Abstract
The purine nucleoside 2-chlorodeoxyadenosine (CdA) is often used in leukemia therapy. Its efficacy, however, is compromised by the emergence of resistant cells. In the present study, 3 CdA-resistant cell lines were generated and characterized. Their ability to accumulate 2-chloroadenosine triphosphate (CdATP) varied, reflecting differences in activities of deoxycytidine kinase (dCK) and deoxyguanosine kinase (dGK). Nonetheless, the selected lines were uniformly resistant to CdA-induced apoptosis, as assessed by caspase activation and DNA fragmentation. In contrast, cytosols from resistant cells were capable of robust caspase activation when incubated in the presence of cytochrome c and dATP. Moreover, replacement of dATP with CdATP also resulted in caspase activation in the parental and some of the resistant cell lines. Strikingly, CdA-induced decreases in mitochondrial transmembrane potential and release of cytochrome c from mitochondria were observed in the parental cells but not in any resistant lines. The lack of cytochrome c release correlated with an increased ability of mitochondria from resistant cells to sequester free Ca2+. Consistent with this enhanced Ca2+buffering capacity, an early increase in cytosolic Ca2+after CdA treatment of parental cells but not resistant cells was detected. Furthermore, CdA-resistant cells were selectively cross-resistant to thapsigargin but not to staurosporine- or Fas-induced apoptosis. In addition, CdA-induced caspase-3 activation and DNA fragmentation were inhibited by the Ca2+ chelator BAPTA-AM in sensitive cells. Taken together, the data indicate that the mechanism of resistance to CdA may be dictated by changes in Ca2+-sensitive mitochondrial events.
Introduction
The nucleoside analog, 2-chlorodeoxyadenosine (CdA, cladribine) is used for the treatment of lymphoproliferative disorders such as hairy cell leukemia and chronic lymphocytic leukemia (CLL) as well as for therapy of multiple sclerosis and autoimmune disorders.1 CdA was initially described as a novel chemotherapeutic agent2 that demonstrated greater activity than previously used analogs. Subsequent experience, however, has shown that its efficacy can be limited by the emergence of cells refractory to treatment. Knowledge about the mechanism of CdA cytotoxicity is limited, and most studies have focused on the activation and/or deactivation of the drug.3 Among other proposed mechanisms of action of CdA are incorporation of the analog into DNA, inhibition of ribonucleotide reductase, inhibition of DNA repair, DNA strand break accumulation, and activation of poly (ADP-ribose) polymerase.4 However, these possibilities require progression of cells through cell cycle, which does not seem to occur in indolent leukemias.
CdA has also been shown to induce apoptosis in leukemic cells in vitro and in vivo.5 Early reports described endonuclease activation in lymphocytes isolated from patients with CLL treated with CdA, which could be blocked by Ca2+ chelators such as BAPTA (1,2-bis(o-aminophenoxy)ethane-N, N, N′, N′-tetraacetic acid tetra(acetoxymethyl) ester).5 More recent studies of nucleoside analog–induced apoptosis have centered around protease activation. The best characterized of the apoptotic proteases, caspases, are cysteine proteases that cleave their substrates (more than 100 identified to date) at a defined peptide sequence with an aspartate residue in the P1 position.6 Two major pathways of caspase activation have been identified. Ligation of death receptors, such as Fas or tumor necrosis factor receptor-1, can activate a caspase cascade, beginning with an apical receptor-associated caspase, such as caspase-8, and culminating in DNA fragmentation mediated by a caspase-3–regulated deoxyribonuclease. Another proposed pathway involves mitochondrial events. Cytochrome c, released from the intermembrane space of mitochondria into the cytosol, binds to Apaf-1, which is a homolog of the proapoptotic nematode protein Ced-4.7 Apaf-1 also contains a binding site for deoxyadenosine triphosphate (dATP); however, this requirement for dATP is not completely stringent. Adenosine triphosphate (ATP) as well as a host of intracellularly phosphorylated nuceloside analogs, including CdATP (2-chloro-2′-deoxyadenosine-5′-triphosphate), can also cooperate with cytochrome c to promote Apaf-1–mediated caspase activation.8 The discovery that CdATP could induce apoptosis via this mechanism was especially provocative because it explained how this drug might function in nonproliferating cells in the absence of incorporation into DNA. However, this proposed mechanism still requires the presence of cytochrome c in the cytosol.
Despite all the reports indicating that cytochrome c release is a critical event during apoptosis, the precise mechanism by which cytochrome c exits mitochondria is poorly understood. One model proposes that mitochondrial permeability transition (MPT) may be responsible for cytochrome c release.9 Induction of MPT is characterized by a sudden increase in the permeability of the mitochondrial inner membrane to small ions and solutes, leading to collapse of the membrane potential and swelling of the mitochondrial matrix. Calcium is a well-known inducer of MPT. Mitochondria protect the cell against damage due to high cytosolic Ca2+concentrations by readily absorbing Ca2+ up to amounts nearing 100 μM. A further increase in mitochondrial calcium concentration can trigger permeability transition.9 Normal lymphocytes and CLL cells treated with nucleoside analogs or glucocorticoid display drops in mitochondrial transmembrane potential and cytochrome c release.10,11 Also in CLL cells and thymocytes, glucocorticoid-induced apoptosis is associated with an early sustained increase in the cytosolic Ca2+concentration, and Ca2+ buffering agents block DNA fragmentation and delay cell death.12 13 These early studies focused on the concept of a Ca2+-dependent endonuclease and did not address the possibility that mitochondria might be a more immediate target for the observed Ca2+changes.
In the present study, we have generated 3 cell lines resistant to CdA. These lines have differing levels of deoxycytidine kinase (dCK) and deoxyguanosine kinase (dGK), the 2 enzymes that purportedly confer activity to the drug. Consequently, the resistant cell lines accumulate different levels of the phosphorylated drug, CdATP, intracellularly. However, our data show that resistance does not correlate solely with CdATP accumulation but also with changes at the mitochondrial level that confer selective resistance to Ca2+-mediated processes.
Materials and methods
Cell lines
The CCRF-CEM cell line was obtained from American Type Culture Collection (Rockville, MD). CdA-resistant subclones were selected from the original parent CCRF-CEM cell line by exposure to increasing concentrations of CdA until final concentrations of 25 nM, 100 nM, and 1 μM were reached in the respective cell lines. Cells were maintained in RPMI 1640 medium containing fetal calf serum (10%), penicillin (100 U/mL), streptomycin (100 μg/mL), and l-glutamine (2 mM) and were kept in humidified air, 5% CO2 at 37°C. The cells were subcultured twice weekly, and the CdA resistance was maintained by adding relevant concentrations of respective drugs. Cells were also routinely tested for mycoplasma contamination. The cells were cultured in drug-free medium for 3 passages before being used for experiments.
Antibodies and chemicals
All antibodies employed are commercially available. A murine cytochrome c–specific antibody was purchased from Pharmingen (San Diego, CA). A murine anti-Bax antibody was purchased from Trevigen (Gaithersburg, MD). Rabbit anti–Bcl-xL, murine anti-Bad, and murine anti-Bid antibodies were from Transduction Labs (Lexington, KY). Rabbit anti-Bak was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A murine Bcl-2 antibody was from Dako (Glostrup, Denmark). CdA was synthesized by Dr Zygmunt Kazimierczuk at the Foundation for the Development of Diagnostics and Therapy (Warsaw, Poland). 8-3H-CdA (4 Ci/mM [1.48 × 1011 Bq]) and [5-3H]-deoxycytidine (16.7 Ci/mM [6.18 × 1011 Bq]) were purchased from Moravek Biochemicals (Brea, CA). Dithiothreitol, staurosporine, phenylmethylsulfonyl fluoride, Nonidet P-40, and deoxycytidine were purchased from Sigma Aldrich (Stockholm, Sweden). RPMI 1640 medium, heat-inactivated fetal calf serum, l-glutamine, and penicillin-streptomycin were from Gibco (Life Technologies, Paisley, United Kingdom). DEVD-AMC (acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin) and LEHD-AMC (acetyl-Leu-Glu-His-Asp-7-amido-4-methylcoumarin) were purchased from the Peptide Institute (Osaka, Japan). Thapsigargin and BAPTA-AM were purchased from Calbiochem (San Diego, CA). Agonistic anti-Fas monoclonal antibodies (clone CH-11) were purchased from Medical & Biological Laboratories (Nagoya, Japan). Kaleidoscope protein standards for Western blot analysis were purchased from Bio-Rad (Hercules, CA).
