Abstract
Cellular interleukin 6 (IL-6) is an important growth factor for Kaposi sarcoma– associated herpesvirus (KSHV)–associated neoplasms, which include human immunodeficiency virus (HIV)–related and -unrelated cases of Kaposi sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman disease (MCD). Increased IL-6 levels are found in tissues affected with these diseases, and KSHV exists in a latent state in the majority of virally infected cells. In addition, acute infection with KSHV up-regulates IL-6 expression in endothelial cells. Thus, the hypothesis was considered that a latent KSHV gene product up-regulates IL-6 expression. To evaluate this hypothesis, the KSHV latency-associated nuclear antigen (LANA) was expressed in human embryonal kidney 293 cells and a bone marrow stromal cell line. LANA up-regulates IL-6 expression by inducing transcription from the IL-6 promoter, and the AP1 response element within the IL-6 promoter is necessary for and mediates IL-6 up-regulation by LANA. Thus, LANA may play a key pathophysiologic role in KSHV-associated neoplasms by functioning to up-regulate expression of IL-6.
Introduction
Kaposi sarcoma–associated herpesvirus (KSHV), also known as human herpesvirus 8, is a novel γ-herpesvirus originally identified in KS tissue.1 Subsequently, multiple studies have confirmed the presence of KSHV in human immunodeficiency virus (HIV)–related and HIV-unrelated cases of Kaposi sarcoma (KS),2 primary effusion lymphoma (PEL),3 and multicentric Castleman disease (MCD).4 In KS, serologic and molecular studies indicate that KSHV represents an etiologic agent rather than a passenger virus.5 In PEL and MCD, the role of KSHV is less well established, in part because these diseases are rare, and seroepidemiologic studies are consequently difficult. However, the association of KSHV infection with PEL is unique among B-cell lymphomas, and the severity of MCD correlates with KSHV viral load.6
Interleukin 6 (IL-6) is a cytokine with pleiotropic effects, and its importance as a growth factor for KSHV-associated neoplasms has been increasingly established. IL-6 is an autocrine growth factor for KS cells,7 and the growth and survival of PEL cells is dependent on the availability of IL-6.8 In MCD, IL-6 levels are elevated in the lymph nodes and serum, and IL-6 appears to be important for the development of MCD in mice.9 10
Because the vast majority of KSHV-infected cells in KS and PEL are latently infected and express cellular IL-6, and acute infection with KSHV up-regulates IL-6 expression in endothelial cells,11-14 we hypothesized that a latent KSHV protein was responsible for up-regulation of IL-6 expression. We initially focused on latency-associated nuclear antigen (LANA), an 1162–amino acid protein encoded by open reading frame 73. Endogenous LANA expressed in PEL cell lines is a large protein that is 222 to 234 kd in size.15 It localizes to the nucleus and contains a leucine zipper structure and other putative transcriptional regulatory domains in its predicted amino acid sequence. In fact, LANA can function as a transcriptional activator,16 as well as a transcriptional repressor.17-20 Given the established role of LANA as a regulator of transcription, we sought to determine if LANA might function to up-regulate cellular IL-6 expression and induce transcription from the IL-6 promoter.
Materials and methods
Cell culture
Human embryonal kidney 293 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in modified Eagle medium (MEM; Omega Scientific, Thousand Oaks, CA) supplemented with 10% fetal bovine serum (FBS; Omega Scientific). The 293 cells were chosen because they are semipermissive for KSHV infection.21 For our studies, we also used an immortalized bone marrow stromal cell line. Bone marrow stromal cells endogenously express IL-6 and complement the use of 293 cells, which lack cellular IL-6 expression. To generate the bone marrow stromal cell line, a bone marrow aspirate was obtained from a healthy volunteer after informed consent. Mononuclear cells were isolated by density sedimentation and cultured in Iscoves modified Dulbecco medium (IMDM; Omega Scientific) supplemented with 10% FBS and 10% donor horse serum (Omega Scientific). The adherent cells were immortalized by transfecting 107 cells with Lipofectamine (Life Technologies, Gaithersburg, MD) with a vector that expresses the SV40 large T antigen (pSV40gpt, ATCC). Thirty days after transfection, healthy colonies were identified and isolated with cloning cylinders. One colony was arbitrarily chosen for our future studies, and the resulting cell line was termed R1T cells.
Cloning of wild-type and deletion mutants of LANA
Genomic DNA from the KSHV+ PEL cell line, KS-1, was used as a template to amplify LANA by polymerase chain reaction (PCR) using the Expand High Fidelity DNA polymerase (Roche Molecular Biochemicals, Indianapolis, IN). The following primers were used: forward, 5′-AGCCTAGAATTCAGACCAGATTTCCCGAGGAT-3′, and reverse, 5′-GTGAACGAATTCTTATGTCATTTCCTGTGGAG-3′;EcoRI sites are underlined, and nonspecific nucleotides were positioned 5′ of restriction site to facilitate enzyme digestion. The amplification product was digested with EcoRI and cloned into the pEGFP-C2 plasmid vector (Clontech, Palo Alto, CA). The construct, pEGFP-LANA, results in the enhanced green fluorescent protein (EGFP) fused in-frame to the 5′ end of LANA. We confirmed the orientation of selected clones by restriction analysis and the reading frame by partial sequencing. A 5′ deletion of LANA was generated in which the amino-terminal 440 amino acids are deleted, and the carboxy terminus remains cloned in-frame with EGFP (Figure1A). Primers to generate this construct, termed LANAΔN440 were as follows: forward, 5′-ACATGAGAATTCATTATGGGCATCCAAAGTTCACAACA-3′ and reverse, 5′-TGTCATGGGCCCTTATGTCATTTCCTGTGGAGA-3′; underlines represent EcoRI and ApaI sites, respectively. The putative transcriptional regulatory domains are maintained in LANAΔN440, but the nuclear localization signals have been removed.
Schematic diagrams of the regulatory domains of LANA and IL-6 promoter constructs.
(A) Wild-type and mutant LANA constructs. (B) Wild-type and IL-6 promoter constructs.
Schematic diagrams of the regulatory domains of LANA and IL-6 promoter constructs.
(A) Wild-type and mutant LANA constructs. (B) Wild-type and IL-6 promoter constructs.
The EGFP-LANA fusion gene was subcloned into the pTRE vector (Clontech) for tetracycline-regulated expression. pEGFP-LANA was digested with NheI, treated with Klenow fragment to create blunt ends, and subsequently digested with XbaI. The pTRE vector was prepared by SacII digestion, followed by blunt ending and then XbaI digestion.
Cloning of wild-type and mutant IL-6 promoter constructs
Genomic DNA was used as a template to amplify a 1200–base pair (bp) region upstream of the transcription start site of theIL6 gene. The primers used were: forward, 5′-GGAAGATCTCTCCTGCAAGAGACACCATCCTGA-3′ and reverse, 5′-CGGGAATTCAGGGCAGAATGAGCCTCAGAGACAT-3′; underlines represent BglII and EcoRI sites, respectively. The PCR product was cloned into the pSEAP-Basic vector (Clontech) to generate pIL6-1200/SEAP. The reporter gene in this vector is secreted alkaline phosphatase (SEAP).
Deletions and point mutations of pIL6-1200/SEAP were generated by PCR. The name of the mutant IL-6 promoter constructs, the nature of the mutation, the purpose for generating the mutation construct, and the primers and technique used to generate the construct are listed in Table 1; a schematic diagram of each construct is shown in Figure 1B.
IL-6 promoter constructs
| Construct name . | Mutation . | Purpose of mutation . | Primers (restriction enzyme site)*,† . |
|---|---|---|---|
| pIL6-1200/SEAP | None | N/A | See text |
| pIL6-327/SEAP | Deletion of −1200 to −327 | Isolate effects of all the known positive regulatory elements located between −327 and +1. | F = 5′-GGAAGATCTCTTCGTGCATGACTTCAGCTTTACT-3′ (BglII) R = 5′-CGGGAATTCAGGGCAGAATGAGCCTCAGAGACAT3-3′ (EcoRI) |
| pIL6-327-AP1mut/ SEAP | Point mutation of an AP1 site | Determine if AP1 site is necessary for LANA-mediated induction of IL-6 expression | F = 5′-TGCAATCAGATCTATGCCAAGTGCTGCAGCACTAATA-3′ (BglII) R = 5′-CGGGAATTCAGGGCAGAATGAGCCTCAGAGACAT3-3′ (EcoRI) |
| pIL6-225/SEAP | Deletion of −327 to −225 | Deletion of AP1 and ETS sites | Generated from pIL6-327/SEAP by digestion with NheI followed by religation. |
| Construct name . | Mutation . | Purpose of mutation . | Primers (restriction enzyme site)*,† . |
|---|---|---|---|
| pIL6-1200/SEAP | None | N/A | See text |
| pIL6-327/SEAP | Deletion of −1200 to −327 | Isolate effects of all the known positive regulatory elements located between −327 and +1. | F = 5′-GGAAGATCTCTTCGTGCATGACTTCAGCTTTACT-3′ (BglII) R = 5′-CGGGAATTCAGGGCAGAATGAGCCTCAGAGACAT3-3′ (EcoRI) |
| pIL6-327-AP1mut/ SEAP | Point mutation of an AP1 site | Determine if AP1 site is necessary for LANA-mediated induction of IL-6 expression | F = 5′-TGCAATCAGATCTATGCCAAGTGCTGCAGCACTAATA-3′ (BglII) R = 5′-CGGGAATTCAGGGCAGAATGAGCCTCAGAGACAT3-3′ (EcoRI) |
| pIL6-225/SEAP | Deletion of −327 to −225 | Deletion of AP1 and ETS sites | Generated from pIL6-327/SEAP by digestion with NheI followed by religation. |
Template for PCR amplification was pIL6-1200/SEAP, unless otherwise stated.
PCR amplification products were cloned intoBglII/EcoRI sites in pSEAP-Basic, unless otherwise stated.
Transient transfection studies
The R1T and 293 cells were plated at 105 cells/well in 24-well plates the day before transfection. All plasmids were transfected with Lipofectamine Plus (Life Technologies) in serum-free medium according to the manufacturer's instructions. Supernatants were harvested at 48 hours for SEAP expression. The SEAP activity was measured with a SEAP assay kit (Clontech) on a tube luminometer (Turner Designs, Sunnyvale, CA) according to the manufacturer's instructions. Transfection efficiency was determined by the percentage of EGFP-expressing cells as determined by cell counting at × 400 magnification in 3 random fields with an inverted phase-contrast UV microscope (TS100-F, Nikon, Melville, NY). Raw data for reporter gene expression was normalized by dividing by the average number of EGFP+ cells per high-power field (3 separate fields were counted for each experiment).
Fluorescence imaging
Fluorescent images were superimposed on brightfield images using Kodak Microscopy Documentation System (Eastman Kodak, Rochester, NY) and Adobe Photoshop, version 5.5, software (Adobe Systems, San Jose, CA).
Generation of 293 lines stably expressing LANA
The 293 cells were transfected with pEGFP-C2 or pEGFP-LANA in 10-cm dishes with Lipofectamine Plus reagent. Forty-eight hours after transfection, 800 μg/mL G418 (Life Technologies) was added for selection of stable transformants. Medium was changed every 4 days until stable transformants were observed. Colonies demonstrating green fluorescence were isolated with cloning cylinders and subsequently combined. Cells were maintained in 400 μg/mL G418.
The 293 Tet-Off cell line was purchased from Clontech. The Tet-Off inducible mammalian expression system allows for regulated expression of a gene of interest by altering the concentration of tetracycline (or doxycycline), such that increasing antibiotic concentrations result in repression of transcription of the gene of interest. EGFP-LANAwas cloned into the pTRE vector, as described above. pTRE-EGFP-LANA was cotransfected with pTK-Hyg (to allow for antibiotic selection with hygromycin) into 293 Tet-Off cells in 10-cm dishes. Forty-eight hours after transfection, cells were selected in 50 μg/mL hygromycin. Green fluorescing colonies were identified after 4 weeks, isolated, and expanded. Colonies were tested for basal and induced expression of EGFP-LANA by immunoblotting with an anti-GFP antibody (Clontech). One colony with absent background and high, induced expression of EGFP-LANA was chosen for future studies.
Cytokine assays
IL-6 and tumor necrosis factor α (TNF-α) were measured on enzyme-linked immunosorbent assay plates (Biotech Diagnostic, Laguna Niguel, CA and R & D Systems, Minneapolis, MN, respectively). Each sample was run in duplicate, and the mean of the 2 results is reported.
Western blot
For detection of EGFP-LANA fusion protein, 20 μg nuclear protein was extracted from transfected cells, electrophoresed on a 4% to 20% polyacrylamide gel, and transferred to a nitrocellulose membrane. Immunoblotting was performed with a polyclonal anti-EGFP antibody (Clontech) at 1:100 dilution, and secondary horseradish peroxidase–conjugated antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:5000 dilution. Bands were identified by chemiluminescence (ECL Western Blotting Detection, Amersham Pharmacia Biotech, Piscataway, NJ).
Results
LANA induction of IL-6 expression
For our experiments, we chose 2 cell lines: 293 cells and a bone marrow stromal cell line (R1T cells). The 293 cells were chosen because they are semipermissive for KSHV infection and lack endogenous IL-6 expression21; the R1T bone marrow stromal cells complement the use of 293 cells, because they do manifest constitutive IL-6 expression. The 293 cells were stably transfected with a vector that expresses LANA fused to the enhanced green fluorescent protein (EGFP-LANA) or the EGFP vector without LANA as a negative control. As expected, EGFP-LANA, which contains a nuclear localization signal, localized to the nucleus (Figure2A). IL-6 expression in culture supernatants was significantly induced by EGFP-LANA, whereas the EGFP control had no effect on IL-6 production (Figure3A). TNF-α concentrations were unaffected by EGFP-LANA expression (Figure 3A), a finding that demonstrates that LANA-mediated IL-6 up-regulation is not due to a generalized increase in protein expression.
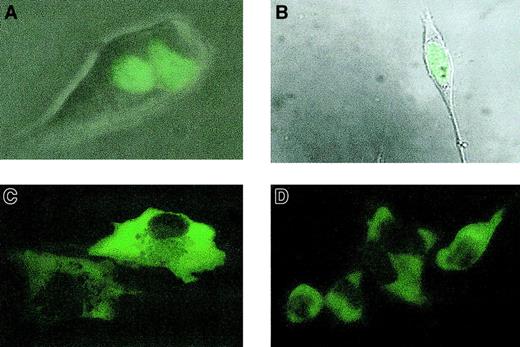
Cellular localization of EGFP-LANA.
(A) Nuclear localization of EGFP-LANA in stably transfected 293 cells. Fluorescent image was superimposed on brightfield image using Kodak Microscopy Documentation System and Adobe Photoshop, version 5.5. (B) Fluorescent image superimposed on brightfield image demonstrates nuclear localization of EGFP-LANA in R1T cells. (C) Cytoplasmic localization and nuclear exclusion of EGFP-LANAΔN440 in R1T cells. (D) Same as panel D, but in 293 cells. (All images taken with a Nikon TS100F phase contrast microscope at × original 400 magnification).
Cellular localization of EGFP-LANA.
(A) Nuclear localization of EGFP-LANA in stably transfected 293 cells. Fluorescent image was superimposed on brightfield image using Kodak Microscopy Documentation System and Adobe Photoshop, version 5.5. (B) Fluorescent image superimposed on brightfield image demonstrates nuclear localization of EGFP-LANA in R1T cells. (C) Cytoplasmic localization and nuclear exclusion of EGFP-LANAΔN440 in R1T cells. (D) Same as panel D, but in 293 cells. (All images taken with a Nikon TS100F phase contrast microscope at × original 400 magnification).
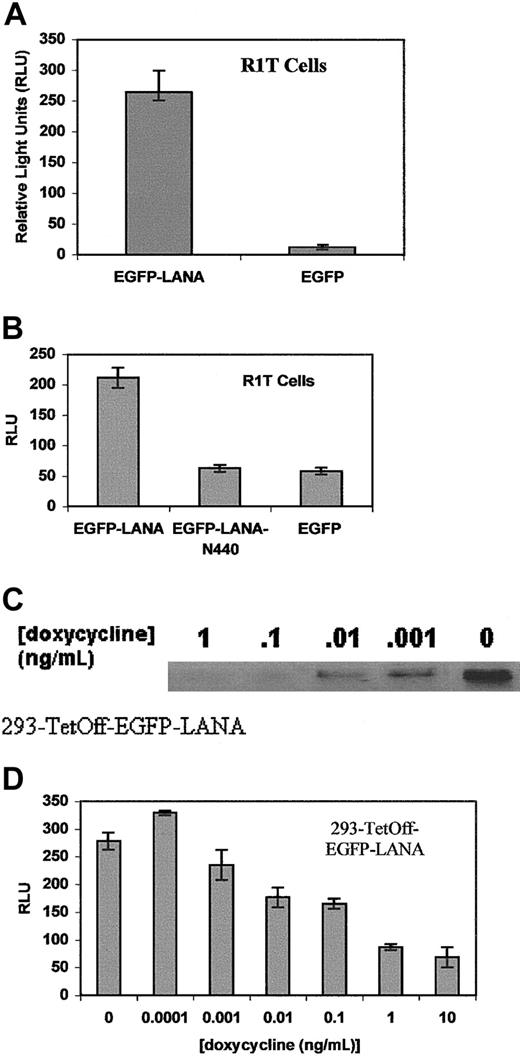
Induction of IL-6 expression by LANA.
(A) IL-6 and TNF-α levels in culture supernatants of 293 cells stably transfected with EGFP-LANA or EGFP blank vectors. Experiments were performed in duplicate. TNF-α levels were undetectable in 293 cells transfected with EGFP-LANA or the EGFP control vector. IL-6 levels were undetectable in 293 cells transfected with the EGFP control vector. (B) IL-6 levels in culture supernatants of R1T cells expressing EGFP-LANA in the tetracycline-regulated system. Various concentrations of doxycycline were used to induce EGFP-LANA expression; 1 μg/mL doxycycline represents a suppressive concentration. Experiments were performed in duplicate.
Induction of IL-6 expression by LANA.
(A) IL-6 and TNF-α levels in culture supernatants of 293 cells stably transfected with EGFP-LANA or EGFP blank vectors. Experiments were performed in duplicate. TNF-α levels were undetectable in 293 cells transfected with EGFP-LANA or the EGFP control vector. IL-6 levels were undetectable in 293 cells transfected with the EGFP control vector. (B) IL-6 levels in culture supernatants of R1T cells expressing EGFP-LANA in the tetracycline-regulated system. Various concentrations of doxycycline were used to induce EGFP-LANA expression; 1 μg/mL doxycycline represents a suppressive concentration. Experiments were performed in duplicate.
To further confirm these findings and establish a correlation between LANA expression and IL-6 production, we used the Tet-Off inducible gene expression system. pTRE-EGFP-LANA, was transiently transfected along with the pTet-Off vector into the R1T bone marrow stromal cell line. The pTet-Off vector (Clontech) expresses a regulatory protein that induces expression from the pTRE vector in the absence of doxycycline. Nuclear localization of the EGFP-LANA fusion protein was observed in the R1T cells (Figure 2B), whereas the EGFP alone was present in both the cytoplasm and nucleus (data not shown). The degree of expression of IL-6 in culture supernatants inversely correlated with the doxycycline concentrations (Figure 3B), indicating that IL-6 levels are augmented with increased expression of LANA.
Induction of the IL-6 promoter by LANA
To investigate whether LANA can induce transcription from the IL-6 promoter, we cotransfected R1T cells with EGFP-LANA or the EGFP control along with the pIL6-1200/SEAP reporter gene construct in which the IL-6 promoter regulates the expression of the SEAP reporter gene. The IL-6 promoter was cloned as the 1200-bp 5′ flanking region of theIL6 gene. EGFP-LANA markedly enhanced reporter gene expression in comparison to the EGFP control (Figure4A). To confirm the specificity of the reporter gene expression by LANA, we also cotransfected EGFP-LANAΔN440 with pIL6-1200/SEAP. LANAΔN440 is a truncation of LANA that contains putative transcriptional regulatory domains, but whose amino-terminal nuclear localization signals have been deleted. As expected, EGFP-LANAΔN440 localized to the cytoplasm rather than the nucleus (Figure 2C) and did not induce reporter gene expression (Figure4B). Similarly, EGFP-LANAΔN440 localized to the cytoplasm in 293 cells (Figure 2D) and did not induce reporter gene expression in those cells (data not shown). Thus, localization of LANA to the nucleus is necessary for activation of the IL-6 promoter.
LANA transactivates the IL-6 promoter in R1T and 293 cells.
(A) Reporter gene expression in R1T cells transiently transfected with pIL6-1200/SEAP and EGFP-LANA or EGFP blank vectors. Results are the means of 3 experiments. (B) Transient transfection of pIL6-1200/SEAP with EGFP-LANA, EGFP-LANAΔN440, or EGFP blank in R1T cells. Experiments performed in duplicate. (C) Western blot for EGFP-LANA on protein extracts from 293-TetOff-EGFP-LANA cells were exposed to various concentrations of doxycycline. Nuclear protein (20 μg) was loaded in each lane and hybridized with an anti-EGFP antibody (Clontech). Coomassie blue staining of gel demonstrated equal loading of protein in each lane. (D) pIL6-1200/SEAP was transiently transfected into 293-TetOff-EGFP LANA cells exposed to various concentrations of doxycycline. Results are means of 2 experiments.
LANA transactivates the IL-6 promoter in R1T and 293 cells.
(A) Reporter gene expression in R1T cells transiently transfected with pIL6-1200/SEAP and EGFP-LANA or EGFP blank vectors. Results are the means of 3 experiments. (B) Transient transfection of pIL6-1200/SEAP with EGFP-LANA, EGFP-LANAΔN440, or EGFP blank in R1T cells. Experiments performed in duplicate. (C) Western blot for EGFP-LANA on protein extracts from 293-TetOff-EGFP-LANA cells were exposed to various concentrations of doxycycline. Nuclear protein (20 μg) was loaded in each lane and hybridized with an anti-EGFP antibody (Clontech). Coomassie blue staining of gel demonstrated equal loading of protein in each lane. (D) pIL6-1200/SEAP was transiently transfected into 293-TetOff-EGFP LANA cells exposed to various concentrations of doxycycline. Results are means of 2 experiments.
We also generated an EGFP-LANA tetracycline-regulated stable cell line in 293 cells (293-TetOff-EGFP-LANA). In this line, doxycycline was able to decrease expression of EGFP-LANA (Figure 4C). When we transiently transfected the pIL6-1200/SEAP into the 293-TetOff-EGFP-LANA cells exposed to various concentrations of doxycycline, the reporter gene expression correlated with the degree of EGFP-LANA expression (Figure4D).
The AP1 response element is necessary but not sufficient for LANA-mediated activation of the IL-6 promoter
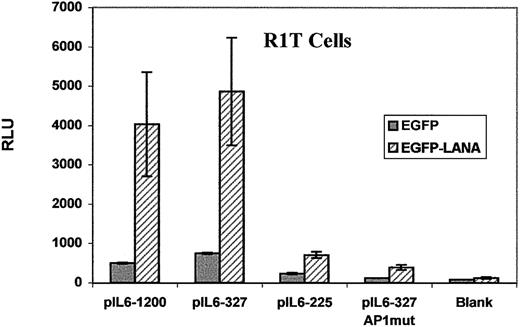
The IL-6 promoter contains numerous cis-acting response elements (REs; Figure 1B), the transactivation of which modulatesIL6 gene transcription. Members of the AP1 family, including jun and fos, are leucine zipper transcription factors that transactivate the IL-6 promoter through the AP1 RE.22 23Because LANA contains a leucine zipper in its predicted amino acid sequence similar to the AP1 family members such as the jun and fos, we postulated that activation of the IL-6 promoter by LANA may be mediated through the AP1 RE. To evaluate this possibility, we generated a series of 5′ deletions and AP1 mutations of the IL-6 promoter construct (Figure 1B and Table 1). We cotransfected these IL-6 promoter mutation constructs with EGFP-LANA or EGFP control vectors into R1T cells. We generated two 5′ deletions of pIL6-1200/SEAP. In the first deletion, pIL6-327/SEAP, the region between −327 and −1200 was deleted, but all of the known positive regulatory elements within the IL-6 promoter remain (Figure 1B). In the second deletion, pIL6-225/SEAP, we removed the region between −225 and −327, which contains the AP1 RE and an ETS site (Figure 1B). Whereas reporter gene expression from pIL6-327/SEAP was equivalent to that of pIL6-1200/SEAP, the expression from pIL6-225/SEAP was significantly reduced, suggesting that the region between −327 and −225, which contains the AP1 RE, was critical to LANA-mediated activation of the IL-6 promoter (Figure5). To establish that the AP1 RE within the −327 and −225 region was necessary for induction of reporter gene expression from the IL-6 promoter by LANA, we created a construct that contained point mutations within the AP1 RE of pIL6-327/SEAP. This construct, pIL6-327-AP1mut/SEAP, yielded significantly reduced reporter gene expression compared to the wild-type construct (Figure 5). These results demonstrate that deletion or point mutations of the AP1 RE results in loss of LANA-induced reporter gene expression and indicate that the AP1 RE is necessary for maximal IL-6 promoter induction by LANA.
The AP1 RE mediates maximal transactivation of the IL-6 promoter by LANA.
R1T cells were cotransfected with an IL-6 promoter construct and either EGFP-LANA or EGFP blank vectors. Reporter gene expression from the blank pSEAP-Basic vector served as the negative control. Results are the means of 2 experiments. See text for details.
The AP1 RE mediates maximal transactivation of the IL-6 promoter by LANA.
R1T cells were cotransfected with an IL-6 promoter construct and either EGFP-LANA or EGFP blank vectors. Reporter gene expression from the blank pSEAP-Basic vector served as the negative control. Results are the means of 2 experiments. See text for details.
Although the data clearly demonstrate a requirement for the AP1 RE for maximal induction of the IL-6 promoter by LANA, transfection of the pIL6-225/SEAP and pIL6-327-AP1mut/SEAP constructs resulted in an increase in reporter gene expression compared to pSEAP-Basic, the blank reporter gene construct, indicating that REs 3′ of −225 are also relevant to LANA-mediated activation of the IL-6 promoter (Figure 5).
To further demonstrate the ability of LANA to induce transcription from the AP1 RE, we cotransfected EGFP-LANA or the EGFP control with a reporter construct that contains 5 copies of the AP1 RE that regulates expression of firefly luciferase (p5xAP1-luc, Figure6). The results of cotransfection of EGFP-LANA with p5xAP1-luc led to a marked increase in luciferase expression compared to the EGFP empty vector. In contrast, the luciferase expression from κ-B (p5xkB-luc) or GAL4 (p5xGAL4-luc) REs was comparable between EGFP-LANA and EGFP (Figure 6). We chose the κ-B RE because nuclear factor (NF)–κB is a potent inducer of IL-6 expression.24 The absence of luciferase expression in these groups confirms that the effect of LANA on IL-6 promoter activation is not a consequence of nonspecific increase in gene transcription.
LANA transactivates the AP1 RE but not the κ-B RE.
R1T cells were cotransfected with the EGFP-LANA or EGFP blank vector and the p5xGAL4-luc, p3xkB-luc, or p5xAP1-luc vector. Luciferase expression was assayed at 48 hours, and experiments were run in duplicate.
LANA transactivates the AP1 RE but not the κ-B RE.
R1T cells were cotransfected with the EGFP-LANA or EGFP blank vector and the p5xGAL4-luc, p3xkB-luc, or p5xAP1-luc vector. Luciferase expression was assayed at 48 hours, and experiments were run in duplicate.
Discussion
Evidence for a causal role for KSHV in the neoplasms associated with this virus has been mounting.5,25 Because KSHV exists predominantly in a latent state,12-14 the use of antibiotics such as ganciclovir and foscarnet, which inhibit replication of virus in the lytic phase of growth, are not likely to have a significant impact on KSHV and its role in the pathogenesis of its associated diseases. Thus, targeted therapy of KSHV-associated diseases requires the identification and inhibition of those KSHV gene products that contribute to the pathogenesis of these diseases.
In this report, we have shown that LANA, encoded by open reading frame 73 of KSHV, up-regulates IL-6 expression by inducing transcription from the IL-6 promoter and that the AP1 RE is necessary for and mediates this function. Because several studies have established the importance of cellular IL-6 as a crucial growth factor for KSHV-associated diseases,7,9,12-14,24,26-31 it follows that LANA is a critical viral gene product that participates in tumorigenesis. IL-6 is an autocrine growth factor for KS cells,7,30 and polymorphisms of the IL-6 promoter that are associated with enhanced expression of IL-6 lead to an augmented risk for KS development in men infected with HIV.31 The growth of PEL cells is dependent on the availability of IL-6, an autocrine growth factor for these cells.8,27 In patients with MCD, the levels of IL-6 are elevated in the lymph nodes and serum.9 Moreover, IL-6 appears to be important for the development of MCD in mice.10 In humans, monoclonal antibodies to IL-6 have resulted in alleviation of symptoms in patients with MCD.31 Thus, by augmenting IL-6 expression, LANA may function in the initiation or progression of KSHV-associated diseases.
We have shown that maximal induction of IL-6 expression by LANA is dependent on the presence of the AP1 RE. The exact mechanism by which LANA mediates this function is currently unknown. LANA may function as a transcription factor that is capable of binding to the AP1 RE and inducing IL6 gene transcription. This possibility is suggested by the fact that LANA contains a leucine zipper similar to endogenous AP1 transcription factors, such as jun and fos, which can induce IL6 gene expression through the AP1 RE.22 23 Alternatively, LANA may function to modulate the activity or expression of other AP1 transcription factors that regulateIL6 gene transcription.
Because the AP1 RE is present in numerous promoters, LANA may also induce expression of factors other than IL-6, including cellular oncoproteins and other cytokines, which may alter cellular growth control in KSHV-associated neoplasms. For example, the vascular endothelial cell growth factor (VEGF) promoter contains an AP1 RE, and VEGF is an established growth factor for KS.30,32 The potential relevance of cytokines like IL-6 and VEGF in the pathogenesis of KSHV-associated diseases is underscored by the fact that infection of primary human endothelial cells by KSHV renders them immortal and tumorigenic, yet only a small subset of the tumor cell population is actually infected with KSHV, suggesting that KSHV-mediated cellular transformation may occur through paracrine mechanisms.33Moreover, conditioned medium from KSHV-infected endothelial cells compared to that of uninfected, early passage endothelial cells, render uninfected endothelial cells more responsive to the proliferative effects of VEGF.33 Thus, paracrine factors, such as cytokines like IL-6 and VEGF, may mediate tumorigenesis in KSHV-associated diseases.
Although we have focused on the AP1 RE, the contribution of LANA to the pathogenesis of KSHV-associated neoplasms may also be mediated by mechanisms independent of the AP1 RE. Indeed, LANA is a large protein with several putative functional domains. For example, LANA represses the transcriptional activity of p53 and inhibits the proapoptotic effects of p53.20 Interestingly, p53 also represses IL6 gene transcription.34 Thus, the ability of LANA to repress the transcriptional activity of p53 may also participate in up-regulated IL-6 expression. In addition, LANA can function as a transcriptional activator of artificial promoters that contain E2F-binding sites as well as the cyclin E promoter and, in conjunction with the cellular oncoprotein, Hras, can induce transformation of rat embryonal fibroblasts and render them tumorigenic.16 These effects may also contribute to the pathogenesis of KSHV-associated neoplasms.
In addition to LANA, other KSHV gene products may play a role in the up-regulation of IL-6 expression and the pathogenesis of KSHV-associated diseases. Open reading frame K13 encodes for Fas-associated death domain (FADD)–like interferon-converting enzyme inhibitory protein (vFLIP). vFLIP can activate the NF-κB pathway,35,36 a potent inducer of IL-6 expression.24 In addition, KSHV also encodes an IL-6–like molecule, viral IL-6 (vIL-6), which retains the biologic activity of its cellular counterpart.37 Although vIL-6 is not a latent protein, it is detectable in patients with KSHV-associated diseases, probably due to the expression of vIL-6 by the small subset of KSHV-infected cells that contain lytic virus.38 Thus, vIL-6 may also function in concert with cellular IL-6 as an autocrine or paracrine tumor cell growth factor.
Because LANA is constitutively expressed in most KS and PEL cells and its expression is unaffected by induction of lytic replication, it is considered a latent protein. The identification of the pathophysiologic effects of latent KSHV proteins may be particularly relevant to potential therapeutic interventions. The development of strategies to interrupt pathogenic latent KSHV proteins, such as LANA, may prove to be effective therapies. The characterization of specific domains of LANA that mediate its functions is required for the development of therapies, such as small molecules, that can block the function of LANA. Therapies that target viral gene products as opposed to cellular proteins offer the promise of a potentially high therapeutic index.
Supported by research funds of the Veterans Administration (VA), including a Career Development Award to M.B.R. and the VA Research Enhancement Award Program (REAP) to A.K.L, G.B., and M.B.R.; the Jonsson Comprehensive Cancer Center at UCLA to M.B.R. and A.K.L.; the American Cancer Society (grant RPG-00-305-01-MBC to M.B.R.); and the American Society of Hematology (Junior Faculty Scholar Award to M.B.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Matthew B. Rettig, VA Greater Los Angeles Healthcare System, 11301 Wilshire Blvd, Bldg 304, Rm E1-108, Los Angeles, CA 90073; e-mail: matthew.rettig@med.va.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal