell lysis by treatment with 8.3% ammonium chloride, and then washed in Hanks balanced salt solution (StemCell Technologies) with 2% fetal calf serum (HF/2). Nonspecific antibody binding was inhibited by first incubating the cells with human serum and 3 μg/mL anti–mouse IgG receptor antibody 2.4G2 for 10 minutes at 0°C to block Fc receptors.35 The cells were then incubated for 30 minutes at 0°C with the following directly labeled antibodies: anti-CD34–fluorescein isothiocyanate (FITC) (8G12),36anti–human CD19–phycoerythrin (PE), and anti–human CD20-PE (Becton Dickinson, San Jose, CA), all in one tube to permit the identification of human CD34−CD19+ and/or CD20+ B-lineage cells (referred to as CD34−CD19/20+ cells); and, in another tube, anti-CD34–PE, anti-CD45–FITC (Hlel) (Becton Dickinson), and anti-CD71–FITC (OKT-9) to allow the total human cell population (referred to as CD45/71+ cells) to be quantified and, at the same time, to permit isolation by fluorescence-activated cell sorting (FACS) of the human CD34+ subset for subsequent assessment of CFCs and LTC-ICs. Cells were then washed twice in HF/2 with 2 μg/mL propidium iodide (PI) (Sigma Chemicals) added to the second wash. Cells were sorted on a FACStar+ (Becton Dickinson) equipped with 5-W argon and 30-mW helium neon lasers. Specific fluorescence of FITC and PE was detected by means of gates that excluded more than 99.9% of cells stained with irrelevant isotype control antibodies labeled with the same fluorochromes. Under these conditions, no positive staining of mouse marrow cells (obtained from NOD/SCID mice that were not transplant recipients) was seen. LYSIS II software (Becton Dickinson) was used for acquisition and analysis of FACS data. For each analysis, a minimum of 20 000 viable (PI−) cells were assessed, and positive staining was scored only when more than 5 positive events were recorded.
In vitro progenitor assays
Human CD34+ cells isolated from the marrow of engrafted mice were assayed for CFCs at concentrations ranging from 250 to 9000 cells per 1.1 mL in duplicate 1.1 mL standard serum containing methylcellulose cultures (MethoCult 4230; StemCell Technologies) supplemented with 3000 U/L (3 U/mL) human erythropoietin (StemCell Technologies); 50 ng/mL Steel factor (SF) (expressed and purified in our laboratory); and 20 ng/mL each of the following: interleukin (IL)–3; granulocyte-macrophage colony-stimulating factor (both from Novartis, Basel, Switzerland); granuloctye colony-stimulating factor (G-CSF) (StemCell Technologies); and IL-6 (Cangene, Mississauga, ON, Canada). Myeloid CFCs (granulocyte-macrophage colony-forming units [CFU-GMs]) and erythroid CFCs (erythroid burst-forming units [BFU-Es]) were subcategorized as HPP and LPP subsets according to previously described criteria.10 Human CD34+ cells were assayed for LTC-ICs by assessment of the number of CFCs produced after 6 weeks in duplicate 1-mL cultures, each initiated with from 960 to 9000 cells. These were cocultured with irradiated mouse marrow fibroblasts previously engineered to produce SF, G-CSF, and IL-3 and maintained in hydrocortisone-supplemented myeloid LTC medium (MyeloCult), as previously described in detail.37 LTC-IC numbers were calculated on the assumption that, on average, each human cord blood–derived LTC-IC produces 28 CFCs and each human marrow–derived LTC-IC produces 18 CFCs after 6 weeks.37
3H-thymidine suicide assay
For assessment of the cycling activity of CFCs, sorted CD34+ cells were resuspended in Iscoves medium, and equal aliquots were then incubated at no more than 2 × 106cells per milliliter with and without 20 μCi/mL (.74 MBq/mL) high–specific activity 3H-thymidine (25 Ci/mmol [925 GBq/mmol]) (Amersham, Mississauga, ON, Canada) for 20 minutes at 37°C, as described in detail previously,10 before being washed and assayed for CFCs in duplicate cultures at the same cell concentrations as the untreated cells. The proportion of a given type of CFC in S-phase at the time of harvest was calculated from the loss of colony-forming activity (percentage killed) measured in the assays of the 3H-thymidine–treated cells versus the control cells. The proliferative activity of the LTC-ICs was similarly determined from CFC assays of duplicate 6-week cultures initiated with cells that had been incubated overnight in serum-free medium supplemented with SF, IL-3, and G-CSF, with or without the addition of3H-thymidine, also as described previously.5
Statistical analyses
Values shown are the mean ± SEM. The Student 2-tailed t test was used to determine significant differences (P < .05).
Results
SDF-1 and HCC-1 block the activation of all primitive CFCs in the adherent cell layer of LTCs initiated with normal adult human marrow
In previous studies, we have shown that the predominantly quiescent HPP-CFCs (both erythroid and granulopoietic) present in the adherent layer of normal human LTCs at least 7 days after a previous half-medium change are stimulated to enter S-phase 2 days following the next addition of fresh medium, and that this response can be blocked by the simultaneous addition of MIP-1α and MCP-1, but not several other chemokines.15,16 In this study, we used the same protocol to investigate whether SDF-1, HCC-1, and I-309 might have similar inhibitory activities. Accordingly, each of these agents was added simultaneously with a half-medium change (resulting in a final concentration of 100 ng/mL in the medium) to LTCs that had been initiated by seeding human marrow cells 7 to 10 days previously onto pre-established irradiated normal human marrow–derived adherent layers. At 2 to 3 days after the addition of new medium (with or without the test agents), when it was anticipated that the HPP-CFCs in the adherent layer of the control cultures would be maximally proliferating, all cultures were harvested and CFC cycling assays performed. As shown in Table 1, the addition of either SDF-1 or HCC-1 prevented the activation (persistance of low percentage killed) of the HPP-CFCs (both the primitive CFU-GMs and the primitive BFU-Es) in the adherent layer of the treated LTCs. I-309 did not have an equivalent effect on the primitive BFU-Es although the decrease in the percentage of the primitive CFU-GMs killed did reach significance (P < .05). In contrast, the proliferative activity of the mature CFU-GMs present in the same chemokine-treated LTC adherent layers and evaluated in the same assays was not affected by any of the treatments. This was shown by the high percentage killed (Table 1). Mature BFU-Es are not found in these cultures in sufficient numbers to allow their cycling status to be reliably assessed.10 Under these conditions, the cytostatic action exerted by SDF-1 and HCC-1 on the HPP-CFCs was completely reversible, as indicated by the finding that the total number of HPP-CFCs (and LPP-CFCs) in the cytokine-treated cultures was the same as in the control cultures (P > .05; data not shown).
SDF-1 is endogenously produced and contributes to the inhibition of HPP-CFC proliferation in the adherent layer of human LTCs
SDF-1 is known to be constitutively produced by many cell types, including endothelial cells and other bone marrow stromal elements38-40 that compose a large fraction of the cells in the adherent layer of LTCs.41,42 Moreover, measurement by ELISA of SDF-1 levels in the medium harvested from variously treated LTCs confirmed that it was present in low but readily detectable concentrations in the supernatants removed 2 to 3 days and 7 days after a first medium change (Table 2). Interestingly, the levels of SDF-1 doubled during this interval, consistent with its continuous endogenous production and replacement of the amounts removed during the previous half-medium change. Surprisingly, the addition of 100 ng/mL exogenous SDF-1 did not alter the levels of SDF-1 detectable in the supernatant of the cultures 2 to 3 days later. However, rapid depletion of exogenously added cytokines from LTC supernatants has been previously documented43 and is presumably due to their variable abilities to be bound to (and possibly degraded by) the cells and extracellular matrix components of the cultures. Thus, the concentrations of cytokines in LTC supernatants can, at best, provide only a relative measure of their effective amounts in the adherent layer, which can be at least 100 × higher.13 43
We then asked whether the endogenously produced SDF-1 has a significant role in regulating the proliferative status of HPP-CFCs in these LTCs, as shown previously for TGF-β and MCP-1.11 15 LTCs were therefore set up as before with normal adult human marrow cells. At 2 to 3 days after the half-medium change on day 7, we added 30 μg/mL anti–SDF-1 antibody (or control immunoglobulin, at a similar final concentration) or 10 μg/mL SDF-1(G2), the SDF-1 antagonist. Then, another 4 to 5 days later, the numbers and cycling status of the CFCs in the adherent layer were assessed to determine whether these treatments would prevent the previously activated HPP-CFCs from reverting to a quiescent state. As anticipated from previous studies, no S-phase HPP-CFCs were detectable in the adherent layer of the control cultures (low percentage killed), although all of the LPP-CFCs present in the assays remained in cycle (high percentage killed; Table3). In contrast, a majority of the HPP-CFCs in the adherent layer of the cultures to which either SDF-1 blocking agent had been added were still in S-phase (high percentage killed; Table 3), without affecting their absolute numbers (data not shown). These results indicate that SDF-1 is another endogenously produced specific inhibitor of normal human HPP-CFC cycling that helps to regulate their rate of turnover in the LTC system.
Insensitivity of CML HPP-CFCs to the cytostatic effect SDF-1 has on normal HPP-CFCs
In past experiments, we have shown that Ph+ HPP-CFCs are usually prevalent for the first 2 to 3 weeks in the adherent layer of LTCs initiated with peripheral blood cells from patients with CML with expanded clones of neoplastic cells (as indicated by the presence of a high WBC count). However, unlike their normal counterparts, these neoplastic HPP-CFCs remain continuously in cycle, even when the original irradiated adherent layer is derived from normal marrow.44 The anomalous proliferative activity of the CML HPP-CFCs in the LTC system can be attributed at least in part to their failure to respond to endogenously produced MCP-1 that inhibits normal HPP-CFC cycling in this system.15 CML HPP-CFCs also fail to respond to the cytostatic action that MIP-1α has on normal progenitors,16 suggesting a broader mechanism of BCR-ABL–mediated interference of signaling events activated by multiple chemokine receptors. Consistent with this hypothesis is the recently demonstrated inability of BCR-ABL–transduced murine BAF/3 cells to respond to SDF-1.45 We therefore predicted that the cycling of CML HPP-CFCs might also be unresponsive to SDF-1. To test this possibility, CML LTCs were initiated by placing cells from 7 patients with CML with high WBC counts onto pre-established irradiated normal marrow feeders. After 7 to 10 days, 100 ng/mL SDF-1 was added, and its effect on the cycling of the CML HPP-CFCs in the adherent layer was assessed another 2 to 3 days later. As a negative control, some cultures were left untouched. As a positive control, 5 ng/mL TGF-β was added to a third set of cultures. As expected, a high proportion of the HPP-CFCs harvested from the adherent layer of the control (untreated) CML LTCs were in S-phase (high percentage killed), and the proliferation of the neoplastic HPP-CFCs was completely, specifically, and reversibly arrested in parallel cultures to which 5 ng/mL TGF-β was added (low percentage of the HPP-CFCs killed, with persisting high percentages of the LPP-CFCs in the same cultures killed; Table4). The addition of SDF-1 had no effect on the cycling of any type of CML CFCs (Table 4), even when the concentration added was increased to 1 μg/mL (data not shown), a 10-fold higher concentration than that shown to be active on normal HPP-CFCs (Table 1). None of the treatments had any effect on absolute CFC numbers (data not shown).
SDF-1 inhibits the cycling of normal human LTC-ICs as well as HPP-CFCs in engrafted NOD/SCID mice
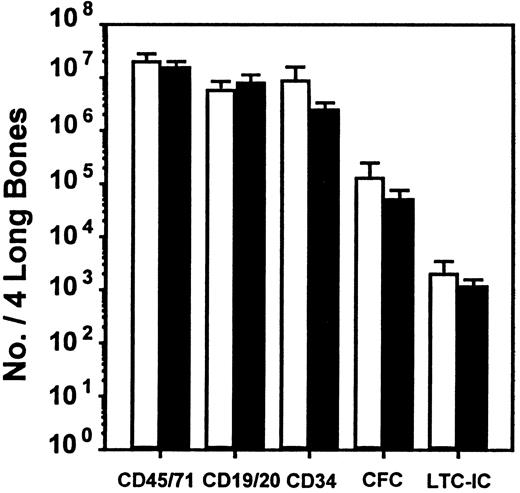
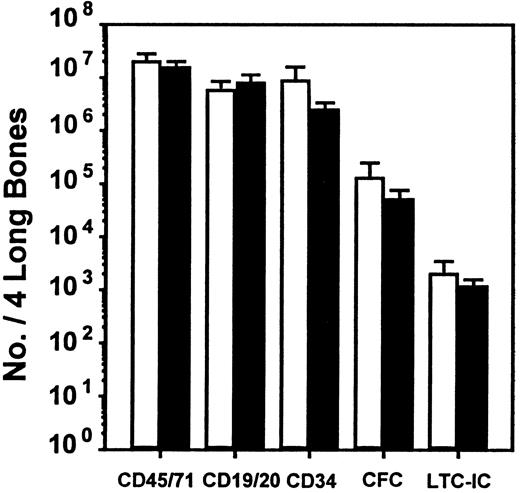
To investigate whether the effect of SDF-1 observed in vitro could be seen in vivo and to extend the evaluation to an even earlier progenitor cell type (LTC-ICs), we used the human NOD/SCID xenograft model. We also used a protocol that had previously allowed the differential in vivo inhibitory effects of MIP-1α, MCP-1, and TGF-β on human CFC and LTC-IC cycling to be demonstrated in this model.21 Accordingly, mice received transplants of 107 human cord blood cells to give reproducibly high levels of engraftment, and 6 to 8 weeks later they were injected on 2 consecutive days with SDF-1 (10 μg per injection per mouse). They were then killed 1 day later for analysis of any effects on human cell engraftment or progenitor cycling activity. The pooled results from 4 such experiments (2 to 4 mice per group per experiment) are shown in Figures 1 and2. The SDF-1 treatment had no significant immediate effects on any of the 5 parameters of human cell engraftment measured (P > .05). These consisted of the total number of human hematopoietic cells present in the marrow of the mice as well as the number of maturing human lymphoid and myeloid cells, the number of more primitive human CD34+cells, and the numbers of CFCs and LTC-ICs detected in the isolated human CD34+ population (Figure 1).
Lack of effect of SDF-1 administration on level of engraftment of NOD/SCID mice with human cells.
■, control mice injected with medium; ▪, SDF-1–treated mice. Values are the mean ± SEM of the total number of human cells of each type shown measured in 4 experiments. In each of these experiments, groups of 2 to 4 mice were injected with cord blood cells pooled from 1 to 3 donors and were then each injected 6 to 8 weeks later with 10 μg SDF-1 (or PBS) per mouse on 2 consecutive days prior to being killed and assessed on the third day. No significant difference (P > .05) was observed between the level of human cells in the control and SDF-1–treated animals for any of the human cell types examined.
Lack of effect of SDF-1 administration on level of engraftment of NOD/SCID mice with human cells.
■, control mice injected with medium; ▪, SDF-1–treated mice. Values are the mean ± SEM of the total number of human cells of each type shown measured in 4 experiments. In each of these experiments, groups of 2 to 4 mice were injected with cord blood cells pooled from 1 to 3 donors and were then each injected 6 to 8 weeks later with 10 μg SDF-1 (or PBS) per mouse on 2 consecutive days prior to being killed and assessed on the third day. No significant difference (P > .05) was observed between the level of human cells in the control and SDF-1–treated animals for any of the human cell types examined.
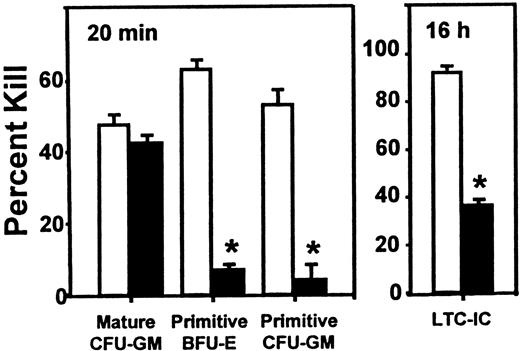
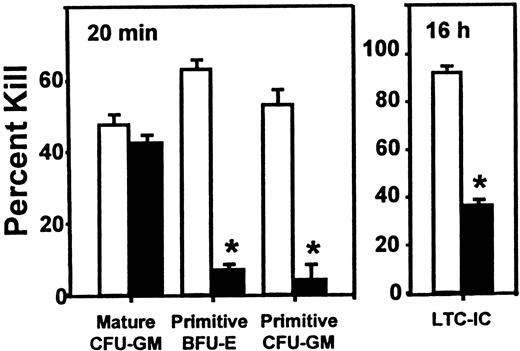
Inhibitory effect of SDF-1 on the cycling activity of human HPP-CFCs and LTC-ICs in engrafted NOD/SCID mice.
Experiments are the same as those used to generate the data shown in Figure 1, but in this case, the values are the mean ± SEM of the percentage killed for each type of progenitor assessed after exposure of sorted human CD34+ cells to high–specific activity3H-thymidine either for 20 minutes (CFCs) or overnight (LTC-ICs). Data marked by asterisks indicate a significant difference from the corresponding control value (P < .001). The total colony counts in the control groups (no 3H-thymidine) ranged from 10 to 134.
Inhibitory effect of SDF-1 on the cycling activity of human HPP-CFCs and LTC-ICs in engrafted NOD/SCID mice.
Experiments are the same as those used to generate the data shown in Figure 1, but in this case, the values are the mean ± SEM of the percentage killed for each type of progenitor assessed after exposure of sorted human CD34+ cells to high–specific activity3H-thymidine either for 20 minutes (CFCs) or overnight (LTC-ICs). Data marked by asterisks indicate a significant difference from the corresponding control value (P < .001). The total colony counts in the control groups (no 3H-thymidine) ranged from 10 to 134.
In contrast, as shown in Figure 2, selective and significant effects on the proliferative activity of different subsets of these human progenitors were observed when the sorted human CD34+ cells were first exposed to high–specific activity 3H-thymidine (for 20 minutes to assess the cycling activity of the CFCs and overnight for the LTC-ICs, as described in “Materials and methods”). In mice given SDF-1, the proportion of S-phase progenitors in all of the primitive progenitor compartments was markedly reduced (P < .001). This included both erythroid and granulopoietic HPP-CFCs subsets as well as the LTC-ICs. In contrast, administration of SDF-1 had no effect on the high cycling activity of the mature CFU-GMs (LPP-CFCs) present in the same mice, as shown by the persistence (P > .05) of a high fraction of S-phase LPP-CFCs (mature CFU-GMs) in the same assays.
Evidence that SDF-1 is an endogenous in vivo regulator of primitive human progenitor proliferation
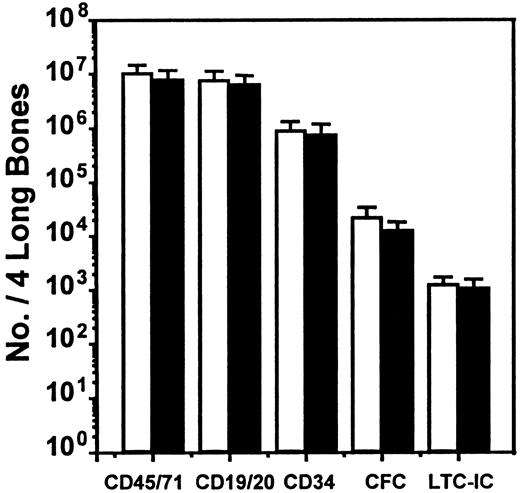
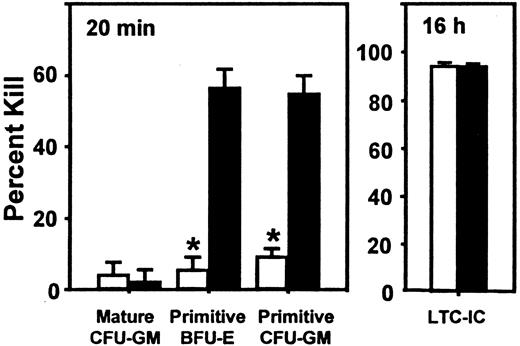
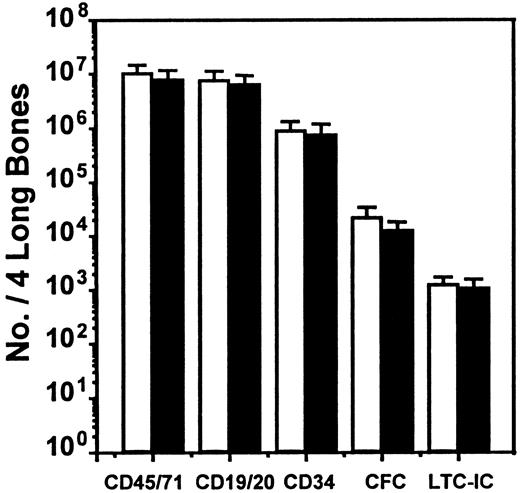
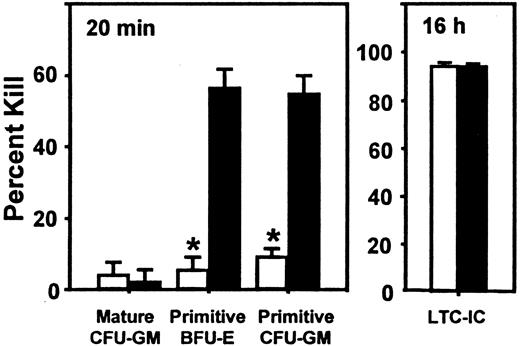
We next asked whether SDF-1 produced endogenously in vivo might contribute to the downregulation of human CFC cycling. This occurs by 10 to 12 weeks after transplantation in NOD/SCID mice engrafted with normal human marrow cells (but not after the transplantation of cord blood cells20) and was previously shown to be mediated in part by MCP-1 and possibly MIP-1α with the use of in vivo treatments of such mice with antagonists of these chemokines.21 We therefore transplanted human marrow cells into mice and then injected each mouse with 2 daily doses of 10 μg SDF-1(G2), a specific antagonist of SDF-1,46 (or with PBS) 10 weeks later, by which time most of the human CFCs have become quiescent,19even though the human LTC-ICs in the same mice continue to proliferate.21 At 1 day later, the mice were killed, and any effects on human cell engraftment or progenitor cycling were determined. As illustrated in Figure 3, this protocol of SDF-1(G2) treatment had no significant effect on any of the 5 parameters of human cell engraftment assessed (P > .05). However, it did cause a specific reactivation of human HPP-CFC (but not LPP-CFC) cycling, as shown by the significantly increased (P < .001) sensitivity of the HPP-CFCs (but not the LPP-CFCs) to a 20-minute exposure to high–specific activity 3H-thymidine (Figure4). In the same mice, the turnover of the human LTC-ICs was already maximal in the absence of any treatment, as found previously,21 and administration of SDF-1(G2) did not change this.
Lack of effect of an SDF-1 antagonist on the level of human cell engraftment in NOD/SCID mice.
The number of different types of human cells present in the marrow of NOD/SCID mice was assessed 10 to 12 weeks after their transplantation with human marrow cells and 1 day after 2 daily injections of 10 μg SDF-1(G2) (▪) or PBS (■) per mouse. Values are the mean ± SEM of the total number of each human cell type determined in 6 experiments. In each of these experiments, groups of 2 to 4 mice were injected with cells from a different normal marrow sample. No significant difference (P > .05) between controls and SDF-1(G2)–treated animals was observed for any of the cell types examined.
Lack of effect of an SDF-1 antagonist on the level of human cell engraftment in NOD/SCID mice.
The number of different types of human cells present in the marrow of NOD/SCID mice was assessed 10 to 12 weeks after their transplantation with human marrow cells and 1 day after 2 daily injections of 10 μg SDF-1(G2) (▪) or PBS (■) per mouse. Values are the mean ± SEM of the total number of each human cell type determined in 6 experiments. In each of these experiments, groups of 2 to 4 mice were injected with cells from a different normal marrow sample. No significant difference (P > .05) between controls and SDF-1(G2)–treated animals was observed for any of the cell types examined.
Effect of administration of an SDF-1 antagonist on the cycling of quiescent human HPP-CFCs in engrafted NOD/SCID mice.
Administration of an SDF-1 antagonist can reactivate the cycling of quiescent human HPP-CFCs in engrafted NOD/SCID mice. Data are from the same mice used for Figure 3, but in this case, the values are the mean ± SEM of the percentage killed as assessed after exposure of sorted human CD34+ cells to high–specific activity3H-thymidine for 20 minutes (or overnight for the LTC-ICs). Data marked by an asterisk indicate a significant difference from the control value (P < .001). The total colony counts in the control groups (no 3H-thymidine) ranged from 10 to 126.
Effect of administration of an SDF-1 antagonist on the cycling of quiescent human HPP-CFCs in engrafted NOD/SCID mice.
Administration of an SDF-1 antagonist can reactivate the cycling of quiescent human HPP-CFCs in engrafted NOD/SCID mice. Data are from the same mice used for Figure 3, but in this case, the values are the mean ± SEM of the percentage killed as assessed after exposure of sorted human CD34+ cells to high–specific activity3H-thymidine for 20 minutes (or overnight for the LTC-ICs). Data marked by an asterisk indicate a significant difference from the control value (P < .001). The total colony counts in the control groups (no 3H-thymidine) ranged from 10 to 126.
Discussion
The chemokines compose a large family of structurally related low-molecular-weight (6- to 14-kd) proteins that function as major regulators of leukocyte migration, activation, and chemotaxis during inflammatory processes.47 A number of chemokines have also been shown to have the ability to inhibit the proliferation of primitive hematopoietic cells.15,16,48 However, the latter effect appears to be quite dependent on the context in which the cells are exposed because the chemokines found to be active as inhibitors of primitive hematopoietic cells in the LTC system15 cover a much more selective spectrum than the chemokines reported to be active in dilute suspension cultures.48 The chemokines have been broadly classified into 4 subgroups on the basis of the position of the first 2 cysteines that form essential disulphide bonds. MIP-1α and MCP-1, the only 2 chemokines previously shown to be inhibitors of normal human HPP-CFC cycling either in the LTC system or in xenografted NOD/SCID mice,21 are both classified as CC chemokines because the first 2 cysteines are adjacent. HCC-1 and I-309, also tested here, are both members of the CC family. On the other hand, SDF-1 is a member of the CXC subset in which these cysteines are separated by another amino acid.
The chemokine receptors belong to the heptahelical receptor superfamily, which, upon ligand binding, couple trimeric guanine-nucleotide binding (G) proteins and activate calcium flux changes. Because the ligand-binding abilities of many chemokine receptors overlap extensively and many individual cell types express a number of different chemokine receptors, the mechanisms by which specific chemokine effects are mediated have often been difficult to elucidate. For example, MIP-1α−/− mice do not show any obvious perturbation of hematopoiesis or other defects in their normal physiology except for a compromised ability to respond to viral infections.49 MCP-1−/− mice have also not been reported to display any alteration in blood cell production50 consistent with the observed overlap in the hematopoietic regulatory activities of these cytokines in vitro. However, although CC receptor 2−/− mice develop normally, a significant defect in monocyte/macrophage recruitment and in interferon-γ production has been seen.51
SDF-1 is different from many of the other chemokines in its apparent specificity for a single receptor, CXCR4 (previously referred to as LESTR, HUMSTER, or fusin),52-54 and its much broader range of action. CXCR4 is expressed on lymphocytes and monocytes,38,55 megakaryocytes,56 and dendritic cells, as well as primitive progenitors in the hematopoietic system, including CFCs and LTC-ICs.22-24,28,57 CXCR4 is also expressed on cells in a wide variety of other organs and tissues, including heart, brain, spleen, liver, and colon.52,53,58-61 SDF-1 is produced constitutively in many tissues, including bone marrow, thymus, spleen, heart, lung, muscle, kidney, and liver.40,62,63 This contrasts with many other chemokines whose expression is highly regulated by proinflammatory cytokines and has led to the idea that SDF-1 plays a role in steady-state homeostatic processes, including leukocyte and hematopoietic stem cell trafficking24-29,38; B lymphopoiesis; establishment of marrow myelopoiesis during embryogenesis; neurogenesis; cardiogenesis; and blood vessel formation.23,60-62,64 65
In this paper, we provide evidence for the first time of a chemokine, SDF-1, that can function as an inhibitor of human LTC-IC cycling. In addition, SDF-1 inhibited the cycling activity of HPP-CFCs, but not the more mature LPP-CFCs, both in vivo and in vitro (in the adherent layer of LTCs). The latter mimic the effects we have previously documented for TGF-β, MCP-1, and MIP-1α tested in the same type of experiment.12,15,16 Interestingly, however, studies in the NOD/SCID model have shown that each of these cytokines regulates the proliferation of a slightly different range of human progenitor types, with only TGF-β and SDF-1 being active on human LTC-ICs.21 These findings support, although they do not prove, a direct action of each of these cytokines on the various subsets of human progenitors affected. The broader range of SDF-1 activity may be explained simply by a potentially broader range of CXCR4 expression on human CD34+ cells. Future studies of chemokine receptor expression on functionally tested purified subpopulations of CD34+ cells should help to address this possibility.
The effect of SDF-1 on the proliferation of hematopoietic cells in other studies appears to be contradictory. In some studies, no effect on progenitor cycling was discerned.25,56,66 On certain myeloid progenitor cell lines, a suppressive effect of SDF-1 was reported,67 although this was not the case for others, in spite of expression of functional CXCR4.68 In combination with other cytokines, SDF-1 was actually found to promote the proliferation of human CD34+ cells purified from normal adult peripheral blood although when SDF-1 was added alone, only survival was supported, not mitogenesis.69 These findings highlight the role other factors can have in influencing the effects chemokines exert on hematopoietic cell proliferation control as well as the importance of the progenitor subset investigated, as is also indicated by earlier evidence of opposing actions of MIP-1α (and TGF-β) in stimulating the proliferation of later progenitor types but inhibiting their more primitive precursors.70-72
The present findings also show that endogenously produced SDF-1 can, like endogenously produced MIP-1α and/or MCP-1, contribute to the negative regulation of primitive adult human marrow–derived CFCs being regenerated in a foreign environment. Nevertheless, evidence of endogenous chemokine-mediated inhibition of proliferation of the human LTC-ICs present in the same mice was not seen although such a response could be elicited when a large dose of exogenous SDF-1 was injected. Given the differences in types and concentrations of other cytokines already known to characterize the responses of hematopoietic progenitors at different stages of differentiation, a differential effect of endogenous SDF-1 on human HPP-CFCs and LTC-ICs in engrafted NOD/SCID mice may be explained in a similar way. It would also be interesting to determine whether the late-onset chemokine-mediated inhibition of human CFC proliferation seen in human marrow–engrafted NOD/SCID mice (and/or the corresponding lack of inhibition of human LTC-IC proliferation) is unique to this model, or whether these are also features of syngeneic or allogeneic transplants.
Several groups have demonstrated the ability of marrow-derived stromal feeder layers and cell lines to inhibit the proliferation of primitive hematopoietic progenitors. In addition, LTC-IC and CFC production has been shown to be increased when direct contact with the stromal layer is prevented,73 and evidence of a role of integrin receptors in the adhesion required to downregulate progenitor cycling has been reported.74 Similarly, it has been suggested that the abnormal proliferative activity of CML progenitors within the adherent layer of the LTC system is caused at least in part by the abnormal adhesive properties of CML progenitors.75-77However, we have shown that primitive CML cells can be stimulated to proliferate autonomously by an autocrine IL-3/G-CSF–mediated mechanism.78 This could also explain the generic ability of primitive CML cells to overcome a common mechanism of chemokine-activated cycling inhibition that may be operative in normal progenitors and that may include potential secondary effects on integrin activation.
Finally, our findings suggest that SDF-1, because of its activity on very primitive hematopoietic cells, may (unlike MIP-1α or MCP-1) have potential therapeutic value as a stem cell protective agent in patients undergoing extensive chemotherapy. Such treatments are generally believed to activate and hence sensitize the stem cell compartment so that the stem cells' acquired sensitivity to later cycles of drugs that target dividing cells becomes dose limiting. The use of SDF-1 in such circumstances could be of particular benefit to patients whose malignant progenitors are unresponsive to SDF-1, as suggested here for CML.
We thank members of the Stem Cell Assay Service for assistance in the initial processing of cord blood, bone marrow, and CML blood cells, and members of the Flow Cytometry Service for assistance in the FACS isolation of human CD34+ cells. We also thank P. Lansdorp, StemCell Technologies, Cangene, Chemokine Therapeutics, and Novartis for generous gifts of reagents, and A. Ahamed for assistance in preparing the manuscript.
Supported by grants from the National Cancer Institute of Canada, with funds from the Canadian Cancer Society and the Terry Fox Run and PO1 55435 (National Heart, Lung, and Blood Institute), and by Canadian Institutes of Health Research grant 36346.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
C. J. Eaves, Terry Fox Laboratory, 601 West 10th Ave, Vancouver, BC, V5Z 1L3, Canada; e-mail:ceaves@bccancer.bc.ca.