Abstract
The adenosine triphosphate binding-site–directed agent STI571 and the tyrphostin adaphostin are undergoing evaluation as bcr/abl kinase inhibitors. The current study compared the effects of these agents on the survival of K562 cells, bcr/abl-transduced FDC-P1 cells, and myeloid progenitors from patients with chronic myelogenous leukemia (CML) compared with healthy donors. Treatment of K562 cells with 10 μM adaphostin resulted in decreased p210bcr/ablpolypeptide levels in the first 6 hours, followed by caspase activation and accumulation of apoptotic cells in less than 12 hours. By 24 hours, 90% of the cells were apoptotic and unable to form colonies. In contrast, 20 μM STI571 caused rapid inhibition of bcr/abl autophosphorylation without p210bcr/abl degradation. Although this was followed by the inhibition of Stat5 phosphorylation and the down-regulation of Bcl-xL and Mcl-1, only 7% ± 3% and 25% ± 9% of cells were apoptotic at 16 and 24 hours, respectively. Instead, the cytotoxic effects of STI571 became more pronounced with prolonged exposure, with IC90values greater than 20 μM and 1.0 ± 0.6 μM after 24 and 48 hours, respectively. Consistent with these results, 24-hour adaphostin exposure inhibited CML granulocyte colony-forming units (CFU-G) (median IC50, 12 μM) but not normal CFU-G (median IC50, greater than 20 μM), whereas 24-hour STI571 treatment had no effect on CML or normal CFU-G. Additional experiments revealed that STI571-resistant K562 cells remained sensitive to adaphostin. Moreover, the combination of STI571 + adaphostin induced more cytotoxicity in K562 cells and in CML CFU-G than either agent alone did. Collectively, these results identify adaphostin as a mechanistically distinct CML-selective agent that retains activity in STI571-resistant cell lines.
Introduction
Approximately 4500 new cases of chronic myelogenous leukemia (CML) occur in the United States each year. In most patients, a characteristic t(9;22) translocation juxtaposes the 5′ end of thebcr gene with the 3′ end of the abl gene, resulting in a unique 210-kd fusion protein, p210bcr/abl.1,2 This constitutively active cytoplasmic kinase is capable of not only transforming murine fibroblasts and hematopoietic cell lines, but also causing a chronic myeloproliferative disorder resembling CML on transduction into mouse marrow.3,4 This p210bcr/abl-induced transformation appears to involve the activation of signaling through the Ras-Raf and phosphatidylinositol-3 kinase/Akt pathways as well as transcription mediated by signal transducer and activator of transcription 5 (Stat5) and nuclear factor κB (NFκB).1,5 Collectively, these events result in the up-regulation of antiapoptotic proteins, including Bcl-xL,6 the X-linked inhibitor of apoptosis protein (XIAP), and survivin,7 contributing to the resistance of p210bcr/abl-expressing cells to a variety of apoptotic stimuli.6,8 9
Until recently, treatment options for CML, which included the use of hydroxyurea, α-interferon with or without cytarabine, or stem cell transplantation, were less than satisfactory.10,11 The almost universal presence of the p210bcr/abl kinase in CML, coupled with evidence implicating this kinase in the pathogenesis of the disorder, made this fusion protein an attractive target for CML-directed therapy. Previous efforts identified multiple p210bcr/abl kinase inhibitors.12-16
The most widely studied p210bcr/abl inhibitor is STI571 (formerly known as CGP 57148),17 a reversible inhibitor that occupies the adenosine triphosphate-binding pocket of p210bcr/abl and stabilizes the kinase in an inactive conformation.18 Preclinical studies demonstrated that STI571 also inhibits the kinase activities of c-abl, platelet-derived growth factor receptor, and the c-kit receptor.15,19,20 Phase 1 studies suggest that STI571 has impressive activity against chronic-phase CML21 but more limited activity against p190bcr/abl-expressing acute lymphocytic leukemia and the blast crisis phase of CML.22Recent studies have demonstrated that mutation and amplification of p210bcr/abl are observed in samples from patients who have relapsed after STI571 therapy.23 Additional preclinical and clinical studies of STI571, alone and in combination with conventional cytotoxic agents, are ongoing.7,24 25
An alternative approach to inhibiting protein kinases involves the use of small molecules that alter the binding of peptide substrates rather than adenosine triphosphate. A chemically diverse group of agents, generically termed tyrphostins, has been synthesized and evaluated as potential tyrosine kinase inhibitors.26 The tyrphostin AG957 has previously been reported to inhibit p210bcr/ablactivity in immune complex kinase assays14 and to cause decreased p210bcr/abl autophosphorylation followed by bcr/abl degradation in intact cells.27,28 Interestingly, AG957 also inhibits T-cell receptor–mediated phosphorylation of the adaptor protein c-Cbl,29 suggesting that kinases other than bcr/abl might also be affected. Despite the lack of absolute specificity for bcr/abl-transformed cell lines, AG957 selectively inhibits the proliferation of CML progenitors compared with normal myeloid progenitors.28,30 Subsequent animal testing has revealed that AG957 has a short serum half-life (S. Stinson, V.L.N., E.A.S., unpublished observations, December 2000). Examination of a series of analogues has demonstrated that adaphostin, the adamantyl ester of AG957, has greater potency in vitro28and a longer serum half-life in vivo (S. Stinson, V.L.N., E.A.S., unpublished observations, December 2000).
In the current study, we compared the actions of STI571 and adaphostin in a number of preclinical models. These studies focused on determining whether similar events occur downstream of bcr/abl kinase inhibition after treatment with the 2 compounds. We also examined the possibility that both of these p210bcr/abl-directed agents might exhibit synergistic or non–cross-resistant effects.
Materials and methods
Materials
Adaphostin was synthesized by the Drug Synthesis and Chemistry Branch, Division of Cancer Treatment and Diagnosis, National Cancer Institute (Bethesda, MD). STI571 was kindly provided by Novartis Pharma AG (Basel, Switzerland). Puromycin and Hoechst 33258 were from Sigma (St Louis, MO).
Antibodies were purchased from the following suppliers: phosphotyrosine and phospho-Stat5 from Upstate Biotechnology (Lake Placid, NY); c-abl from Oncogene Research (Cambridge, MA); Stat5, procaspase-3, XIAP, and Mcl-1 from Transduction Labs (Lexington, KY); and Bcl-xLand FADD from PharMingen (San Diego, CA). Rabbit antiserum that recognizes cleaved caspase-3 was from Tamie Chilcote (Elan Pharmaceutics, San Francisco, CA). All other materials were obtained as previously indicated.28
Cell lines
K562 cells (American Type Culture Collection, Manassas, VA) were passaged in RPMI 1640 containing 5% heat-inactivated fetal bovine serum, 100 U/mL penicillin G, 100 μg/mL streptomycin, and 2 mM glutamine (medium A). STI571-resistant K562 cells31 were maintained in medium A containing or lacking 0.5 μM STI571.
To derive FDC-P1bcr/abl cells, log-phase FCD-P1 murine interleukin-3 (IL-3)–dependent myeloid cells (kindly provided by Larry Karnitz, Mayo Clinic, Rochester, MN) growing in medium B, which consisted of RPMI 1640, 10% (vol/vol) heat-inactivated fetal calf serum, 10% (vol/vol) WEHI-conditioned medium, 100 U/mL penicillin G, 100 μg/mL streptomycin, and 2 mM glutamine, were incubated for 20 hours with the retrovirus pBabe/Puro containing p210bcr/ablcDNA behind the SV40 early promoter (kindly provided by Ruibao Ren, Brandeis University, Waltham, MA), washed twice, incubated for 48 hours in medium B, and diluted with medium B containing 5 μg/mL puromycin. Puromycin-resistant clones were subsequently isolated by limiting dilution and subjected to immunoblotting to confirm the expression of p210bcr/abl. Thereafter, cells were grown in medium B in the absence (parental FDC-P1) or presence (FDC-P1bcr/abl) of 5 μg/mL puromycin.
Clonogenic assays
Aliquots containing 0.5 × 106 K562 cells in 1 mL medium A were incubated with diluent, STI571, or adaphostin for the indicated lengths of time, sedimented at 100g for 5 minutes, diluted, and plated in gridded 35-mm plates in the medium of Pike and Robinson32 containing 0.3% (wt/vol) Bacto agar. After incubation for 10 to 14 days at 37°C, colonies containing at least 50 cells were counted on an inverted phase-contrast microscope.
Clinical samples were studied under the aegis of a protocol approved by the Institutional Review Board of the Mayo Clinic in accordance with the policies of the US Department of Health and Human Services. To evaluate the effects of adaphostin and STI571 on normal versus CML CFU-G, 10 mL peripheral blood was drawn from healthy volunteers and consenting patients with previously untreated chronic-phase CML using EDTA as an anticoagulant. Mononuclear cells (density = 1.077 g/cm3) harvested from Ficoll-Hypaque step gradients were cultured for 24 hours at a density of 1 × 106/mL in Iscoves modified Dulbecco medium containing 20% (vol/vol) fetal bovine serum (medium C) supplemented with increasing concentrations of adaphostin or STI571. At the completion of the incubation, samples were sedimented at 200g for 10 minutes, resuspended at a concentration of 1 × 106 cells/mL in medium C containing 50 ng/mL G-CSF, plated in 0.3% agar, and incubated for 7 to 8 days. Colonies containing at least 32 cells were counted on an inverted phase-contrast microscope.
Immunoblotting
K562 cells were incubated with the indicated concentration of STI571 or adaphostin in medium A for 1 to 48 hours as indicated, sedimented at 200g for 10 minutes, washed in serum-free RPMI 1640 containing 10 mM HEPES (pH 7.4), and lysed in 6 M guanidine hydrochloride under reducing conditions.33 Aliquots containing 50 μg total cellular protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) containing 5% to 15% polyacrylamide gradients, transferred to nitrocellulose, and probed with primary antibodies followed by horseradish peroxidase–coupled secondary antibodies using standard procedures.34
Immunoprecipitation
Ten million cells were incubated in the absence or presence of 10 μM adaphostin or 20 μM STI571 for 3 hours, sedimented at 200g for 10 minutes, resuspended in 1 mL lysis buffer containing 0.5% Nonidet P-40, 50 mM Tris-HCl (pH 8.0), 0.1 mM EDTA, 150 mM NaCl, 1 mM sodium orthovanadate and 1 mM dithiothreitol supplemented with one Complete Mini protease inhibitor tablet (Boehringer Mannheim, Indianapolis, IN) per 10 mL of lysis buffer immediately before use, incubated on a rotating shaker at 4°C for 1 hour, and centrifuged at 16 000g for 15 minutes. Additional incubations were performed at 4°C with gentle rotation. After pellets were discarded, supernatants were supplemented with 20 μL protein A and 20 μL protein G beads, incubated for 1 hour, and sedimented for 5 minutes at 700g to preclear the lysates. Supernatants were transferred to fresh tubes, incubated overnight with 0.2 μg anti-Stat5 antibody, supplemented with 20 μL protein A and protein G beads, and incubated for 1 hour. After sedimentation for 5 minutes at 700g, bound immunoprecipitates were washed 3 times in 1 mL lysis buffer, solubilized in 50 μL SDS sample buffer, heated to 100°C for 5 minutes, and loaded onto 7.5% polyacrylamide gels. After transfer to nitrocellulose, immunoprecipitates were probed with antiphospho-Stat5 or anti-Stat5 antibodies essentially as described above.
Fluorescence microscopy
For morphologic analysis, cells were fixed in 3:1 (vol/vol) methanol:acetic acid, stained with 1 μg/mL Hoechst 33258 in 50% (vol/vol) glycerol, and examined under epi-illumination using a Zeiss Axioplan microscope (Carl Zeiss, Thornwood, NY). Two hundred to 300 cells per sample were scored for apoptotic changes (peripheral chromatin condensation or nuclear fragmentation) as described.28
Transient transfections
Plasmids encoding enhanced green fluorescence protein (pEGFP-N1), dominant negative caspase-9, or crmA were obtained from Clontech (Palo Alto, CA), Emad Alnemri (Thomas Jefferson University, Philadelphia, PA), and Charles Young (Mayo Clinic, Rochester, MN), respectively. Log-phase K562 or Jurkat cells were transfected in the buffer described by van den Hoff et al35 using a T840 square-wave electroporator (BTX, San Diego, CA) delivering a 240-V pulse for 10 msec. After 24-hour incubation, 30% to 40% of the cells displayed green fluorescence. The brightest 10% to 12% of the total cell population was isolated by fluorescence-activated cell sorting (FACS), exposed to drug or diluent as indicated in the legend to Figure4A, fixed, and examined for apoptotic morphologic changes.
Statistical analysis
Data are expressed as the mean ± standard deviation of the indicated number of replicate experiments. Differences between samples were analyzed using 2-sided t tests. Changes in paired samples were analyzed using 2-sided paired ttests.36 The distributions of IC50 values for CFU-G from control subjects and patients with CML were compared using a 2-sided Mann-Whitney-Wilcoxon Utest.36
Results
Delayed induction of apoptosis by STI571
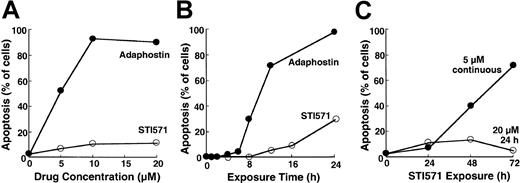
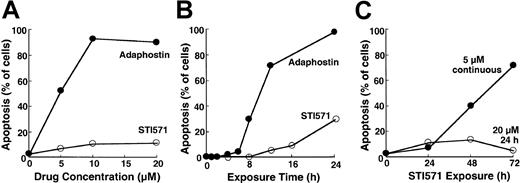
To compare the cellular effects of adaphostin and STI571, we initially incubated K562 cells, a cell line that expresses p210bcr/abl,37 with varying concentrations of adaphostin or STI571 for 24 hours. At the completion of the treatment, cells were sedimented and examined for apoptotic morphologic changes. Results of this analysis indicated that adaphostin induced apoptosis in a dose- and time-dependent manner. Representative experiments are shown in Figure 1A and 1B. After the addition of 10 μM adaphostin to the culture, 67% ± 7% of the cells (mean ± SD, n = 3) exhibited apoptotic morphologic changes at 16 hours and 85 ± 10% (n = 10) appeared apoptotic at 24 hours. Moreover, after treatment with lower doses of adaphostin, the percentage of apoptotic cells continued to increase even after drug withdrawal (data not shown). In striking contrast, 20 μM STI571 induced apoptosis more slowly, with only 7% ± 3% (n = 3) and 25% ± 9% (n = 9) of the cells appearing apoptotic at 16 hours and 24 hours, respectively (Figure 1A-B). If STI571 was removed after 24 hours, the percentage of apoptotic cells did not increase over time (Figure 1C). Prolonging the STI571 exposure to 48 hours or more, however, dramatically enhanced the induction of apoptosis (Figure 1C). These results suggest that the induction of apoptosis by the 2 agents is fundamentally different. In particular, adaphostin relatively rapidly initiates a process that continues to proceed even after drug withdrawal. In contrast, the induction of apoptosis by STI571 appears to be slower and to depend on the continuous presence of drug.
Induction of apoptosis by adaphostin or STI571 in K562 cells.
(A) Cells were treated for 24 hours with the indicated concentration of adaphostin (closed circles) or STI571 (open circles), sedimented, fixed, stained with Hoechst 33258, and examined for apoptotic morphologic changes. (B) Cells were treated for the indicated length of time with 10 μM adaphostin (closed circles) or 20 μM STI571 (open circles), sedimented, fixed, stained with Hoechst 33258, and examined for apoptotic morphologic changes. Cells analyzed in this panel correspond to samples blotted in Figures 3 and 4. (C) Cells were incubated for 24 hours with 20 μM STI571, sedimented at unit gravity, and incubated in drug-free medium for up to 48 more hours (open circles). Alternatively, cells were exposed to 5 μM continuously for the indicated length of time (closed circles). At the end of the incubation cells were fixed, stained, and examined for apoptotic morphologic changes. Results shown in each panel are from 1 of at least 3 representative experiments.
Induction of apoptosis by adaphostin or STI571 in K562 cells.
(A) Cells were treated for 24 hours with the indicated concentration of adaphostin (closed circles) or STI571 (open circles), sedimented, fixed, stained with Hoechst 33258, and examined for apoptotic morphologic changes. (B) Cells were treated for the indicated length of time with 10 μM adaphostin (closed circles) or 20 μM STI571 (open circles), sedimented, fixed, stained with Hoechst 33258, and examined for apoptotic morphologic changes. Cells analyzed in this panel correspond to samples blotted in Figures 3 and 4. (C) Cells were incubated for 24 hours with 20 μM STI571, sedimented at unit gravity, and incubated in drug-free medium for up to 48 more hours (open circles). Alternatively, cells were exposed to 5 μM continuously for the indicated length of time (closed circles). At the end of the incubation cells were fixed, stained, and examined for apoptotic morphologic changes. Results shown in each panel are from 1 of at least 3 representative experiments.
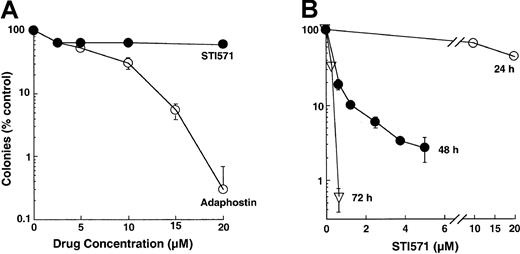
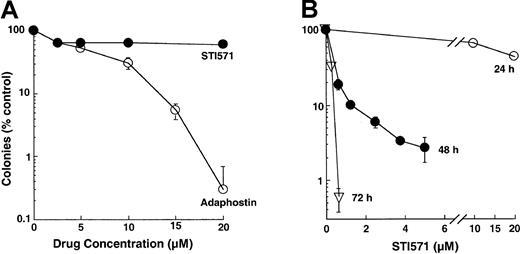
These differences in the kinetics of induction of apoptosis were also reflected in the results of colony-forming assays. Treatment with adaphostin for 24 hours reduced the ability of K562 cells to form colonies in soft agar by several logs (Figure2A), with an IC90 of 10 ± 2 μM (n = 17). In contrast, treatment with varying concentrations of STI571 for 24 hours had a much less dramatic effect (Figure 2A-B), with 70% ± 10% (n = 7) of clonogenic cells retaining the ability to form colonies after 24-hour exposure to 10 μM STI571. Nonetheless, K562 cells were relatively sensitive to more prolonged STI571 exposures, with an IC90 of 1.0 ± 0.6 μM after 48 hours (n = 5) and less than 1 μM after 72 hours (Figure 2B).
Effect of adaphostin or STI571 on colony-forming ability of K562 cells.
(A) Log-phase cells were exposed to the indicated concentration of adaphostin or STI571 for 24 hours, washed, and plated in soft agar. Colony formation was examined 10 to 14 days later. (B) cells were exposed to the indicated concentrations of STI571 for 24, 48, or 72 hours, washed, and plated in soft agar. Results shown are representative of 7 to 17 (A) or 5 (B) experiments. Error bars, mean ± 1 SD from quadruplicate plates treated with the indicated drug concentration in the experiments presented.
Effect of adaphostin or STI571 on colony-forming ability of K562 cells.
(A) Log-phase cells were exposed to the indicated concentration of adaphostin or STI571 for 24 hours, washed, and plated in soft agar. Colony formation was examined 10 to 14 days later. (B) cells were exposed to the indicated concentrations of STI571 for 24, 48, or 72 hours, washed, and plated in soft agar. Results shown are representative of 7 to 17 (A) or 5 (B) experiments. Error bars, mean ± 1 SD from quadruplicate plates treated with the indicated drug concentration in the experiments presented.
Effect of adaphostin and STI571 on p210bcr/abl
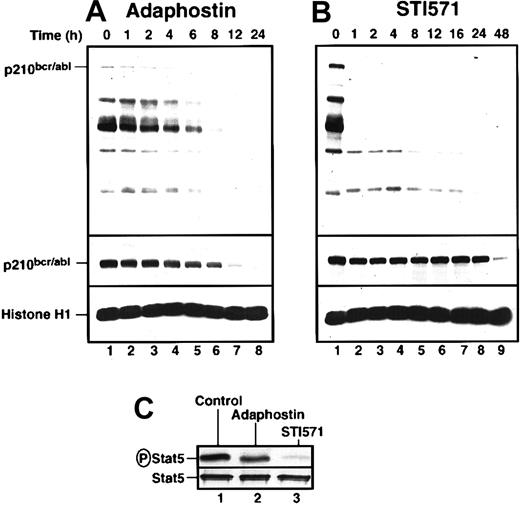
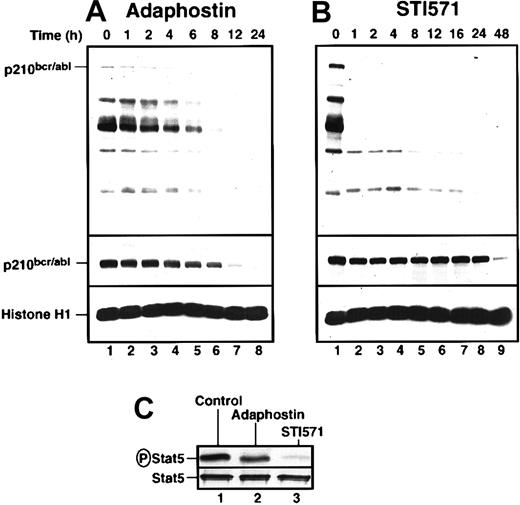
To investigate the biochemical basis for the differences seen in Figures 1 and 2, we examined the effects of adaphostin and STI571 on p210bcr/abl phosphorylation, p210bcr/ablpolypeptide content, and selected downstream signals. K562 cells were incubated with the drugs for increasing lengths of time, harvested, and subjected to immunoblotting with reagents that recognize phosphotyrosine or the p210bcr/abl polypeptide (top and middle panels, respectively, Figure3A-B). Cells treated with 10 μM adaphostin showed gradual and progressive decreases in phosphorylation of bcr/abl and other tyrosine phosphorylated polypeptides, with readily detectable phosphorylation persisting for at least 6 hours (Figure 3A, top panel). In contrast, 20 μM STI571 abolished p210bcr/abl phosphorylation within the first hour and caused marked decreases in tyrosine phosphorylation of many other polypeptides over the same time frame (Figure 3B). Despite its lower toxicity (Figures 1, 2), STI571 appeared to produce more robust inhibition of bcr/abl kinase activity in situ. Consistent with this conclusion, STI571 inhibited phosphorylation of the p210bcr/abl substrate Stat538 39 by more than 85% within 3 hours, whereas adaphostin had significantly less effect (Figure 3C).
Adaphostin and STI571 alter p210bcr/abl-induced signaling in K562 cells in different ways.
K562 cells were treated with 10 μM adaphostin (A) or 20 μM STI571 (B) for the indicated length of time. Lysates containing 50 μg total cellular protein were subjected to SDS-PAGE followed by blotting with antiphosphotyrosine (top panel), anti-abl (middle panel), or anti-histone H1 (bottom panel) as a loading control. Percentages of cells that were apoptotic at each time point of this experiment are indicated in Figure 1B. (C) After K562 cells were treated with diluent (lane 1), 10 μM adaphostin (lane 2), or 20 μM STI571 (lane 3) for 3 hours, cell lysates were subjected to immunoprecipitation with anti-Stat5 monoclonal antibody and probed with anti-phosphoStat5 antiserum (top panel) or anti-Stat5 antibody (bottom panel). Results shown in each panel are representative of at least 3 independent experiments.
Adaphostin and STI571 alter p210bcr/abl-induced signaling in K562 cells in different ways.
K562 cells were treated with 10 μM adaphostin (A) or 20 μM STI571 (B) for the indicated length of time. Lysates containing 50 μg total cellular protein were subjected to SDS-PAGE followed by blotting with antiphosphotyrosine (top panel), anti-abl (middle panel), or anti-histone H1 (bottom panel) as a loading control. Percentages of cells that were apoptotic at each time point of this experiment are indicated in Figure 1B. (C) After K562 cells were treated with diluent (lane 1), 10 μM adaphostin (lane 2), or 20 μM STI571 (lane 3) for 3 hours, cell lysates were subjected to immunoprecipitation with anti-Stat5 monoclonal antibody and probed with anti-phosphoStat5 antiserum (top panel) or anti-Stat5 antibody (bottom panel). Results shown in each panel are representative of at least 3 independent experiments.
These 2 agents also differed in their effects on p210bcr/abl polypeptide levels. Treatment with 10 μM adaphostin resulted in a gradual decrease of p210bcr/abl in the first 8 hours (Figure 3A). In contrast, STI571 had no effect on p210bcr/abl levels in the first 24 hours, though p210bcr/abl diminished markedly at 48 hours when 99% of the cells were apoptotic (Figure 3B).
Apoptotic biochemical changes induced by adaphostin and STI571
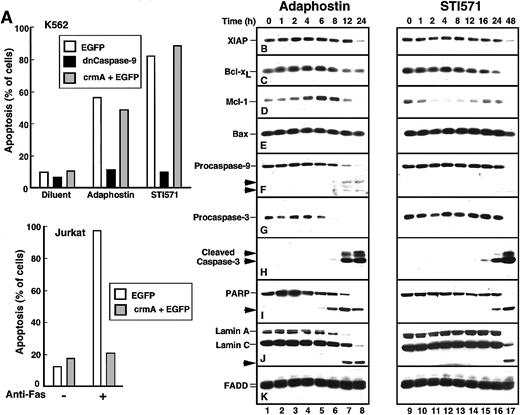
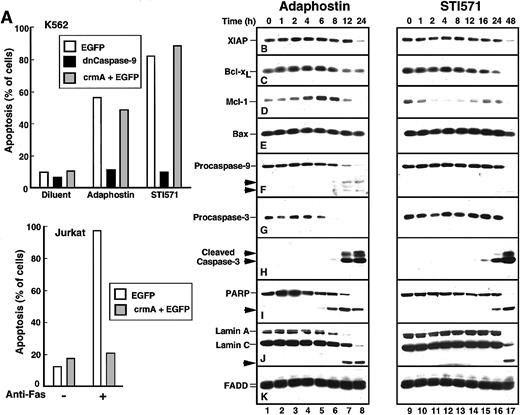
Additional experiments were performed to identify changes occurring between the drug-induced alteration of p210bcr/abl and the appearance of apoptotic morphologic changes. When cells were transiently transfected with a plasmid encoding dominant-negative caspase-9,40 the induction of apoptosis by either adaphostin or STI571 was prevented (Figure4A). In contrast, transfection with the caspase-8 inhibitor crmA41 had no effect on the induction of apoptosis by either of these agents (Figure 4A), even though the same crmA construct prevented Fas-mediated apoptosis in Jurkat cells (Figure 4A, bottom panel). These results suggest that adaphostin and STI571 both induce apoptosis through the mitochondrial pathway of caspase activation.42 43
Adaphostin and STI571 induce apoptotic biochemical changes at different rates.
(A) Identification of caspase-9 as the initiating caspase for adaphostin and STI571. K562 cells were transiently transfected with plasmids encoding EGFP, dominant-negative procaspase-9 fused to EGFP, or a 5:1 ratio of plasmids encoding crmA and EGFP as described in “Materials and methods.” After 24-hour incubation, cells expressing EGFP were isolated by FACS, suspended in medium A, treated for 48 hours with 2.5 μM adaphostin or 625 nM STI571, fixed, and examined for apoptotic morphologic changes. As a control, Jurkat cells transfected with EGFP or a 5:1 ratio of crmA and EGFP were isolated by FACS, treated for 24 hours with 20 ng/mL CH-11 agonistic anti-Fas antibody, fixed, and examined for apoptotic morphologic changes. (B-K) Changes in polypeptide levels during adaphostin- or STI571-induced apoptosis. Cells were treated with 10 μM adaphostin (left) or 20 μM STI571 (right) for the indicated length of time. Lysates containing 50 μg total cellular protein were subjected to SDS-PAGE followed by blotting with reagents that recognize XIAP (B), Bcl-xL (C), Mcl-1 (D), Bax (E), procaspase-9 (F), procaspase-3 (G), cleaved caspase-3 (H), poly(ADP-ribose) polymerase (I, PARP), lamins A and C (J) or, as a loading control, FADD (K). In each panel, lanes 1 to 17 correspond to nonadjacent lanes from a single blot. The same blots used in Figure 3were probed in this figure. Arrows, cleaved fragments corresponding to previously described43 caspase cleavage products. Percentages of cells that were apoptotic at each time point of this experiment are indicated in Figure 1B. Results shown are representative of at least 3 independent experiments.
Adaphostin and STI571 induce apoptotic biochemical changes at different rates.
(A) Identification of caspase-9 as the initiating caspase for adaphostin and STI571. K562 cells were transiently transfected with plasmids encoding EGFP, dominant-negative procaspase-9 fused to EGFP, or a 5:1 ratio of plasmids encoding crmA and EGFP as described in “Materials and methods.” After 24-hour incubation, cells expressing EGFP were isolated by FACS, suspended in medium A, treated for 48 hours with 2.5 μM adaphostin or 625 nM STI571, fixed, and examined for apoptotic morphologic changes. As a control, Jurkat cells transfected with EGFP or a 5:1 ratio of crmA and EGFP were isolated by FACS, treated for 24 hours with 20 ng/mL CH-11 agonistic anti-Fas antibody, fixed, and examined for apoptotic morphologic changes. (B-K) Changes in polypeptide levels during adaphostin- or STI571-induced apoptosis. Cells were treated with 10 μM adaphostin (left) or 20 μM STI571 (right) for the indicated length of time. Lysates containing 50 μg total cellular protein were subjected to SDS-PAGE followed by blotting with reagents that recognize XIAP (B), Bcl-xL (C), Mcl-1 (D), Bax (E), procaspase-9 (F), procaspase-3 (G), cleaved caspase-3 (H), poly(ADP-ribose) polymerase (I, PARP), lamins A and C (J) or, as a loading control, FADD (K). In each panel, lanes 1 to 17 correspond to nonadjacent lanes from a single blot. The same blots used in Figure 3were probed in this figure. Arrows, cleaved fragments corresponding to previously described43 caspase cleavage products. Percentages of cells that were apoptotic at each time point of this experiment are indicated in Figure 1B. Results shown are representative of at least 3 independent experiments.
Recent results have suggested that decreased synthesis of Bcl-xL, a Stat5-induced antiapoptotic regulator of the mitochondrial pathway, contributes to STI571-induced apoptosis.44 Additional observations have suggested that survivin and XIAP, 2 antiapoptotic products of p210bcr/abl-induced45 NFκB activation,46 are also down-regulated in response to STI571.7 To determine whether similar effects would also be induced by adaphostin, K562 cells treated for increasing lengths of time with STI571 or adaphostin were subjected to immunoblotting using reagents that recognize numerous inhibitor of apoptosis proteins and Bcl-2 family members (Figure 4B-E).
As previously reported,7 treatment with STI571 resulted in decreased levels of the antiapoptotic protein XIAP. This was most prominent, however, at 48 hours (Figure 4B, lane 17), a time when 99% of the cells in this experiment were apoptotic. In contrast, the Stat transcriptional target Bcl-x was down-regulated after 16 to 24 hours of treatment with STI571 (Figure 4C, lanes 15-16). When levels of Mcl-1, a more rapidly turning-over Stat transcriptional target,48 were examined, decreased levels were evident within 2 hours (Figure 4D, lane 11), though Mcl-1 appeared subsequently to be up-regulated (Figure 4D, lanes 15-16) through a presumably Stat5-independent mechanism (see below). In contrast, STI571 had no effect on levels of the proapoptotic Bcl-2 family members Bax (Figure4E) and Bak (data not shown).
Despite the down-regulation of Bcl-xL and Mcl-1, most STI571-treated cells remained viable for up to 24 hours, as demonstrated by the limited loss of proliferative potential (Figures1A, 2). Consistent with this interpretation, we observed that cleavage of procaspases-9 and -3 to active fragments (Figure 4F-H) and digestion of the caspase substrates poly(ADP-ribose) polymerase and lamin A (Figure 4I-J) was limited in the first 24 hours but became more extensive at 48 hours.
After treatment with adaphostin, XIAP levels again did not change until most of the cells were apoptotic (Figure 4B, lane 8). The behavior of other polypeptides, however, was considerably different after adaphostin treatment. In contrast to the down-regulation of Bcl-xL and Mcl-1 seen with STI571, adaphostin treatment caused a consistent up-regulation of these antiapoptotic proteins at 2 to 6 hours (Figure 4C-D, lanes 3-5), followed by a down-regulation at later time points as the cells became apoptotic. Because these changes occurred at a time when Stat5 phosphorylation was minimally altered (Figure 3C, lane 2), these changes appeared to be independent of increased Stat5 activation. Despite the elevation of these antiapoptotic proteins, proteolytic cleavage of procaspases-9 and -3 to active species was evident by 8 hours (Figure 4F, H, lane 6). This was accompanied by cleavage of poly(ADP-ribose) polymerase and lamin A by 8 and 12 hours, respectively. In short, adaphostin was able to induce apoptosis without the down-regulation of NFκB-regulated inhibitor of apoptosis proteins or Stat-regulated antiapoptotic Bcl-2 family members.
Comparison of the effects of adaphostin and STI571 in FDC-P1 and p210bcr/abl-transduced FDC-P1
The striking differences in the manner in which adaphostin and STI571 induced apoptosis in K562 cells raised the possibility that these agents might differ in other respects. To assess this possibility, we examined the selectivity of the agents for bcr/abl-expressing cells in vitro using 2 model systems.
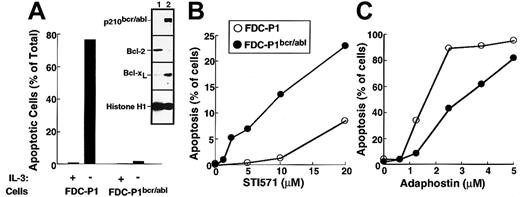
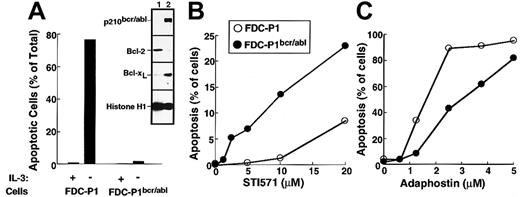
The first set of experiments was performed using the murine myeloid line FDC-P1 transduced with a retrovirus encoding p210bcr/abl. Consistent with changes reported in other cell types transduced with bcr/abl kinases,6,49 50 the expression of p210bcr/abl resulted in the up-regulation of Bcl-xL (inset, Figure 5A) and decreased sensitivity to IL-3 withdrawal (Figure 5A) relative to parental FDC-P1 cells. Transduction with p210bcr/abl also sensitized the FDC-P1 cells to the cytotoxic effects of STI571 (Figure5B). In contrast, adaphostin was at least as toxic to parental cells as to p210bcr/abl-expressing cells (Figure 5C).
Effects of STI571 and adaphostin on FDC-P1 and FDC-P1/p210bcr/abl cells.
(A) Characterization of FDC-P1–transduced cells. Parental and p210bcr/abl-expressing cells were sedimented at unit gravity, washed, and resuspended in medium containing or lacking WEHI-conditioned medium as a source of IL-3. After 24 hours, cells were fixed and examined for apoptotic morphologic changes. (Inset) Expression of p210bcr/abl, Bcl-2, Bcl-xL and histone H1 (as a loading control) in parental (lane 1) and p210bcr/abl-transduced (lane 2) cells. (B, C) Cells were incubated for 48 hours with the indicated concentration of STI571 or adaphostin and examined for apoptotic morphologic changes. Results shown in each panel are representative of at least 3 independent experiments.
Effects of STI571 and adaphostin on FDC-P1 and FDC-P1/p210bcr/abl cells.
(A) Characterization of FDC-P1–transduced cells. Parental and p210bcr/abl-expressing cells were sedimented at unit gravity, washed, and resuspended in medium containing or lacking WEHI-conditioned medium as a source of IL-3. After 24 hours, cells were fixed and examined for apoptotic morphologic changes. (Inset) Expression of p210bcr/abl, Bcl-2, Bcl-xL and histone H1 (as a loading control) in parental (lane 1) and p210bcr/abl-transduced (lane 2) cells. (B, C) Cells were incubated for 48 hours with the indicated concentration of STI571 or adaphostin and examined for apoptotic morphologic changes. Results shown in each panel are representative of at least 3 independent experiments.
Effects on normal and CML CFU-G
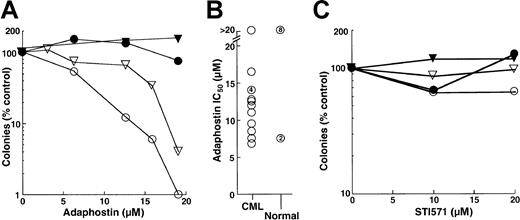
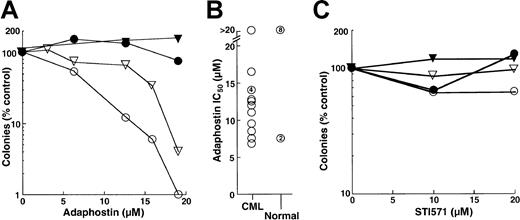
The results obtained in the FDC-P1 model appeared to be at odds with previous reports that AG957 and adaphostin exhibit selectivity for CML myeloid progenitors, including granulocyte erythroid macrophage megakaryocyte-CFU (CFU-GEMM), granulocyte macrophage-CFU (CFU-GM) and CFU-G, compared to the normal counterparts.28 30 To further evaluate this issue, the effects of adaphostin and STI571 on circulating CFU-G from patients with chronic-phase CML and from healthy control subjects were compared. In the 14 patients with CML who provided samples for this study, we observed an overwhelming dependence on CML clones for hematopoiesis. This was evidenced by the fact that 99%± 2.5% of the cells were Philadelphia chromosome–positive in the 12 patients who underwent bone marrow cytogenetics and that 97%± 2.8% of the cells were positive for bcr/abl rearrangement in the 7 patients who underwent fluorescence in situ hybridization studies. Results obtained using cells from 2 CML patients and the simultaneously analyzed healthy control subjects are shown in Figure 6A. A 24-hour exposure to adaphostin inhibited subsequent granulocyte colony formation in a dose-dependent manner, with 90% inhibition at 12 and 17 μM, respectively (Figure 6A, open symbols). In contrast, there was no inhibition of granulocyte colony formation in the corresponding normal samples (Figure 6A, closed symbols). As summarized in Figure 6B, the 24-hour adaphostin exposure inhibited subsequent granulocyte colony formation in 13 of 14 CML samples, with IC50 values ranging from 7 to 16 μM. In contrast, the effects on healthy control subjects were significantly different (P < .02 by Mann-Whitney-Wilcoxon U test). In particular, adaphostin did not affect CFU-G in 8 of 10 healthy control subjects examined. Although adaphostin inhibited colony formation by approximately 50% in 2 other control subjects, this was observed at all concentrations ranging from 6 to 20 μM and appeared to reflect technical difficulties with the diluent-treated samples rather than a true dose-dependent inhibition (data not shown).
Effects of adaphostin and STI571 on CFU-G from patients with chronic-phase CML or from healthy volunteers.
(A, C) Peripheral blood mononuclear cells from 2 patients with CML (open symbols) and corresponding healthy control subjects (closed symbols) were incubated for 24 hours with the indicated concentration of adaphostin (A) or STI571 (C), washed, and plated under drug-free conditions in the presence of 50 ng/mL G-CSF. After 7 to 8 days, granulocyte colonies were counted. (B) Summary of IC50values when samples from 14 patients with CML and 10 healthy control subjects were treated with adaphostin and plated in G-CSF, as illustrated in panel A. Unnumbered circles represent single samples. Numbers within circles denote multiple separate samples with the indicated IC50 values.
Effects of adaphostin and STI571 on CFU-G from patients with chronic-phase CML or from healthy volunteers.
(A, C) Peripheral blood mononuclear cells from 2 patients with CML (open symbols) and corresponding healthy control subjects (closed symbols) were incubated for 24 hours with the indicated concentration of adaphostin (A) or STI571 (C), washed, and plated under drug-free conditions in the presence of 50 ng/mL G-CSF. After 7 to 8 days, granulocyte colonies were counted. (B) Summary of IC50values when samples from 14 patients with CML and 10 healthy control subjects were treated with adaphostin and plated in G-CSF, as illustrated in panel A. Unnumbered circles represent single samples. Numbers within circles denote multiple separate samples with the indicated IC50 values.
When the same samples shown in Figure 6A and 6B were incubated with STI571, a different picture emerged. Even though 24-hour adaphostin treatment inhibited granulocyte colony formation by more than 90% (Figure 6A), 24-hour STI571 exposure had limited effect (Figure 6C). Examination of 12 separate CML samples indicated that treatment with 20 μM STI571 for 24 hours inhibited subsequent granulocyte colony formation only modestly, with 68% ± 23% (median, 61%) of CML CFU-G surviving. Effects on normal CFU-G were even more modest, with a median colony count of 118% of diluent-treated control subjects after 24-hour STI571 exposure.
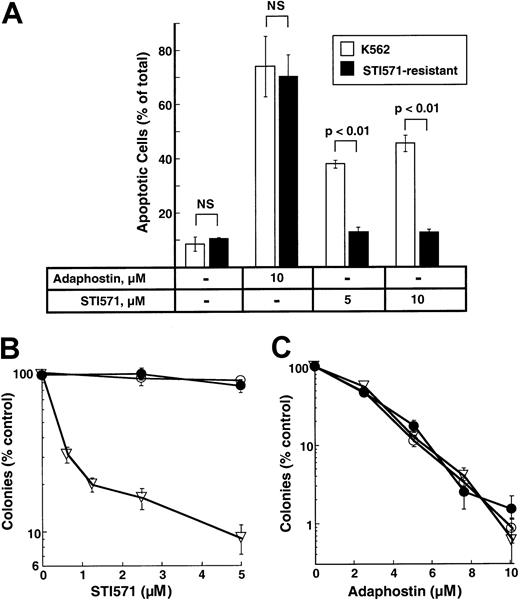
Effect of adaphostin on STI571-resistant cells
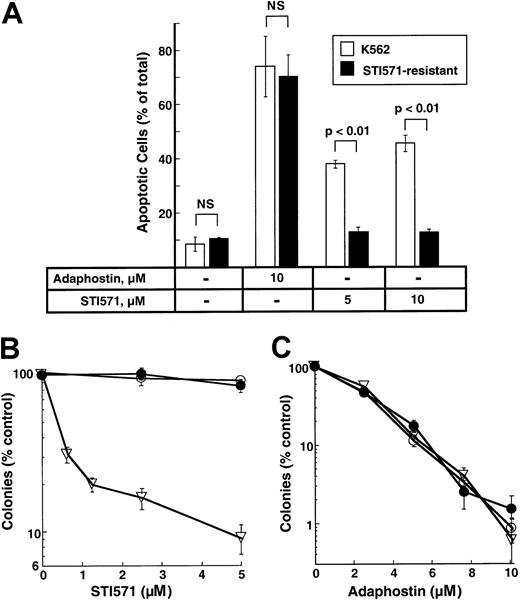
K562 cell lines resistant to STI571 have been described and characterized.31 To determine whether these cells were cross-resistant to adaphostin, parental and STI-resistant cells were treated with 10 μM adaphostin for 24 hours and examined for apoptotic morphologic changes. As indicated in Figure7A, STI571-resistant cells retained their sensitivity to adaphostin-induced apoptosis. In colony-forming assays, STI571-resistant cells (Figure 7B) also retained their sensitivity to the antiproliferative effects of adaphostin (Figure 7C).
Effects of adaphostin on STI571-resistant cells.
(A) Induction of apoptosis. Parental or STI571-resistant K562 cells were incubated for 24 hours with diluent or the indicated concentration of adaphostin or STI571. Cells were then fixed, stained with Hoechst 33258, and examined for apoptotic nuclear changes. Results shown are the mean ± 1 SD from 3 independent experiments. Brackets indicate samples compared by t tests. NS indicates differences were not statistically significant. (B, C) Effects of STI571 and adaphostin on colony formation. Parental cells (open triangles) or STI571-selected cells maintained in the absence (open circles) or presence (closed circles) of 0.5 μM STI571 were incubated with the indicated concentration of STI571 for 48 hours (B) or adaphostin for 24 hours (C), washed, and plated in 0.3% agar in the absence of drug. Error bars, mean ± 1 SD for quadruplicate plates treated with the indicated drug concentration. Results from this experiment are representative of 3 independent experiments.
Effects of adaphostin on STI571-resistant cells.
(A) Induction of apoptosis. Parental or STI571-resistant K562 cells were incubated for 24 hours with diluent or the indicated concentration of adaphostin or STI571. Cells were then fixed, stained with Hoechst 33258, and examined for apoptotic nuclear changes. Results shown are the mean ± 1 SD from 3 independent experiments. Brackets indicate samples compared by t tests. NS indicates differences were not statistically significant. (B, C) Effects of STI571 and adaphostin on colony formation. Parental cells (open triangles) or STI571-selected cells maintained in the absence (open circles) or presence (closed circles) of 0.5 μM STI571 were incubated with the indicated concentration of STI571 for 48 hours (B) or adaphostin for 24 hours (C), washed, and plated in 0.3% agar in the absence of drug. Error bars, mean ± 1 SD for quadruplicate plates treated with the indicated drug concentration. Results from this experiment are representative of 3 independent experiments.
Synergy between adaphostin and STI571
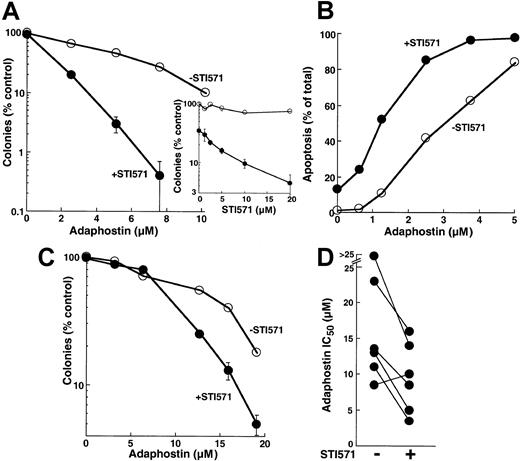
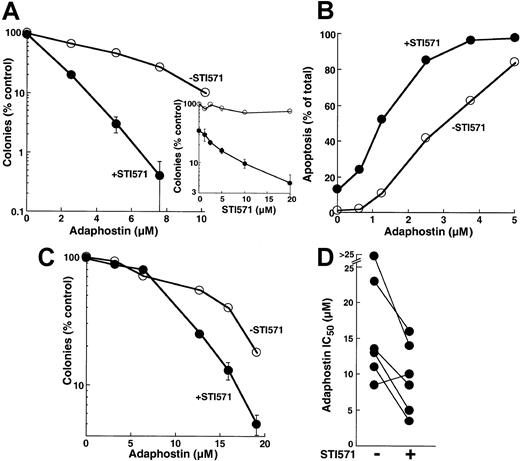
Because adaphostin works, at least in part, by down-regulating levels of bcr/abl (Figure 3A) and because decreased levels of bcr/abl would be expected to increase the sensitivity of cells to STI571,31,51 52 we evaluated the effects of combining these 2 agents. When parental K562 cells were treated with increasing concentrations of adaphostin, the addition of STI571 enhanced the antiproliferative effects at all adaphostin concentrations analyzed (Figure 8A). This enhancement was evident at STI571 concentrations as low as 2.5 μM (inset, Figure 8A). Further examination revealed that the enhanced inhibition of colony formation reflected an induction of apoptosis in a greater percentage of cells treated with the combination than of cells treated with either drug alone (Figure 8B). During treatment with 2.5 μM adaphostin, for example, the addition of 10 μM STI571 increased the percentage of apoptotic cells from 45% ± 6% to 84% ± 10% (n = 3,P < .01 by t test).
Effect of treating cells with the combination of adaphostin and STI571.
(A) K562 cells were incubated for 24 hours with the indicated concentration of adaphostin in the presence of diluent (open circles) or 20 μM STI571 (closed circles). At the completion of the incubation, cells were washed, plated in 0.3% agar, and incubated for 10 to 14 days before colony formation was assessed. Error bars, mean ± 1 SD for quadruplicate plates treated with the indicated drug concentration. (Inset) Cells were incubated for 24 hours with the indicated concentration of STI571 in the absence (open circles) or presence (closed circles) of 5 μM adaphostin. At the completion of the incubation, colony-forming ability was assessed as described for panel A. (B) K562 cells were incubated for 24 hours with the indicated concentration of adaphostin in the presence of diluent (open circles) or 10 μM STI571 (closed circles), fixed, and examined for apoptotic morphologic changes. Results in panels A and B are representative of 3 independent experiments. (C) Circulating mononuclear cells from a patient with CML were treated for 24 hours with the indicated concentrations of adaphostin in the absence (open circles) or presence (closed circles) of 20 μM STI571, washed, and plated in 0.3% agar containing 50 ng/mL G-CSF. After 8 days, granulocyte colonies were counted. (D) Summary of results obtained when the experiment depicted in panel C was performed on 6 CML samples.
Effect of treating cells with the combination of adaphostin and STI571.
(A) K562 cells were incubated for 24 hours with the indicated concentration of adaphostin in the presence of diluent (open circles) or 20 μM STI571 (closed circles). At the completion of the incubation, cells were washed, plated in 0.3% agar, and incubated for 10 to 14 days before colony formation was assessed. Error bars, mean ± 1 SD for quadruplicate plates treated with the indicated drug concentration. (Inset) Cells were incubated for 24 hours with the indicated concentration of STI571 in the absence (open circles) or presence (closed circles) of 5 μM adaphostin. At the completion of the incubation, colony-forming ability was assessed as described for panel A. (B) K562 cells were incubated for 24 hours with the indicated concentration of adaphostin in the presence of diluent (open circles) or 10 μM STI571 (closed circles), fixed, and examined for apoptotic morphologic changes. Results in panels A and B are representative of 3 independent experiments. (C) Circulating mononuclear cells from a patient with CML were treated for 24 hours with the indicated concentrations of adaphostin in the absence (open circles) or presence (closed circles) of 20 μM STI571, washed, and plated in 0.3% agar containing 50 ng/mL G-CSF. After 8 days, granulocyte colonies were counted. (D) Summary of results obtained when the experiment depicted in panel C was performed on 6 CML samples.
Additional experiments revealed that the effects of combining adaphostin and STI571 were not limited to K562 cells. Treatment with STI571 enhanced the antiproliferative effects of adaphostin on circulating CML myeloid progenitors as well (Figure 8C). When 6 separate CML samples were treated with adaphostin in the absence or presence of STI571, the IC50 values observed in the presence of STI571 were significantly lower (P = .01 using paired t test), with 5 of 6 samples showing readily detectable sensitization (Figure 8D).
Discussion
In the current study, the effects of the bcr/abl-directed agents adaphostin and STI571 were compared in a number of model systems. Results of this analysis demonstrated that adaphostin was capable of inducing apoptosis relatively rapidly in K562 cells, whereas more prolonged exposures to STI571 were required to induce the same effect. This difference in the tempo of the cytotoxic effects reflected mechanistic differences between the 2 agents. In additional experiments, adaphostin failed to demonstrate selectivity for bcr/abl-transformed tissue culture cell lines, whereas STI571 required bcr/abl expression for its toxicity. Despite this apparent lack of selectivity in established tissue culture cell lines, adaphostin demonstrated selectivity for CML CFU-G compared with normal progenitors in vitro. Additional studies showed that adaphostin was active against STI571-resistant cells. Moreover, enhanced cytotoxic effects resulted when the 2 agents were combined. Collectively, these results not only provide evidence of clear-cut differences between the 2 agents, but also suggest potentially important implications for further development of both agents.
Although STI571 and adaphostin both inhibit bcr/abl activity in vitro,14,15 the effects of these agents on p210bcr/abl-expressing K562 cells differed substantially. A 24-hour exposure to adaphostin was capable of reducing survival (Figure 1A-B) and colony-forming ability (Figure 2A) by several logs. In contrast, a 24-hour exposure to STI571 had a more limited effect on cell viability as assessed in colony-forming assays (Figure 2) or assays of apoptosis (Figures 1 and 4). On more prolonged exposure, however, STI571 became highly cytotoxic (Figures 1B, 2B, 4). These results parallel unpublished observations recently cited by Druker17 and support the decision to administer STI571 on prolonged schedules in the clinical studies.
It is important to stress that these differences between adaphostin and STI571 did not reflect an inability of STI571 to inhibit p210bcr/abl activity in situ. On the contrary, STI571 induced rapid inhibition of bcr/abl activity, as indicated by the decrease in p210bcr/abl autophosphorylation, the decrease in tyrosine phosphorylation of other polypeptides, and the decrease in phosphorylation of Stat5 (Figure 3B-C). These changes were followed several hours later by down-regulation of the antiapoptotic proteins Bcl-xL and Mcl-1 (Figure 4C-D). These observations not only confirm recent reports that Bcl-xL is down-regulated in STI571-treated cells,7,44 but extend the results in 2 important ways. First, we have observed that Mcl-1, another antiapoptotic protein with a Stat-responsive element in its promoter,48 is also down-regulated by STI571 (Figure 4D). Second, our results indicate that the STI571-induced down-regulation of Mcl-1 is transient, with a subsequent up-regulation that remains unexplained but is unable to rescue the cells (Figure 4D).
In contrast to STI571, adaphostin induces a much slower decrease in p210bcr/abl signaling (Figure 3A). This does not lead to an appreciable decrease in Stat5 phosphorylation in the first several hours (Figure 3C), nor does it result in an initial decrease in Bcl-xL or Mcl-1 (Figure 4C-D). On the contrary, Bcl-xL and Mcl-1 transiently increase during the first few hours of adaphostin treatment. Our results are consistent with previous reports that Mcl-1 can be induced by various types of cellular stress,53 54 and they raise the possibility that Bcl-xL might be similarly regulated. These elevated levels decline at later time points when caspases are activated (Figure 4C, lanes 6-8).
Such differences in the actions of STI571 and adaphostin suggest that adaphostin is killing cells, at least in part, by a mechanism that does not involve the inhibition of p210bcr/abl-mediated signaling. Consistent with this conclusion, Losiewicz et al29 reported that AG957, a closely related analogue of adaphostin, is capable of inhibiting T-cell receptor–mediated c-Cbl phosphorylation in Jurkat cells. Because c-Cbl is a substrate of bcr/abl,55 the observation that c-Cbl phosphorylation is inhibited in AG957-treated lymphoid cells raises the possibility that AG957 and its derivative, adaphostin, are exerting their effects by inhibiting other kinases that phosphorylate c-Cbl and p210bcr/abl. The possibility of an entirely different and unsuspected target for adaphostin, however, cannot be ruled out.
Although adaphostin does not require bcr/abl expression to kill established tissue culture cell lines (Figure 5), this agent does exhibit selectivity for CFU-G from CML patients compared with healthy donors (Figure 6). These results parallel previous reports that the parent drug AG957 selectively inhibits proliferation of CFU-GEMM, CFU-GM and CFU-G from CML patients.28 30 The basis for this selectivity is unknown.
Resistance to STI571 has been observed in tissue culture cell lines31,51,52 and in patients treated for chronic-phase CML or blast crisis.23,56 57 The apparent differences in mechanisms of action prompted us to determine whether STI571-resistant cells were cross-resistant to adaphostin. Interestingly, STI571-resistant K562 cells retained their sensitivity to adaphostin (Figure 7), raising the possibility that these agents might have different mechanisms of resistance.
In additional experiments, the effect of combining these 2 agents was examined. Treatment with the combination killed many more K562 cells than either agent did alone (Figure 8A-B). In addition, the combination inhibited the outgrowth of CML CFU-G more than either agent alone (Figure 8C-D). Combined with the results in Figure 7, these observations suggest that adaphostin should undergo further preclinical testing to evaluate its potential as a therapeutic agent in settings in which STI571 has limited efficacy, including STI571-resistant chronic-phase CML, blast-crisis CML, and bcr/abl-positive acute lymphocytic leukemia. In addition, the ability of adaphostin to sensitize p210bcr/abl-expressing cells to STI571 (Figure 8) suggests that this combination might be worthy of further preclinical and possible clinical testing.
We thank Novartis for providing STI571, Tamie Chilcote and Guy Poirier for gifts of antibodies, Ruibao Ren for viruses, Larry Karnitz for FDC-P1 cells, Judith Karp for encouragement, and Deb Strauss for secretarial assistance.
Supported in part by R01 CA85972, R01 CA69008, and T32 CA09441.
B.M.F.M. and J.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Scott H. Kaufmann, Div of Oncology Research, Guggenheim 1301, Mayo Clinic, 200 First St SW, Rochester, MN 55901; e-mail: kaufmann.scott@mayo.edu.