Quantitation of dCK and dGK enzyme activities
Cells were suspended at 106/100 μL in an extraction buffer containing 50 mM Tris (pH 7.6), 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 20% glycerol, and 0.5% Nonidet P-40. The suspended cells were then frozen and thawed repeatedly 3 times and centrifuged at 14 000g in an Eppendorf centrifuge for 5 minutes at 4°C to remove cell debris. The supernatant was collected and used as a source of protein for the enzyme assays. The substrates used were deoxycytidine and CdA. The concentration was chosen to be 10 times higher than the Km value for the principle kinase phosphorylating the substrate. The assays were initiated by addition of 2 to 3 μg protein to a reaction mixture containing 50 mM Tris (pH 7.6), 5 mM MgCl2, 5 mM ATP, 4 mM dithiothreitol, 10 mM sodium fluoride, and substrate in a total volume of 25 μL. After incubation at 37°C for 30 minutes, 10-μL aliquots were withdrawn and spotted on Whatman DE81 papers. Filters were then washed as described by Spasokoukotskaja et al,14 eluted by 100 μL 0.4 M perchloric acid, and counted in 3 mL Ecoscint scintillation fluid in a liquid scintillation counter (RackBeta, LKB Wallac, Turku, Finland). Conditions to maintain the linear reaction rate were determined in preliminary experiments. The specific activity of the enzymes was expressed in picomoles per milligram of protein per minute. The activity of dGK was determined by using CdA in the presence of 1 mM deoxycytidine to block the dCK enzyme.
High-performance liquid chromatography analysis of CdA nucleotides
The accumulation of CdA nucleotides in cells was measured according to a previously described method.15 Briefly, 10 × 106 to 20 × 106 exponentially growing cells were exposed to 10 μM CdA for 12 hours. The cells were centrifuged at 1200g for 5 minutes, washed 2 times with cold phosphate-buffered saline (PBS), and CdA nucleotides were extracted by addition of 200 μL ice-cold 0.4 M perchloric acid containing 0.08 M triethylammonium phosphate. The suspension was mixed and brought to pH 6.2 by addition of 100 μL ice-cold 1.2 M KOH/0.5 M NH4H2PO4. After centrifugation at 13 100g for 5 minutes at 4°C, a 100-μL aliquot of the supernatant was injected into the Ultrasphere ODS (250 × 4.6 mm, 5 μm; Beckman Instruments, Fullerton, CA) column with the Guard Pak precolumn (Bondapak C18; Millipore, Milford, MA). The mobile phase consisted of triethylammonium phosphate buffer (0.08 M, pH 6.1) and methanol (89:11, vol/vol). The elution was carried out at flow rates of 1.5 mL/min at the ambient temperature (22°C). The temperature of the autosampler was maintained at 8°C. The concentration of the CdA nucleotides was determined at 265 nm by comparing the peak area of the CdA nucleotides in the cells to those of the standard substances.
Quantitation of DNA fragmentation
Quantification of apoptosis by propidium iodide staining and fluorescence-activated cell sorting (FACS) analysis was performed as described previously.16 Following incubation with various doses of CdA, cells were pelleted by centrifugation and resuspended in PBS containing 50 μg/mL propidium iodide, 0.1% Triton X-100, and 0.1% sodium citrate. Samples were read on the FL-3 channel (FACScan, Becton Dickinson, Mountain View, CA). A total of 10 000 events were quantitated.
Measurement of caspase-3 and caspase-9 activity
The measurement of DEVD-AMC and LEHD-AMC cleavage was performed in a fluorometric assay modified from Nicholson et al.17Cell lysates (generated from 1.0 × 106 cells) and substrate were combined in a standard reaction buffer: 100 mM HEPES (DEVD-AMC) or 100 mM 2-(N-morpholino)-ethanesulfonic acid for LEHD-AMC; 10% sucrose, 5 mM dithiothreitol, 0.0001% Nonidet P-40, and 0.1% 3-[(3-cholamidopropyl) dimethylammonio] propane-1-sulphonic acid, pH 7.25 (for DEVD-AMC) or pH 6.8 (for LEHD-AMC). This mixture was added in duplicate to a microtiter plate. The cleavage of the fluorogenic peptide substrate DEVD-AMC or LEHD-AMC (50 μM) was monitored by AMC liberation in a Fluoroscan II plate reader (LabSystems, Stockholm, Sweden) using 355-nm excitation and 460-nm emission wavelengths. Fluorescence was measured every 70 seconds during a 30-minute period, and fluorescence units were converted to picomoles of AMC using a standard curve generated with free AMC. Data from duplicate samples were then analyzed by linear regression.
Determination of cytochrome c release from mitochondria
Release of cytochrome c from mitochondria was measured by immunoblotting. Cells were incubated in the absence or presence of 100 nM CdA or 1 μM CdA for 12 hours, harvested by centrifugation, and gently lysed in an ice-cold STE buffer containing 250 mM sucrose, 25 mM Tris, and 1 mM ethylenediaminetetraacetic acid, pH 6.8. Lysates were immediately centifuged for 15 minutes at 15 000g. Supernatants were mixed with 2 × Laemmli reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and extracts from equal numbers of cells (10 × 106) were resolved by 12% SDS-PAGE. Polypeptides were transferred to nitrocellulose membranes (0.2 μM, Schleicher & Scheull, Keene, NH), and cytochrome c was detected with a monoclonal antibody (Pharmingen).
Quantitation of changes in ΔΨmito
The potential-sensitive fluorochrome tetramethylrhodamine ethyl ester (TMRE; Molecular Probes, Eugene, OR) was used to measure ΔΨmito. Cells were obtained by centrifugation and resuspended in HEPES buffer (10 mM HEPES-NaOH, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2, pH 7.4) containing 25 nM TMRE for 30 minutes at 37°C in the dark. Cells were then analyzed on a Becton Dickinson FACScan flow cytometer on the FL2 channel.
Isolation of cytosol and reconstitution of a cell-free model for caspase activation
Cytosols were prepared by lysing cells in STE buffer as described above. Cytosols were then incubated in the absence or presence of 1 mM dATP or 1 mM CdATP and 40 nM cytochrome c (Sigma, St Louis, MO) for 1 hour at 30°C. Samples were then analyzed for caspase-3–like activity as described above.
Induction of MPT in permeabilized cells
Cells were washed in Ca2+-free PBS and resuspended in a buffer containing 0.15 M KCl, 5 mM KH2PO4, 1 mM MgSO4, 5 mM succinate, 5 mM Tris, pH 7.4. After 2 minutes, cells were permeabilized with 0.005% digitonin, and 5 μM rotenone was added. MPT was induced by sequential additions of Ca2+ as previously described.18Ca2+ fluxes were monitored with a Ca2+-selective electrode.
Quantitation of intracellular Ca2+
Cells were incubated for 30 minutes at 37°C in RPMI containing 1 μM Indo-1 AM (1H-Indole-6-carboxylic acid, 2-[4-[bis[2-[(acetyloxy)methoxy]-2-oxoethyl]amino]-3-[2-[2-[bis[2-[(acetyloxy)methoxy]-2-oxoetyl]amino]-5-methylphenoxy]ethoxy]phenyl] (acetyloxy)methyl ester) (Molecular Probes). Cells were then harvested and washed in 1 mL of Ca2+-free PBS. Cells were resuspended in 1 mL Ca2+-free PBS and read on a Becton Dickinson FACS Vantage SE using a UV laser on the FL4 channel (excitation 351 nm, emission 530 nm).
Immunoblotting for Bcl-2 family proteins
For detection of Bax, Bcl-2, Bcl-xL, Bad, Bak, and Bid, cells were lysed for 1 hour at 4°C in a buffer containing 150 mM NaCl, 1% Triton X-100, a cocktail of protease inhibitors (Complete Mini tablets; Boehringer-Mannheim, Indianapolis, IN), and 25 mM Tris (pH 7.5). Insoluble material was sedimented by centrifugation for 5 minutes at 12 000g, and the supernatants were solubilized for 5 minutes at 100°C in Laemmli SDS-PAGE sample buffer containing 100 mM dithiothreitol. Polypeptides were resolved at 130 V on a 12% gel and electrophoretically transferred to 0.2- μm nitrocellulose membranes for 2 hours at 100 V. Membranes were blocked overnight in a TBS-T buffer (25 mM Tris [pH 8.0], 150 mM NaCl, and 0.5% Tween 20) containing 5% (wt/vol) nonfat dried milk. Blots were then probed for 4 hours with primary antibody and developed using a horseradish peroxidase–coupled antimouse (Bax, Bcl-2, Bad) or antirabbit (Bcl-xL, Bid, Bak) secondary antibody by enhanced chemiluminescence (ECL, Amersham, Uppsala, Sweden) according to the supplier's instructions.
Results
To investigate molecular mechanisms underlying CdA resistance, cell lines that were able to survive in the presence of CdA were generated and characterized. In brief, CEM cells were incubated in the presence of increasing concentrations of CdA to confer resistance. Thus, to generate the CEM CdA25 cell line, parental CEM 0 cells were treated with 5-nM increments of CdA, increasing every 2 weeks until the cells were in a final concentration of 25 nM CdA. To generate the CEM CdA100 cell line, some of the CEM CdA25 cells were further treated with 5-nM increments of CdA every 2 weeks until the cells were finally cultured in 100 nM CdA. The CEM CdA1000 cell line was generated in a similar manner, except we began with CEM CdA100 cells and treated them with 50 nM increasing increments every 2 weeks. Finally, a cell line resistant to 9-β-D-arabinofuranosyl-2-fluoroadenine (FaraA, fludarabine) that demonstrated cross-resistance to CdA was used for some of the studies described below. These cells were designated CEM FaraA1000 and were generated in an identical manner as the CEM CdA1000 cells, except they were treated with FaraA instead of CdA.
The conversion of CdA to its phosphorylated form is initiated by the generation of CdAMP from CdA. This reaction can be catalyzed by either dCK or dGK. We measured the activity of these enzymes in our cell lines using radioactive CdA as a substrate (Table1). In both CEM CdA100 and CdA1000 cells, dCK enzyme activity was significantly lower than levels seen in CEM 0 cells. However, in CEM CdA25 cells, dCK activity was only slightly less than levels in sensitive cells. We also measured dGK enzyme activity and found that all the resistant cell lines had varying degrees of heightened dGK activity (Table 1). Despite the increased dGK activity, intracellular CdATP levels were lower in resistant cell lines than in parental CEM cells (Table 1). In fact, in the case of the CdA1000 cells, levels of CdATP were extremely low and below the detection level of our UV-based high-performance liquid chromatography assay.
Intracellular drug activity and bioactivation enzymes vary among resistant cell lines
| . | CEM/wt . | CEM/CdA25 . | CEM/CdA100 . | CEM/CdA1000 . |
|---|---|---|---|---|
| CdATP formation (μM) after 12-h incubation with 1 μM CdA | 7.6 ± 0.6 | 4.5 ± 0.4 | 3.7 ± 0.6 | ND |
| dCK activity (pmole/mg/min) | 245 ± 18 | 204 ± 5 | 142 ± 14 | 4 ± 1 |
| dGK activity (pmole/mg/min) | 313 ± 24 | 428 ± 48 | 502 ± 4 | 426 ± 15 |
| . | CEM/wt . | CEM/CdA25 . | CEM/CdA100 . | CEM/CdA1000 . |
|---|---|---|---|---|
| CdATP formation (μM) after 12-h incubation with 1 μM CdA | 7.6 ± 0.6 | 4.5 ± 0.4 | 3.7 ± 0.6 | ND |
| dCK activity (pmole/mg/min) | 245 ± 18 | 204 ± 5 | 142 ± 14 | 4 ± 1 |
| dGK activity (pmole/mg/min) | 313 ± 24 | 428 ± 48 | 502 ± 4 | 426 ± 15 |
Levels of CdATP were quantitated by high-performance liquid chromatography as described in “Materials and methods” after cells were incubated in the presence of 1 μM CdA for 12 hours. Enzyme activity of dCK and dGK was measured in extracts made from sensitive and resistant cells. Radioactive CdA was used as a substrate to measure both dCK and dGK activities. In the case of dGK enzyme activity, measurements were conducted in the presence of excess (1 mM) deoxycytidine to block dCK enzyme activity. The specific activities of dCK and dGK are expressed in picomoles per milligram of protein per minute. Data shown are from 1 typical experiment performed 3 times in duplicate.
ND indicates not detectable.
Resistant cell lines do not undergo DNA fragmentation when treated with CdA and are defective in caspase-3– and caspase-9–like activities
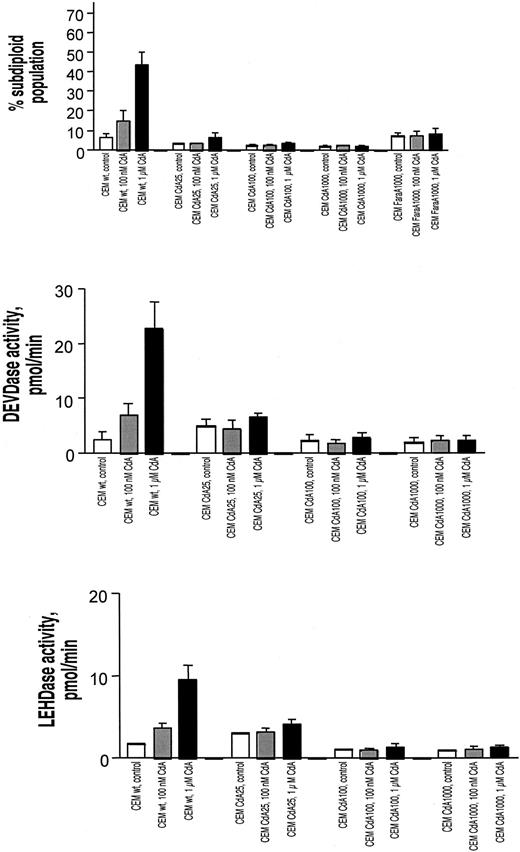
Several reports indicate that CdA induces apoptosis in parental CEM cells. To ascertain the degree of resistance in the various cell lines, we quantitated a classical biochemical event associated with apoptosis: the cleavage of chromatin into oligonucleosomal fragments. By staining cells with propidium iodide to visualize cell cycle distribution, cells with subdiploid DNA content were designated to have undergone cleavage of chromatin into oligonucleosomal fragments.16 From Figure 1A it is apparent that none of the resistant cell lines underwent DNA fragmentation after treatment with CdA. Notably, all of the resistant cell lines, including the FaraA1000 cells, demonstrated similarly low levels of DNA fragmentation despite the earlier-mentioned differences in CdATP formation.
DNA fragmentation, caspase-3–like activity, and caspase-9–like activity in sensitive and resistant cell lines treated with CdA.
All cells were treated in the absence or presence of 100 nM or 1 μM CdA. (A) DNA fragmentation. Cells were incubated in the presence or absence of drug for 48 hours. Subsequent propidium iodide staining and FACS analysis (as described in “Materials and methods”) was conducted to quantitate the number of cells with subdiploid amounts of DNA. This subdiploid population was indicative of cells with fragmented DNA and is depicted graphically. Open bars depict cells treated with diluent alone, gray bars represent cells treated with 100 nM CdA, and black bars show cells treated with 1 μM CdA. (B) Caspase-3–like activity. Cells were incubated in the presence or absence of CdA for 12 hours. DEVDase activity was measured using a commercially available labeled peptide, DEVD-AMC. Cleavage of this fluorescently labeled peptide and consequent release of free AMC, which was measurable with the use of a spectrofluorimeter, indicated the presence of caspase-3–like proteases in cell extracts. Color designation of bars is identical to that for panel A. (C) Caspase-9–like activity. Cells were incubated in the presence or absence of CdA for 12 hours. LEHDase activity was quantitated using the caspase-9 substrate, LEHD-AMC. Cleavage of this fluorescently labeled peptide and consequent release of free AMC, which was measurable with the use of a spectrofluorimeter, indicated the presence of caspase-9–like proteases in cell extracts. Results shown for panels A-C are representative of 4 separate experiments.
DNA fragmentation, caspase-3–like activity, and caspase-9–like activity in sensitive and resistant cell lines treated with CdA.
All cells were treated in the absence or presence of 100 nM or 1 μM CdA. (A) DNA fragmentation. Cells were incubated in the presence or absence of drug for 48 hours. Subsequent propidium iodide staining and FACS analysis (as described in “Materials and methods”) was conducted to quantitate the number of cells with subdiploid amounts of DNA. This subdiploid population was indicative of cells with fragmented DNA and is depicted graphically. Open bars depict cells treated with diluent alone, gray bars represent cells treated with 100 nM CdA, and black bars show cells treated with 1 μM CdA. (B) Caspase-3–like activity. Cells were incubated in the presence or absence of CdA for 12 hours. DEVDase activity was measured using a commercially available labeled peptide, DEVD-AMC. Cleavage of this fluorescently labeled peptide and consequent release of free AMC, which was measurable with the use of a spectrofluorimeter, indicated the presence of caspase-3–like proteases in cell extracts. Color designation of bars is identical to that for panel A. (C) Caspase-9–like activity. Cells were incubated in the presence or absence of CdA for 12 hours. LEHDase activity was quantitated using the caspase-9 substrate, LEHD-AMC. Cleavage of this fluorescently labeled peptide and consequent release of free AMC, which was measurable with the use of a spectrofluorimeter, indicated the presence of caspase-9–like proteases in cell extracts. Results shown for panels A-C are representative of 4 separate experiments.
Activation of caspases is generally considered to be a requisite event during apoptosis. Fourteen mammalian caspases have been described to date and can be grouped by their substrate specificity. Fluorescently tagged versions of these preferred amino acid substrates can be used to investigate the presence of these enzyme activities. We conducted such assays for caspase-3–like (DEVDase) (Figure 1B) and caspase-9–like (LEHDase) (Figure 1C) activity. The results showed that upon drug treatment, resistant cell lines displayed uniformly low levels of DEVDase and LEHDase activities.
Cytosols isolated from resistant cell lines are capable of activating caspases
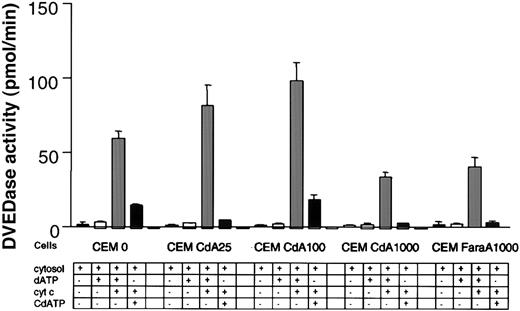
Because our data indicated that CdA treatment was not accompanied by activation of caspase-3 and -9 in resistant cells, we explored whether caspases could be activated in a cell-free system in these cells. Cytosols were prepared from all cell lines and incubated with dATP and cytochrome c. DEVDase activity was then monitored spectrofluorometrically. In the absence of added cytochrome c and/or dATP, none of the cytosols displayed any caspase-3–like activity. This indicates that mitochondrial release of cytochrome c did not occur during preparation of the cytosols. When cytosols incubated in the presence of added cytochrome c and dATP were tested for caspase-3–like activity, we observed robust responses in cytosols from both sensitive and resistant cells (Figure 2). In fact, in 2 of the resistant cell lines (CdA25 and CdA100), DEVDase activity was higher than that seen in parental cells. We included cytosols from the FaraA1000 cells in these experiments and noted caspase activity comparable to that seen in the CdA1000 cells.
Caspase activity in a cell-free system.
Cytosols were isolated using an STE buffer as described in “Materials and methods.” Cytosols were then incubated at 30°C for 1 hour in the absence or presence of 1 mM dATP or 1 mM CdATP and in the absence or presence of 40 nM cytochrome c. Extracts were then plated onto a 96-well plate, and caspase-3–like activity was quantitated with the DEVD-AMC reagent on a spectofluorometer as described previously. Results are characteristic of 3 independent experiments.
Caspase activity in a cell-free system.
Cytosols were isolated using an STE buffer as described in “Materials and methods.” Cytosols were then incubated at 30°C for 1 hour in the absence or presence of 1 mM dATP or 1 mM CdATP and in the absence or presence of 40 nM cytochrome c. Extracts were then plated onto a 96-well plate, and caspase-3–like activity was quantitated with the DEVD-AMC reagent on a spectofluorometer as described previously. Results are characteristic of 3 independent experiments.
Others have shown that ATP as well as a number of purine deoxyribonucleotide analogs can cooperate with cytochrome c to activate caspases.8 In fact, this has been put forth as a potential explanation for the activity of these nucleoside analogs in indolent lymphoproliferative diseases. We tested the validity of this hypothetical mechanism in the context of our resistant cells. Incubation of cytosols from parental CEM cells with CdATP and cytochrome c did result in caspase-3–like activity, although the response was not as high as that seen with dATP and cytochrome c. In the cytosols isolated from resistant cell lines incubated with cytochrome c and CdATP, DEVDase activity varied greatly (Figure 2). The CEM CdA100 cells exhibited a response similar to that seen in the parental CEM cells. However, none of the other cell lines showed appreciable levels of caspase-3–like activity. These diverse responses suggest that an inability of CdATP to activate caspases via binding to Apaf-1 does not account for resistance to CdA.
Lack of mitochondrial perturbations in all resistant cell lines
A drop in mitochondrial transmembrane potential (ΔΨmito), which can be quantitated with commercially available lipophilic cationic dyes, appears to be a frequently occurring event during apoptosis.19 Another, possibly independent mitochondrial perturbation associated with apoptosis is the release of cytochrome c, which is normally sequestered in the intermembrane space of mitochondria, into the cytosol. This event is considered essential for caspase activation mediated by the apoptosome pathway.
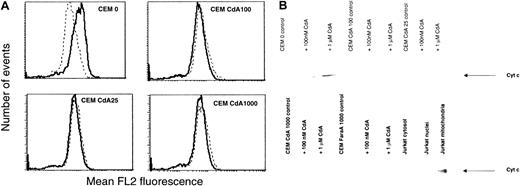
We used TMRE, a dye that loses its fluorescence upon dissipation of mitochondrial inner membrane potential, to quantitate the effect of CdA treatment on ΔΨmito. In parental CEM 0 cells, 1 μM CdA caused a marked decrease in fluorescence, suggesting a drop in ΔΨmito (Figure 3A). However, in resistant cell lines, CdA treatment had no effect on ΔΨmito.
Effect of CdA treatment on mitochondria in sensitive and resistant cell lines.
(A) Effect of CdA treatment on ΔΨmito. Cells were incubated in the absence (solid line) or presence (dotted line) of 1 μM CdA for 12 hours and then stained with the cationic, lipophilic, membrane potential-sensitive fluorochrome TMRE (25 nM) for 30 minutes. Samples were read on the FL2 channel of a Becton Dickinson FACScan. Data shown are representative of 3 independent experiments. (B) Effect of CdA treatment on release of cytochrome c from mitochondria into cytosol. Cells were incubated in the absence or presence of 100 nM or 1 μM CdA for 12 hours. Cytosols were isolated as described in “Materials and methods,” and the presence of cytochrome c was determined by Western blotting. Jurkat subcellular fractions were included as a positive control to illustrate that although there was no cytochrome c release in resistant cells, the protein was detectable in mitochondrial fractions of untreated Jurkat cells.
Effect of CdA treatment on mitochondria in sensitive and resistant cell lines.
(A) Effect of CdA treatment on ΔΨmito. Cells were incubated in the absence (solid line) or presence (dotted line) of 1 μM CdA for 12 hours and then stained with the cationic, lipophilic, membrane potential-sensitive fluorochrome TMRE (25 nM) for 30 minutes. Samples were read on the FL2 channel of a Becton Dickinson FACScan. Data shown are representative of 3 independent experiments. (B) Effect of CdA treatment on release of cytochrome c from mitochondria into cytosol. Cells were incubated in the absence or presence of 100 nM or 1 μM CdA for 12 hours. Cytosols were isolated as described in “Materials and methods,” and the presence of cytochrome c was determined by Western blotting. Jurkat subcellular fractions were included as a positive control to illustrate that although there was no cytochrome c release in resistant cells, the protein was detectable in mitochondrial fractions of untreated Jurkat cells.
To determine whether cytochrome c was released from mitochondria upon CdA treatment, we prepared cytosols from all the cell lines and conducted Western blot analysis using a specific antibody to cytochrome c. This experiment revealed that in sensitive cells, CdA treatment resulted in the appearance of a 14-kd band corresponding to cytochrome c in the cytosolic fraction (Figure 3B). The band was stronger in cytosol from cells treated with 1 μM CdA (lane 3) than in cytosol from cells treated with 100 nM CdA (lane 2). The absence of a similar band in any of the resistant cell lines, including FaraA1000 cells, treated with CdA indicates that cytochrome c release did not occur.
Protein levels of antiapoptotic Bcl-2 family members are similar in sensitive and resistant cells
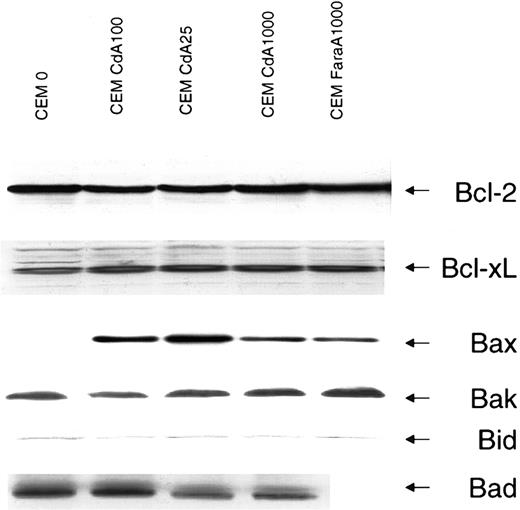
Overexpression of Bcl-2 abrogates apoptosis in numerous settings and also protects against apoptosis induced by microinjection of low doses of cytochrome c into cytosol.20 Bcl-2 family members have been ascribed pore-forming abilities, and several studies have shown localization of these proteins to mitochondrial membranes.21 In fact, it has been suggested that proapoptotic Bcl-2 homologs may potentiate cytochrome c release by inserting into mitochondria and forming a channel either alone or in combination with constituents of the MPT. Alternatively, antiapoptotic family members may attenuate apoptosis by binding and inactivating these pores. We measured levels of Bcl-2 in parental and resistant cell lines and found no difference in expression (Figure4). Bcl-xL levels also did not differ in sensitive or resistant cells (Figure 4).
Protein levels of Bcl-2 family members in sensitive and resistant cells.
Protein levels were detected by Western blotting as described in “Materials and methods.” The antiapoptotic Bcl-2 family members, Bcl-2 and Bcl-xL, and the proapoptotic members, Bax, Bak, Bid, and Bad, were investigated because of their relevance to the process of cytochrome c release. Equal loading is evident from the Bcl-2, Bcl-xL, Bak, and Bid blots. Protein levels for Bcl-2, Bcl-xL, Bax, Bak, and Bid were also measured in a cell line developed with resistance to FaraA (CEM FaraA1000) but displaying cross-resistance to CdA. Data shown are representative of that obtained in 3 independent experiments.
Protein levels of Bcl-2 family members in sensitive and resistant cells.
Protein levels were detected by Western blotting as described in “Materials and methods.” The antiapoptotic Bcl-2 family members, Bcl-2 and Bcl-xL, and the proapoptotic members, Bax, Bak, Bid, and Bad, were investigated because of their relevance to the process of cytochrome c release. Equal loading is evident from the Bcl-2, Bcl-xL, Bak, and Bid blots. Protein levels for Bcl-2, Bcl-xL, Bax, Bak, and Bid were also measured in a cell line developed with resistance to FaraA (CEM FaraA1000) but displaying cross-resistance to CdA. Data shown are representative of that obtained in 3 independent experiments.
The proapoptotic Bcl-2 family members Bax, Bak, Bid, and Bad have been reported to promote cytochrome c release. A recent study using cells from mice deficient in both Bax and Bak reported that murine embryonic fibroblasts from these animals are resistant to several diverse triggers of apoptosis.22 We sought to determine the status of these 2 proteins in our cell lines by Western blot. Interestingly, the proapoptotic family member Bax was virtually undetectable in parental cells, while in all resistant cells Bax levels were significantly higher (Figure 4). Bak, however, was present in equivalent amounts in both sensitive and resistant cells. Bid and Bad are 2 proapoptotic Bcl-2 family members that contain only the BH3 domain. Expression of neither Bid nor Bad differed between sensitive and resistant cells (Figure 4).
Lack of cytochrome c release correlates with decreased susceptibility of mitochondria to undergo MPT in resistant cells
Apart from the contribution of Bcl-2 family members, an alternative mechanism of cytochrome c release is due to MPT with subsequent swelling and rupture of the outer membrane.9 As described above, resistant cells did not release cytochrome c or lose ΔΨmito upon CdA treatment. Thus, we hypothesized that this might be due to a decreased susceptibility to MPT induction in resistant cells.
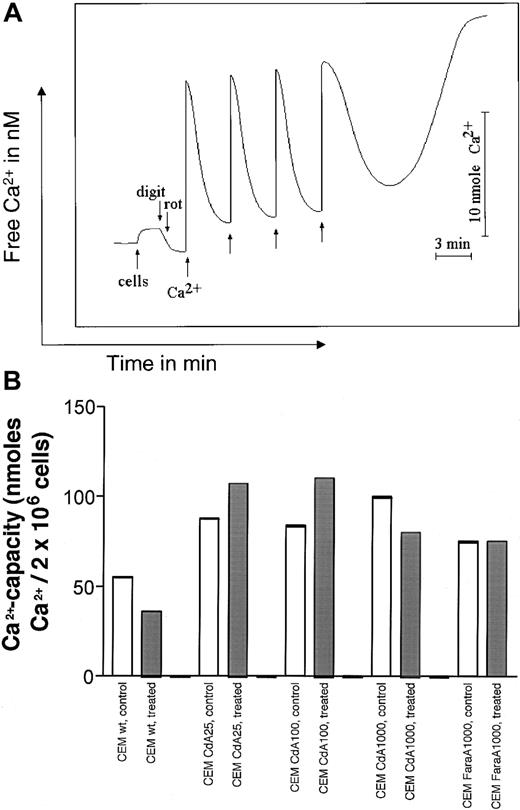
It is well known that Ca2+ is an obligatory component for MPT induction.9 Accumulation of Ca2+ by mitochondria results in mitochondrial swelling, dissipation of membrane potential, and induction of MPT, which is accompanied by release of accumulated cations and low molecular weight components (such as adenine and pyridine nucleotides).9 In Figure5B, MPT was induced in digitonin-permeabilized cells by sequential additions of Ca2+. Permeabilization of plasma membrane by digitonin allows energized mitochondria to accumulate Ca2+ when added to the cell suspension.18 As can be seen in Figure 5A, addition of Ca2+ to permeabilized cell suspensions resulted in a rapid elevation of the Ca2+ concentration in the buffer followed by the restoration of the initial level due to accumulation of Ca2+ in mitochondria. Aliquots of Ca2+ were added to the permeabilized cells until spontaneous Ca2+ release occurred (Figure 5A). In Figure5B, the amount of Ca2+ required to trigger spontaneous Ca2+ release (indicative of MPT induction) was tallied and is represented graphically. The amount of Ca2+ necessary for MPT induction in resistant untreated cells was higher than in untreated sensitive cells (Figure 5B), indicating that the mitochondria of CdA-resistant cells are able to buffer higher amounts of Ca2+ before undergoing MPT induction. Interestingly, CdA treatment sensitized control cells, making them even more vulnerable toward MPT, whereas in CdA25 and CdA100 cells, more Ca2+was necessary to induce MPT after CdA treatment.
Quantitation of Ca2+-induced MPT induction in sensitive and resistant cells.
(A) Illustration of method of detection. Curves were generated using a Ca2+-sensitive electrode. Two million cells were resuspended in a buffer containing 0.15 mM KCl, 5 mM KH2PO4, 1 mM MgSO4, 5 mM succinate, and 5 mM Tris, pH 7.4 (see “Materials and methods”). After 2 minutes, cells were permeabilized with 0.005% digitonin and 5 μM rotenone was added to keep mitochondrial pyridine nucleotides in a reduced state. Ca2+ addition, in 20 nM increments, resulted in a steep increase in Ca2+, followed by restoration of the initial level due to Ca2+ accumulation in mitochondria, at which point 20 nM Ca2+ was again added. The Ca2+ capacity was calculated by adding the total amount of Ca2+ that could be taken up in mitochondria before MPT induction occurred, and mitochondrial Ca2+ was released. (B) Ca2+ capacity in sensitive and resistant cells treated with diluent (open bars) or 1 μM CdA (gray bars) for 12 hours. MPT was measured as described in panel A, and the amount of Ca2+ required for MPT induction is expressed graphically. Data shown are representative of 3 separate experiments.
Quantitation of Ca2+-induced MPT induction in sensitive and resistant cells.
(A) Illustration of method of detection. Curves were generated using a Ca2+-sensitive electrode. Two million cells were resuspended in a buffer containing 0.15 mM KCl, 5 mM KH2PO4, 1 mM MgSO4, 5 mM succinate, and 5 mM Tris, pH 7.4 (see “Materials and methods”). After 2 minutes, cells were permeabilized with 0.005% digitonin and 5 μM rotenone was added to keep mitochondrial pyridine nucleotides in a reduced state. Ca2+ addition, in 20 nM increments, resulted in a steep increase in Ca2+, followed by restoration of the initial level due to Ca2+ accumulation in mitochondria, at which point 20 nM Ca2+ was again added. The Ca2+ capacity was calculated by adding the total amount of Ca2+ that could be taken up in mitochondria before MPT induction occurred, and mitochondrial Ca2+ was released. (B) Ca2+ capacity in sensitive and resistant cells treated with diluent (open bars) or 1 μM CdA (gray bars) for 12 hours. MPT was measured as described in panel A, and the amount of Ca2+ required for MPT induction is expressed graphically. Data shown are representative of 3 separate experiments.
CdA-induced apoptosis requires an increase in intracellular Ca2+ and is inhibited by the intracellular Ca2+chelator BAPTA-AM
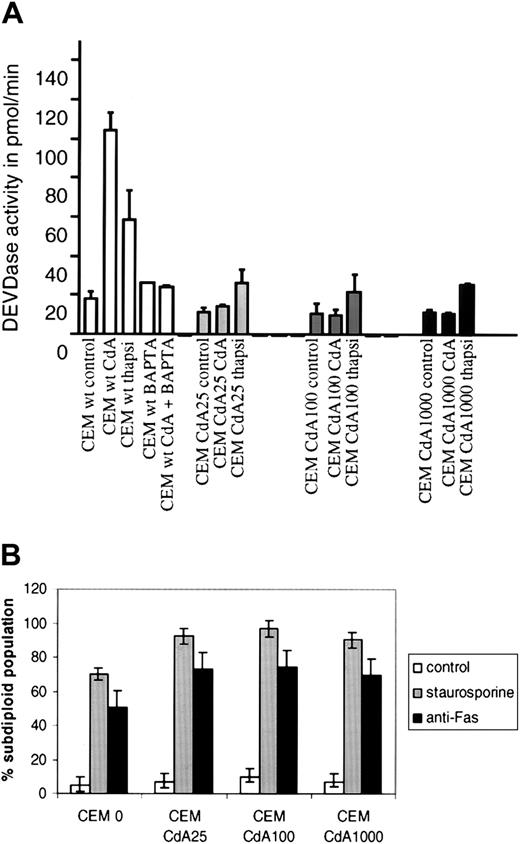
In further experiments, we explored the potential importance of these differences in Ca2+ buffering capacity. We first examined the contribution of Ca2+ to the effects of CdA in sensitive parental CEM cells. If CdA-induced apoptosis requires an increase in intracellular Ca2+ levels, agents that bind Ca2+ should reverse the effects of CdA in these cells. To determine whether this was the case, we pretreated parental cells with a cell-permeant Ca2+ chelator, BAPTA-AM, for 15 minutes prior to CdA treatment. We found that CdA-induced caspase-3–like activity (Figure6A) and DNA fragmentation (data not shown) were completely abrogated by 50 μM BAPTA-AM in CEM 0 cells.
Inhibition of CdA-induced apoptosis by BAPTA-AM and selective cross-resistance to thapsigargin.
(A) Cells were treated with 1 μM thapsigargin or 1 μM CdA for 12 hours, and DEVDase activity was quantitated as described in “Materials and methods.” CdA-induced caspase-3 activity was abrogated by preincubating the cells for 15 minutes in the presence of 50 μM BAPTA-AM, a cell-permeant Ca2+chelator. (B) Cells were treated with diluent alone, staurosporine (100 nM) for 6 hours, or anti-Fas mAb (250 ng/mL) for 3 hours. DNA fragmentation was assessed by propidium iodide staining and subsequent FACS analysis on the FL3 channel of a Becton Dickinson FACScan.
Inhibition of CdA-induced apoptosis by BAPTA-AM and selective cross-resistance to thapsigargin.
(A) Cells were treated with 1 μM thapsigargin or 1 μM CdA for 12 hours, and DEVDase activity was quantitated as described in “Materials and methods.” CdA-induced caspase-3 activity was abrogated by preincubating the cells for 15 minutes in the presence of 50 μM BAPTA-AM, a cell-permeant Ca2+chelator. (B) Cells were treated with diluent alone, staurosporine (100 nM) for 6 hours, or anti-Fas mAb (250 ng/mL) for 3 hours. DNA fragmentation was assessed by propidium iodide staining and subsequent FACS analysis on the FL3 channel of a Becton Dickinson FACScan.
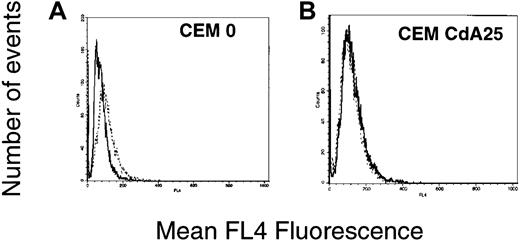
The preceding results suggest that changes in intracellular Ca2+ should be detectable in sensitive cells after CdA treatment. Moreover, if the altered Ca2+ buffering capacity of mitochondria contributes to resistance, the Ca2+ fluxes should be blunted in the resistant cells. To address these possibilities, Indo-1 AM, a cell-permeant UV light–excitable Ca2+ indicator that can be detected using a flow cytometer, was used to assess changes in cytosolic-free Ca2+. Within 30 minutes after addition of CdA, an increase in Indo-1 AM fluorescence was apparent. (Figure7). At later time points, this increase was not detectable. In contrast, CdA25 cells did not display an increase in Indo-1 fluorescence after CdA treatment, indicating that the rise in intracellular Ca2+ levels was, as predicted, blunted in these cells (Figure 7). CdA100 and CdA1000 cells likewise failed to exhibit increases in cytosolic Ca2+ (data not shown).
Quantitation of intracellular Ca2+ changes in sensitive and resistant cells.
Cells were treated with diluent alone (solid line) or 1 μM CdA (dotted line) for 30 minutes. Five minutes into the incubation, 1 μM Indo-1 AM was added to the culture dishes. At the end of the incubation, cells were washed with and then resuspended in Ca2+-free PBS and read on a Becton Dickinson FACS Vantage using a UV laser. Histograms depict Indo-1 fluorescence, which indicates intracellular Ca2+ levels.
Quantitation of intracellular Ca2+ changes in sensitive and resistant cells.
Cells were treated with diluent alone (solid line) or 1 μM CdA (dotted line) for 30 minutes. Five minutes into the incubation, 1 μM Indo-1 AM was added to the culture dishes. At the end of the incubation, cells were washed with and then resuspended in Ca2+-free PBS and read on a Becton Dickinson FACS Vantage using a UV laser. Histograms depict Indo-1 fluorescence, which indicates intracellular Ca2+ levels.
CdA-resistant cells are cross-resistant to thapsigargin
Because CdA sensitivity appears to rely on intracellular Ca2+ increases that are blocked in the CdA-resistant lines, we postulated that the CdA-resistant cells would also be resistant to other apoptosis inducers that rely on perturbations of intracellular Ca2+ homeostasis. Thapsigargin is a cell-permeable lactone that releases endoplasmic reticular (ER) stores of Ca2+ by inhibiting ER Ca2+-ATPases. In thymocytes and a number of other cells, thapsigargin is known to induce apoptosis.23 In response to thapsigargin, parental CEM cells readily underwent apoptosis as measured by caspase-3 activation (Figure 6A) and DNA fragmentation (data not shown). In contrast, CdA-resistant cells were resistant to the apoptotic effects of this agent (Figure 6A). However, CdA-resistant cells were not pan-resistant to diverse triggers of apoptosis and in fact appeared to be more sensitive to Fas or staurosporine treatment than sensitive cells (Figure 6B).
Discussion
Although the development of nucleoside analogs has led to better management of indolent lymphoproliferative disorders, the emergence of refractory disease remains an obstacle. A poor understanding of the mechanism of CdA action has compounded this problem. In the present study, we have developed a series of CdA-resistant cell lines and examined the possibility that the resistance was related to changes in metabolic activation. Because there was not a good correlation between CdATP formation and sensitivity, we examined the action of CdA in the sensitive and resistant cells in greater detail. Results of this analysis demonstrated that caspase-3 could be activated by addition of cytochrome c and dATP to cytosol from sensitive or resistant cells. However, cytochrome c release, MPT, and caspase-3 activation were impaired in the resistant cells. Further experiments indicated that the resistant cells had increased mitochondrial Ca2+ buffering capacity and were cross-resistant to thapsigargin, an agent that triggers apoptosis by increasing cytosolic free Ca2+concentrations. These results have important implications for the current understanding of the action of CdA in both sensitive and resistant cells.
In previous studies of CdA resistance, enzymes controlling the activity of nucleoside analogs have been extensively studied, but contradictory results have been reported. In some studies dCK levels correlate with patient responses to CdA,24 whereas in other studies no such correlations were found.25 In fact, leukemia patients have been shown to exhibit varying and heterogenous levels of dCK that are not always indicative of in vivo responses.25Consistent with this clinical situation, our resistant cell lines displayed varying levels of dCK that did not correlate with CdA sensitivity. Other enzymatic factors important in CdA biotransformation include dGK and 5′ nucleotidase (5′NT). The ability of dGK to efficiently phosphorylate CdA and the mitochondrial localization of this enzyme26 prompted us to examine levels of this enzyme in our cell lines. However, we observed that CdA-resistant cells display various degrees of heightened dGK activity, which did not correlate with CdATP formation (Table 1). A recent study illustrated that overexpression of mitochondrial dGK caused increased sensitivity to several purine nucleoside analogs, including CdA, in solid tumor cell lines.27 Based on this observation, it was proposed that incorporation of CdA into mitochondrial DNA was a potential mechanism of drug action and dGK facilitated this process. However, a separate study concluded that mitochondrial DNA content was not affected by CdA.28 Moreover, in indolent leukemias, incorporation of analogs into mitochondrial DNA is unlikely. Other activities that participate in CdA biotransformation include a 5′NT enzyme, which opposes dCK and dGK phosphorylation of CdA. Although heightened 5′NT activity has been linked to drug resistance, overexpression of 5′NT in a human kidney cell line did not increase resistance to CdA.29 Because the sensitivity of our cell lines did not correlate with levels of CdATP formed (Table 1), we considered it unlikely that alterations in CdA biotransformation provided the sole explanation for the resistance.
The recent suggestion that CdATP may directly participate in caspase activation by binding to Apaf-130 prompted us to examine the possibility that CdATP-induced caspase activation might be specifically lost in the resistant cells due to metabolic inactivation of CdATP or altered sensitivity of Apaf-1. We found that CdATP was a poor trigger of caspase activation in sensitive cells and that cytosols made from some CdA-resistant cells could also elicit caspase activation when incubated with CdATP and cytochrome c (Figure 2). Although we could not rule out the possibility that altered sensitivity of the apoptotic apparatus to CdATP contributes to the inhibition of apoptosis in some of the resistant lines, it cannot contribute in all of them.
In contrast, all of the resistant lines displayed diminished CdA-induced release of cytochrome c, the other requirement for caspase activation by the Apaf-1–mediated pathway. Other mitochondrial perturbations associated with apoptosis—notably, loss of ΔΨmito—also failed to occur in the resistant cells. Direct estimation of MPT induction in permeabilized cells revealed that resistant cells demonstrated greater resistance toward MPT induction by Ca2+.
One model for cytochrome c release cites the ability of Bcl-2 family proteins to insert into mitochondrial membranes, thus creating a pore capable of shuttling cytochrome c from the intermembrane space of mitochondria into the cytosol.21 Bax, Bid, Bik, and Bak have all been implicated in cytochrome c release by this mechanism, and their effects are antagonized by antiapoptotic members such as Bcl-2 and Bcl-xL. In the current study, there was no difference in either Bcl-2 or Bcl-xL expression between sensitive and resistant lines (Figure 4). Unexpectedly, we saw that expression of the proapoptotic family member Bax was virtually undetectable in parental cells. Because these cells release cytochrome c without appreciable levels of Bax, this polypeptide clearly is dispensable for CdA-induced cytochrome c release. Bak, Bid, and Bad, all of which were detected in the parental cells, may contribute to CdA-induced cytochrome c release in the absence of Bax. Surprisingly, we observed Bax levels in all of the resistant lines. Although the up-regulation of Bax is unlikely to contribute to the observed CdA and thapsigargin resistance (Figure 6A), it might explain the slightly enhanced apoptotic responses observed when the CdA-selected cells were treated with staurosporine and agonistic Fas antibody (Figure 6B). In any case, it does not appear that changes in the Bcl-2 family members examined explained the CdA resistance.
Although the present study has focused on caspase activation via a mitochondrial route, death receptor ligation represents a second way in which a caspase cascade may be initiated. Up-regulation of the Fas-FasL system has been proposed to contribute to the cytotoxic effects of certain drugs.31 Whether CdA–induced apoptosis is triggered in this manner has been a matter of some controversy. One recent study contends that up-regulation of Fas and FasL occurs during CdA-induced apoptosis in MOLT-4 cells, a lymphoma cell line.32 Other studies using CEM cells state that the Fas pathway is not required for CdA-induced apoptosis, because preincubation with blocking antibodies to Fas (ZB4) or FasL (NOK-1) does not inhibit CdA-induced apoptosis.33,34 Our results complement the results of these latter studies by showing that CdA-resistant cells retain sensitivity to Fas ligation (Figure 6B). Also, our data corroborate and extend a recent study showing that CdA directly affects mitochondria.35
Key experiments in the present study have included a cell line developed specifically with resistance to FaraA but exhibiting cross-resistance to CdA (FaraA1000). Like the CdA-resistant cells, these cells also did not undergo cytochrome c release (Figure 3B) or Ca2+-induced MPT (Figure 5B) while expressing high levels of Bax protein (Figure 4). This suggests that mitochondrial events may not only mediate CdA sensitivity but also control responses to other nucleoside analogs. Interestingly, our observations mirror a clinical study in which CdA treatment had no effect in patients with FaraA-resistant disease.36
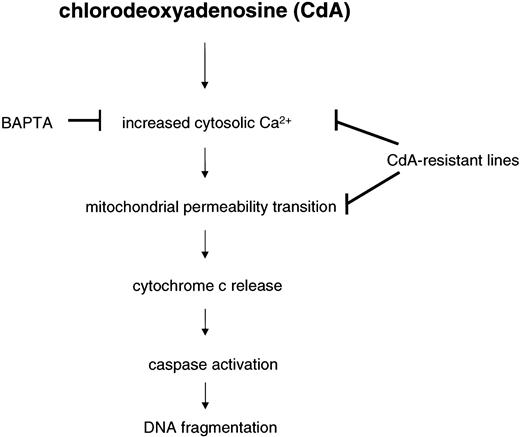
Mitochondria from CdA-resistant cells not only display diminished release of cytochrome c upon CdA treatment but are also less susceptible to Ca2+-induced MPT. Several additional observations indicate that an enhanced Ca2+ binding capacity might contribute to the CdA resistance. First, we observed increased cytosolic free Ca2+ within minutes of CdA treatment in sensitive cells (Figure 7). Second, treatment of the sensitive cells with the Ca2+ chelator BAPTA prevented CdA-induced apoptosis (Figure 6A), providing evidence that the transient Ca2+ increase normally plays a role in the cytotoxicity of CdA. Third, the increase in cytosolic free Ca2+ is blunted in the resistant cells, as would be predicted if they had increased Ca2+ uptake into mitochondria (Figure 7). Finally, the CdA-resistant cells are selectively cross-resistant to thapsigargin, which triggers apoptosis by elevating cytosolic free Ca2+, but not to staurosporine or anti-Fas antibody (Figure 6B). Collectively, these observations support a model (Figure 8) in which transient CdA-induced elevations in Ca2+ lead to MPT, cytochrome c release, and caspase activation, whereas enhanced Ca2+ buffering capacity leads to resistance.
Proposed scheme for CdA-induced apoptosis in CEM cells.
Resistance to CdA is characterized by a lack of cytochrome c release, which is preceded by an abrogation of cytosolic Ca2+elevations and subsequent induction of MPT. CdA treatment causes a decrease in mitochondrial Ca2+ buffering capacity in sensitive CEM 0 cells but not in any of the resistant cell lines. Consequently, there is no caspase-3–like or caspase-9–like activity in CdA-resistant cells. The cell-permeable Ca2+ chelator, BAPTA-AM (50 μM), blocks CdA-induced caspase activation and DNA fragmentation in parental cells, suggesting that mitochondria may be a target for Ca2+-dependent apoptotic events initiated by CdA.
Proposed scheme for CdA-induced apoptosis in CEM cells.
Resistance to CdA is characterized by a lack of cytochrome c release, which is preceded by an abrogation of cytosolic Ca2+elevations and subsequent induction of MPT. CdA treatment causes a decrease in mitochondrial Ca2+ buffering capacity in sensitive CEM 0 cells but not in any of the resistant cell lines. Consequently, there is no caspase-3–like or caspase-9–like activity in CdA-resistant cells. The cell-permeable Ca2+ chelator, BAPTA-AM (50 μM), blocks CdA-induced caspase activation and DNA fragmentation in parental cells, suggesting that mitochondria may be a target for Ca2+-dependent apoptotic events initiated by CdA.
The model depicted in Figure 8 explains not only the data presented above but also the previous demonstration that Ca2+chelators can prevent CdA-induced apoptosis in lymphocytes isolated from CLL patients.5 On the other hand, it also raises several important questions. In attempting to understand how transient elevations in cytosolic-free Ca2+ lead to MPT, we performed additional experiments that rule out a number of possibilities. Nur77 is a Ca2+-dependent transcription factor that has recently been reported to translocate from nuclei to mitochondria, where it promotes cytochrome c release.37 In preliminary experiments we have not observed any differences between Nur77 levels in mitochondria prepared from sensitive versus resistant cells after CdA treatment (J.C., unpublished observations, April 2001). Calcineurin is a Ca2+-sensitive phosphatase that is capable of dephosphorylating the proapoptotic protein, Bad—thus allowing it to translocate to mitochondria, where it binds to and neutralizes Bcl-xL.38 Preliminary observations have failed to demonstrate any clear-cut differences between levels of Bad present in mitochondria of sensitive versus resistant cells after CdA treatment (J.C., unpublished observations, April 2001). Calpains are a family of Ca2+-dependent cysteine proteases that have been reported to cleave Bax and other apoptotic regulators.39 In experiments designed to examine the role of calpains in CdA-induced apoptosis, we observed that the calpain inhibitor calpeptin did not affect CdA-induced DNA fragmentation or cytochrome c release (data not shown). Further experiments are therefore required to understand the manner in which elevated cytosolic-free Ca2+ contributes to CdA-induced apoptosis. Additional studies are likewise required to determine the molecular nature of the change that renders mitochondria from CdA-resistant cells better able to withstand Ca2+-induced MPT. Nonetheless, the present results help frame these questions by providing the unexpected finding that Bcl-2–independent alterations in mitochondrial function contribute to CdA resistance. If this resistance can be better understood, it is possible that strategies for modulating this resistance might ultimately be developed.
Supported by grants from the Swedish Medical Research Council, the Swedish Children Cancer Foundation, and the Swedish Cancer Foundation. J.C. is the recipient of a Wenner-Gren Foundation Fellowship for Visiting Scientists.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joya Chandra, Guggenheim 1394, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: chandra.joya@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal