Abstract
The exact mechanism of apoptosis in neutrophils (PMNs) and the explanation for the antiapoptotic effect of granulocyte colony-stimulating factor (G-CSF) in PMNs are unclear. Using specific fluorescent mitochondrial staining, immunofluorescent confocal microscopy, Western blotting, and flow cytometry, this study found that PMNs possess an unexpectedly large number of mitochondria, which are involved in apoptosis. Spontaneous PMN apoptosis was associated with translocation of the Bcl-2–like protein Bax to the mitochondria and subsequent caspase-3 activation, but not with changes in the expression of Bax. G-CSF delayed PMN apoptosis and prevented both associated events. These G-CSF effects were inhibited by cycloheximide. The general caspase inhibitor z-Val-Ala-DL-Asp-fluoromethylketone (zVAD-fmk) prevented caspase-3 activation and apoptosis in PMNs, but not Bax redistribution. PMN-derived cytoplasts, which lack a nucleus, granules, and mitochondria, spontaneously underwent caspase-3 activation and apoptosis (phosphatidylserine exposure), without Bax redistribution. zVAD-fmk inhibited both caspase-3 activation and phosphatidylserine exposure in cultured cytoplasts. Yet, G-CSF prevented neither caspase-3 activation nor apoptosis in cytoplasts, confirming the need for protein synthesis in the G-CSF effects. These data demonstrate that (at least) 2 routes regulate PMN apoptosis: one via Bax-to-mitochondria translocation and a second mitochondria-independent pathway, both linked to caspase-3 activation. Moreover, G-CSF exerts its antiapoptotic effect in the first, that is, mitochondria-dependent, route and has no impact on the second.
Introduction
During the last decade, apoptosis, or programmed cell death, has attracted great interest. Whole families of molecules have been described as regulators of apoptosis. The Bcl-2 family of proteins and the family of caspase proteases are internal key regulators of the cell fate.1,2 Recent work has demonstrated that these 2 groups of proteins are intimately connected at the level of mitochondria: the Bcl-2 homologues govern the activity of caspases by exerting their effect through the regulation of the mitochondrial function.3 Proapoptotic Bcl-2 proteins, such as Bax, disturb the mitochondrial membrane integrity by forming channels, which facilitates the subsequent release of cytochromec and the activation of Apaf-1 and downstream caspases.4-7 Bax moves from the cytosol to the mitochondria on initiation of apoptosis.8-11 This translocation seems to precede caspase activation.12 Some authors have suggested a critical role for Bax movement to mitochondria in the execution of the apoptotic program.11,13 Moreover, the antiapoptotic protein Bcl-2 is believed to mediate, at least partially, its effect through the inhibition of Bax redistribution.11 14
A downstream event of Bax-mediated mitochondrial dysfunction is the activation of caspases. This family of proteases executes the cleaving of specific targets, which, finally, leads to cell disassembly and death. Among these proteases, caspase-3 stands out for the great number of substrates that it destroys. These targets include nuclear proteins,15 cytoplasmic structures,16 and cytoskeleton elements.17 Bcl-2 and its antiapoptotic homologue, Bcl-XL, are also substrates for caspase-3.18 19 For this reason, caspase-3 is called the main executioner of apoptosis.
In the present study, we have investigated the apoptotic process of mature human neutrophils (PMNs). These cells have a constitutively short life span and rapidly undergo spontaneous apoptosis within hours.20 Survival of PMNs can be extended by delaying apoptosis with a wide variety of agents, including colony-stimulating factors, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF).21-25 To date, the mechanisms of their prosurvival effect remained unclear. Some authors have suggested that active protein synthesis is required for agents such as GM-CSF to produce their prosurvival effect, whereas blocking of protein synthesis promotes PMN apoptosis and abrogates the effect of these agents.25-27 Recently, it has been proposed that the antiapoptotic action of these cytokines may be connected with Bcl-2–related proteins and with regulation of caspase-3 activity. GM-CSF has been suggested to induce expression of the antiapoptotic Bcl-2 homologue Mcl-1,23 to maintain the level of Bcl-XL, and to influence caspase-3 activity,28 thus preventing apoptosis. Down-regulation of Bax expression by G-CSF and GM-CSF has been considered to delay apoptosis.24
Regarding a role of Bcl-2 proteins in this process, one should realize that PMNs are considered to possess no or only few rudimentary mitochondria, which do not play a role in the active life of the cell.29,30 Therefore, PMNs have been considered to be “a nonmitochondrial scene” of apoptosis.23 On the other hand, mitochondria constitute a crossroad of apoptosis regulation between members of the Bcl-2 family and the caspases. In the present study, we show that PMNs do contain mitochondria, in contrast to what was believed earlier. Also, we observed Bax movement to mitochondria and a strong correlation of this event with caspase-3 activation and spontaneous PMN apoptosis. The prosurvival effect of the prosurvival factor G-CSF was demonstrated to coincide with prevention of Bax-to-mitochondria translocation and with inhibition of caspase-3 processing in a transcription-dependent manner. At the same time, using PMN cytoplasts, which lack mitochondria, granules, and nuclei,31 we found a mitochondria-independent pathway of caspase-3 activation and subsequent apoptosis. Moreover, G-CSF had no effect on this last route of apoptosis.
Materials and methods
PMN preparation and culturing
The PMNs were isolated from heparinized blood of healthy donors as described.32 Briefly, 20 mL blood was diluted with 20 mL 10% trisodium citrate/phosphate-buffered saline (PBS). Mononuclear cells and platelets were removed by density gradient centrifugation over isotonic Percoll (Pharmacia, Uppsala, Sweden) with a specific gravity of 1.076 g/mL. Erythrocytes were lysed by short treatment of the pellet fraction with ice-cold isotonic NH4Cl solution (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.4). The remaining PMNs were washed once in PBS and used for further manipulations. In all cases purity was more than 97%. PMNs were resuspended at a final concentration of 2 × 106/mL in Iscoves modified Dulbecco medium (IMDM; Biowhittaker, Brussels, Belgium) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco BRL, Paisley, United Kingdom), penicillin 100 IU/mL (Yamanouchi, Tokyo, Japan), streptomycin 100 μg/mL (Gibco BRL), and glutamine 300 μg/mL. One milliliter of cell suspension was put in each well of 24-well plates (NUNC Brand Products, Roskilde, Denmark) and was incubated overnight (20-22 hours) in a 5% CO2 incubator at 37°C. PMNs were cultured without additions (no stimulus), with 500 ng/mL G-CSF (Neupogen, Amgen, Breda, The Netherlands), with 400 μmol/L z-Val-Ala-DL-Asp-fluoromethylketone (zVAD-fmk, Alexis Biochemicals, San Diego, CA), with 5 μg/mL cycloheximide (CHX; Calbiochem, Bad Soden, Germany), or with a combination of indicated doses of G-CSF and CHX.
Cytoplast preparation and culturing
The PMNs were isolated from the buffy coat of 500 mL fresh blood from volunteer donors, as described above. Cytoplasts were prepared from 108 PMNs as described previously.33Briefly, PMNs were centrifuged through a discontinuous Ficoll-70 (Sigma, St Louis, MO) gradient (12.5%, 16%, 25%) prewarmed to 37°C, containing 5 μg/mL cytochalasin B (Sigma). Centrifugation was performed for 30 minutes at 34°C in a model L2-65B ultracentrifuge with an AH-629 rotor (Beckman Instruments, Fullerton, CA) at 81 000g. After centrifugation, the upper band of cellular material was collected. This band was composed of 99% of cytoplasts, as assessed by light microscopy of cytospins stained with May-Grünwald-Giemsa stain. Cytoplasts were recognized by their absence of nuclei. Following several washings in PBS, cytoplasts were resuspended at a final concentration of 8 × 106/mL in the culture medium and were incubated as indicated for PMNs (see above).
Measurement of apoptosis
Apoptosis of PMNs was measured by flow cytometry with the annexin-V–fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis assay kit (Bender Medsystems, Vienna, Austria).34 About 105 fresh cells or cells after overnight incubation were washed once in ice-cold Hepes medium with 2.5 mM Ca++. All further steps were performed in this medium on ice. The cells were then incubated for 45 minutes with annexin-V–FITC, 1:250, which specifically binds phosphatidylserine residues on the cell membrane. During the last 10 minutes PI was added to a final concentration of 1 μg/mL. PI is a fluorescent dye that intercalates into DNA once the cell membrane has become permeable. After incubation, the cells were washed once and analyzed by FACScan (Becton Dickinson, San Jose, CA). Viable cells were defined as negative for annexin-V–FITC and PI staining. Cell survival was expressed as a percentage of viable cells in relation to the total number of counted cells. Cytoplast apoptosis was assessed in the same way, except for the PI step, with 4 × 105 cytoplasts for each preparation.
Measurement of protein expression of Bcl-2 family members
The expression levels of Bax, Bak, Mcl-1, and Bcl-XLwere determined by FACS analysis, according to Van Vliet et al,35 with some modifications. After isolation and culture, 105 PMNs were washed once in PBS. The cells were then fixed with 2% (wt/vol) paraformaldehyde in PBS for 15 minutes at room temperature, washed twice in PBS, and resuspended in staining buffer containing 0.1% saponin (wt/vol; Calbiochem) and 1% (vol/vol) bovine serum albumin (BSA; Boseral, Organon Teknika, Eppelheim, Germany) in PBS. All further steps were performed in this solution at room temperature. The cells were distributed over a 96-well plate with conical bottoms, incubated for 10 minutes for permeabilization, and were spun down. Supernatant was removed, and PMNs were resuspended in 50 μL of the appropriate primary antibody or isotype control antibody solution. The polyclonal rabbit antibodies against human Bax, Mcl-1 (Pharmingen, San Diego, CA) or Bcl-XL (Calbiochem) were used at a final dilution of 1:250. The monoclonal mouse antihuman Bak antibody (Calbiochem) was used at a final concentration of 25 μg/mL. After 45 minutes of incubation the cells were washed twice and were resuspended in 50 μL of the secondary antibody, Alexa-488–conjugated goat-antirabbit/mouse IgG (Molecular Probes, Eugene, OR), at a final concentration of 2.5 μg/mL. Incubation with the secondary antibody took 45 minutes. After this procedure, the PMNs were washed twice and were analyzed by FACScan. Expression levels of the proteins of interest were assessed by measuring the mean fluorescence intensity (MFI) of bound antibodies.
Confocal laser scanning microscopy
For confocal laser scanning microscopy (CLSM) analysis, PMNs and cytoplasts were fixed and permeabilized as described for flow cytometry. To investigate the staining patterns of Bcl-2–related proteins, the cells were incubated with the appropriate primary antibodies (see above) with subsequent secondary staining with Alexa-568–conjugated goat-antirabbit/mouse IgG (final concentration 2.5 μg/mL). All incubations were performed as described above. After the last wash step, the cells were resuspended in 30 μL PBS, brought on microscope glass slides, and covered by coverslips. The slides were analyzed by a confocal laser scanning microscope (LSM510, Carl Zeiss, Heidelberg, Germany) equipped with Ar and HeNe lasers.
To study the activation of caspase-3, incubations were performed with the monoclonal rabbit antibody against active human caspase-3 (Pharmingen) at a final dilution of 1.5 μg/mL, and AlexaFluor488-conjugated goat-antirabbit IgG.
For the mitochondrial staining MitoTracker GreenFM (Molecular Probes) was used. About 105 fresh or cultured PMNs (or 4 × 105 cytoplasts) were incubated in IMDM for 30 minutes in a 5% CO2 incubator at 37°C with 100 nmol/L MitoTracker Green FM. Then the cells were spun down, resuspended in 30 μL stain-free medium, placed on microscope glass slides, and analyzed by CLSM.
To obtain simultaneous staining of mitochondria and intracellular antigens, the cells were stained with 1 μmol/L MitoTracker Green FM, fixed, permeabilized, labeled for proteins of interest, and analyzed as described above.
Western blotting
The cleavage of procaspase-3 was determined by Western blotting as described elsewhere.28 Briefly, whole cell lysates were obtained by boiling 5 × 105 PMNs or 2 × 106 cytoplasts in sodium dodecyl sulfate (SDS) sample buffer with 4% mercaptoethanol for 5 minutes. Proteins were resolved on 12.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and were electrotransferred to nitrocellulose membrane (Hybond-ECL; Amersham, Arlington Height, IL). The blots were incubated for 1 hour with 1:1000 diluted polyclonal rabbit antihuman caspase-3 antibodies (Pharmingen), which recognize both inactive procaspase-3 and its cleavage product. Thereafter, the blots were incubated for 1 hour with swine antirabbit-IgG conjugated with horseradish peroxidase (HRP; Dako, Glostrup, Denmark), followed by band visualization with enhanced chemiluminescence as described by the manufacturer (Amersham). As a positive control, Jurkat T-cell lysates were used (Pharmingen), which contain caspase-3 as a 32-kd proenzyme.
Measurement of G-CSF receptor expression in PMNs and cytoplasts
The PMNs (105) or cytoplasts (4 × 105) were washed once in PBS and were incubated with a monoclonal antibody (mAb) against human G-CSF receptor (Pharmingen) at a final concentration of 5 μg/mL or with an isotype control. After a 45-minute incubation, the cells were washed twice and stained for 45 minutes with R-phycoerythrin-labeled secondary antibody (Dako) at a final dilution of 10 μg/mL. After washing, the antibody binding was assessed by FACS analysis.
Statistics
Where applicable, the Student t test was used to evaluate the significance of differences between the sample means. Statistical significance was defined as P less than .05.
Results
G-CSF and zVAD-fmk effectively delay PMN apoptosis
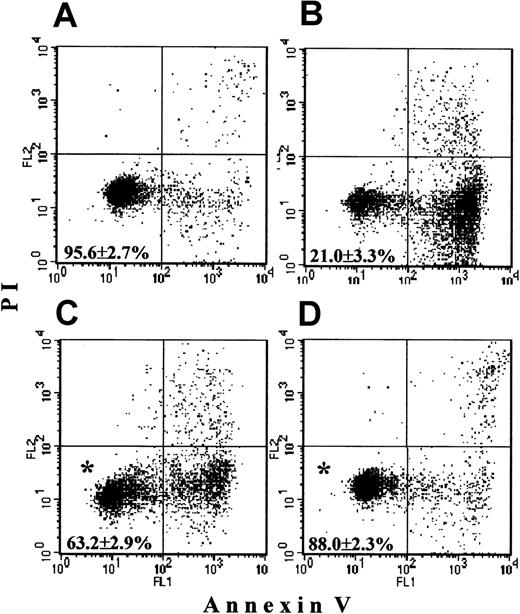
Freshly isolated PMNs contained 95.6% ± 2.7% live cells, that is, negative for both annexin-V and PI (Figure1A). PMNs cultured overnight without additions underwent spontaneous apoptosis, with a survival not higher than 21.0% ± 3.3% (Figure 1B). In the presence of G-CSF, apoptosis was delayed, resulting in 63.2% ± 2.9% surviving cells (Figure1C). In the presence of zVAD-fmk (Figure 1D) apoptosis was minimal, and 88.0% ± 2.3% PMNs were alive. Both agents produced a significant delay of apoptosis when compared with control incubations (P < .05).
Survival of PMNs.
Freshly isolated PMNs as well as cells cultured overnight without additions, with 500 ng/mL G-CSF or with 400 μM zVAD-fmk were stained with annexin-V (x-axis) and PI (y-axis) and were analyzed by FASCscan. Cells without annexin V and PI staining were counted as viable cells (lower left quadrant on each plot). Cell survival was expressed as the percentage of viable cells in the total cell population. (A) Almost all fresh cells were alive. (B) Overnight-cultured untreated cells underwent spontaneous apoptosis. (C,D) Addition of G-CSF or zVAD-fmk, respectively, significantly increased PMN survival (*P < .05 versus untreated cells). Values represent means ± SEM of 8 separate experiments.
Survival of PMNs.
Freshly isolated PMNs as well as cells cultured overnight without additions, with 500 ng/mL G-CSF or with 400 μM zVAD-fmk were stained with annexin-V (x-axis) and PI (y-axis) and were analyzed by FASCscan. Cells without annexin V and PI staining were counted as viable cells (lower left quadrant on each plot). Cell survival was expressed as the percentage of viable cells in the total cell population. (A) Almost all fresh cells were alive. (B) Overnight-cultured untreated cells underwent spontaneous apoptosis. (C,D) Addition of G-CSF or zVAD-fmk, respectively, significantly increased PMN survival (*P < .05 versus untreated cells). Values represent means ± SEM of 8 separate experiments.
PMNs do contain mitochondria: structural changes during apoptosis
To check whether PMNs contain mitochondria, we performed mitochondrial staining with MitoTracker Green FM. In fresh PMNs (Figure2A), the mitochondria showed a tubular shape, whereas in overnight-cultured control PMNs (Figure 2B) the mitochondria had changed into large unstructured aggregates. G-CSF (Figure 2C) prevented the mitochondrial aggregate formation, but addition of zVAD-fmk (Figure 2D) did not influence the aggregation of the mitochondria. The identity of the mitochondria was confirmed with an antibody against a component of the mitochondrial cytochrome oxidase system (mouse mAb antihuman cytochrome c oxidase complex IV, Molecular Probes; data not shown).
Localization and staining patterns of mitochondria in PMNs.
PMNs were incubated as described in Figure 1. Then the cells were stained with 100 nM MitoTracker Green FM and analyzed with CLSM. (A) In fresh cells the mitochondria showed a distinct tubular shape. (B) In PMNs cultured without additions the mitochondria relocalized into large unstructured aggregates. (C) G-CSF prevented the mitochondrial aggregate formation. (D) zVAD-fmk did not influence mitochondrial aggregation. Bar is 5 μm. Results are representative of at least 3 independent experiments.
Localization and staining patterns of mitochondria in PMNs.
PMNs were incubated as described in Figure 1. Then the cells were stained with 100 nM MitoTracker Green FM and analyzed with CLSM. (A) In fresh cells the mitochondria showed a distinct tubular shape. (B) In PMNs cultured without additions the mitochondria relocalized into large unstructured aggregates. (C) G-CSF prevented the mitochondrial aggregate formation. (D) zVAD-fmk did not influence mitochondrial aggregation. Bar is 5 μm. Results are representative of at least 3 independent experiments.
PMN apoptosis and caspase-3 activation: inhibition by G-CSF or zVAD-fmk
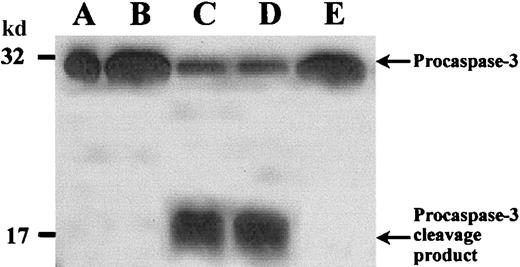
We studied caspase-3 processing during spontaneous apoptosis by Western blotting and CLSM. Caspase-3 is expressed in normal PMNs as a 32-kd inactive precursor, which is proteolytically cleaved into an active form on mediation of apoptosis.36 This cleavage generates a large (17-20 kd) and a small (3-12 kd) subunit. In fresh PMNs (Figure 3, lane B) caspase-3 was found mainly as a 32-kd proenzyme. In contrast, PMNs cultured overnight without additions, undergoing spontaneous apoptosis (Figure 3, lane C), demonstrated decreased amounts of the proenzyme and a band of an approximately 17-kd cleavage product. G-CSF (Figure 3, lane D) inhibited caspase-3 activation, whereas zVAD-fmk–treated PMNs (Figure3, lane E) showed no cleavage product but only inactive 32-kd proenzyme, indicating that caspase-3 activation was inhibited in these cells.
Procaspase-3 cleavage in PMNs.
PMNs were incubated as described in Figure 1. Then whole cell lysates were subjected to SDS-PAGE, and Western blot was performed with an anticaspase-3 polyclonal antibody and an HRP-conjugated secondary antibody. (A) Positive control with procaspase-3 (Jurkat T cells). (B) Fresh PMNs. (C) In PMNs incubated without stimuli procaspase-3 was cleaved, and a 17-kd cleavage product appeared. (D) Addition of G-CSF considerably prevented procaspase-3 cleavage. (E) zVAD-fmk completely inhibited procaspase-3 processing. Results are representative of 3 independent experiments.
Procaspase-3 cleavage in PMNs.
PMNs were incubated as described in Figure 1. Then whole cell lysates were subjected to SDS-PAGE, and Western blot was performed with an anticaspase-3 polyclonal antibody and an HRP-conjugated secondary antibody. (A) Positive control with procaspase-3 (Jurkat T cells). (B) Fresh PMNs. (C) In PMNs incubated without stimuli procaspase-3 was cleaved, and a 17-kd cleavage product appeared. (D) Addition of G-CSF considerably prevented procaspase-3 cleavage. (E) zVAD-fmk completely inhibited procaspase-3 processing. Results are representative of 3 independent experiments.
We also stained PMNs for active caspase-3 with a mAb against a unique epitope of active caspase-3. This mAb does not recognize the inactive form of the enzyme. CLSM analysis showed that fresh PMN (Figure4A) demonstrated only very weak staining, probably reflecting background staining or a negligible binding to inactive enzyme. In contrast, untreated, overnight-cultured PMN (Figure4B) showed a bright punctate staining for active caspase-3, demonstrating a significant amount of the active form of the enzyme in these cells. G-CSF (Figure 4C) considerably inhibited caspase-3 activation, because the cells were only slightly positive. As expected, zVAD-fmk–treated PMNs (Figure 4D) were as dark as fresh cells, demonstrating the absence of active caspase-3. Hence, Western blotting and CLSM results were in compliance with each other, demonstrating a strong correlation between PMN spontaneous apoptosis and caspase-3 activation, and a coincidence of the antiapoptotic effect of G-CSF and zVAD-fmk with inhibition of caspase-3 activation.
Activation of caspase-3 in PMNs.
PMNs were cultured as described in Figure 1. Then the cells were fixed, permeabilized, stained with mAb specific for active caspase-3, and analyzed by CLSM. (A) Fresh PMNs showed faint background staining. (B) Overnight-cultured untreated PMNs revealed a bright punctate staining representing active caspase-3. (C) G-CSF strongly inhibited caspase-3 activation. (D) Cells treated with zVAD-fmk did not demonstrate active caspase-3. Bar is 5 μm. Results are representative of at least 3 independent experiments.
Activation of caspase-3 in PMNs.
PMNs were cultured as described in Figure 1. Then the cells were fixed, permeabilized, stained with mAb specific for active caspase-3, and analyzed by CLSM. (A) Fresh PMNs showed faint background staining. (B) Overnight-cultured untreated PMNs revealed a bright punctate staining representing active caspase-3. (C) G-CSF strongly inhibited caspase-3 activation. (D) Cells treated with zVAD-fmk did not demonstrate active caspase-3. Bar is 5 μm. Results are representative of at least 3 independent experiments.
Spontaneous PMN apoptosis associated with Bax-to-mitochondria translocation
Next, we investigated a number of Bcl-2–related proteins. Levels of expression of Bax, Bak, Mcl-1, and Bcl-XL were studied by flow cytometry with antibodies specific for each protein to be tested. We found that the expression levels of Bax were not significantly different between fresh PMNs and overnight-incubated PMNs. Neither G-CSF nor zVAD-fmk had any effect on its expression (Figure 5). Western blotting with anti-Bax antibody confirmed the flow cytometry data; Bax levels remained unchanged under all conditions tested (data not shown). Similar results were obtained for Bak, Mcl-1, and Bcl-XL(data not shown). We also did not find a correlation between the expression of these Bcl-2 homologues and cell survival. Therefore, we conclude that the expression levels of the Bcl-2–related proteins studied by us were not subject to modulation during PMN apoptosis or its delay.
Expression levels of Bax in PMNs.
PMNs were cultured as described in Figure 1. Then the cells were fixed, permeabilized, stained with antibodies specific for Bax or with isotype control antibodies (control bars), and analyzed by FACScan. After overnight culturing, the expression of Bax had slightly increased, but this change was not significant. Treatment of the cells with either G-CSF or with zVAD-fmk had no effect on Bax expression. Data represent means ± SEM of MFI from 6 independent experiments.
Expression levels of Bax in PMNs.
PMNs were cultured as described in Figure 1. Then the cells were fixed, permeabilized, stained with antibodies specific for Bax or with isotype control antibodies (control bars), and analyzed by FACScan. After overnight culturing, the expression of Bax had slightly increased, but this change was not significant. Treatment of the cells with either G-CSF or with zVAD-fmk had no effect on Bax expression. Data represent means ± SEM of MFI from 6 independent experiments.
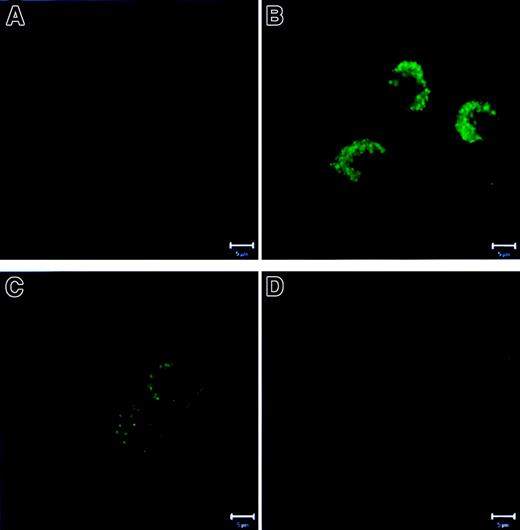
Because we did not find quantitative changes in these proteins, we investigated possible qualitative changes. Recent studies have shown that Bax moves from the cytosol to the mitochondria on execution of apoptosis.8-11 Using CLSM with costaining for mitochondria and Bax, we found that fresh PMNs (Figure6A) showed a separate localization of Bax (red) and mitochondria (green). (Note: due to the fixation/permeabilization procedure, mitochondrial staining showed a more diffuse cytoplasmic pattern than the tubular structures shown in Figure 2, panels A and C.) In contrast, in overnight cultured, untreated PMNs (Figure 6B), mitochondria and Bax colocalized into large aggregates with a shift in fluorescence to yellow. G-CSF (Figure 6C) prevented this aggregate formation and colocalization of Bax with mitochondria, whereas zVAD-fmk (Figure 6D) did not. Bak, Mcl-1, and Bcl-XL did not change their punctate localization separate from mitochondria under any condition tested (data not shown).
Relocalization of Bax to the mitochondria in PMNs.
PMNs were cultured as described in Figure 1. Then the cells were stained with 1 μM MitoTracker Green FM, fixed, permeabilized, stained with antibody specific for Bax, and were analyzed with CLSM. (A) In fresh PMN Bax and the mitochondria were localized separately. (B) Overnight-cultured untreated cells demonstrated fusion of Bax and mitochondria into large aggregates (right panel in yellow). (C) Addition of G-CSF preserved the separate localization of Bax and mitochondria. (D) zVAD-fmk did not prevent aggregate formation and colocalization of Bax and mitochondria. (Comment: due to the fixation and permeabilization procedures, the mitochondrial staining [green] showed a more diffuse cytoplasmic pattern than the tubular structures shown in Figure 2, panels A and C). Bar is 5 μm. This figure is representative of at least 4 independent experiments.
Relocalization of Bax to the mitochondria in PMNs.
PMNs were cultured as described in Figure 1. Then the cells were stained with 1 μM MitoTracker Green FM, fixed, permeabilized, stained with antibody specific for Bax, and were analyzed with CLSM. (A) In fresh PMN Bax and the mitochondria were localized separately. (B) Overnight-cultured untreated cells demonstrated fusion of Bax and mitochondria into large aggregates (right panel in yellow). (C) Addition of G-CSF preserved the separate localization of Bax and mitochondria. (D) zVAD-fmk did not prevent aggregate formation and colocalization of Bax and mitochondria. (Comment: due to the fixation and permeabilization procedures, the mitochondrial staining [green] showed a more diffuse cytoplasmic pattern than the tubular structures shown in Figure 2, panels A and C). Bar is 5 μm. This figure is representative of at least 4 independent experiments.
Normal externalization of phosphatidylserine and caspase-3 activation in cytoplasts
Recently, a mitochondria-independent route of caspase activation has been proposed to exist.37 To check this possibility in PMNs, we generated so-called cytoplasts, that is, enucleated PMNs that lack granules and mitochondria. Thus, cytoplasts are vesicles filled with cytoplasm, which maintain the integrity of the plasma membrane.31
Cytospins stained with May-Grünwald-Giemsa stain showed that more than 99% of the cytoplasts indeed had no nucleus. Neither MitoTracker nor the mAb against a cytochrome c oxidase component revealed the presence of any mitochondria in cytoplasts (not shown). Annexin-V staining was negative in 96.1% ± 3.3% of freshly prepared cytoplasts (Figure 7A). Similar to PMNs, during overnight culture without stimuli cytoplasts underwent membrane “flip-flop,” with exposure of phosphatidylserine residues on the outer layer of the plasma membrane, leading to 64.5% annexin-V+ cytoplasts (35.5% ± 3.3% annexin-V− cells [Figure 7B; compare with PMN data in Figure 1B]). Next, we studied caspase-3 activation in cytoplasts by Western blotting. Fresh cytoplasts (Figure8, lane B) contained only the 32-kd proenzyme. After overnight culturing, cytoplasts (Figure 8, lane C) demonstrated caspase-3 processing with appearance of the same 17-kd cleavage product as in neutrophils. CLSM data supported these findings, showing no active caspase-3 in fresh cytoplasts (Figure9A) and a bright punctate distribution of active caspase-3 in cytoplasts undergoing spontaneous apoptosis (Figure9B). Hence, caspase-3 was “normally” activated even in the absence of a nucleus and mitochondria. Further, we investigated Bax behavior in cytoplasts. This protein appeared to be present in fresh cytoplasts, but did not change its punctate distribution after overnight culturing without additions (figure not shown). We conclude that cytoplasts possess an intact apoptotic machinery, which induces “flip-flop” of the phosphatidylserine residues on the outer layer of the plasma membrane and caspase-3 activation in the absence of a nucleus and mitochondria, but does not require Bax redistribution.
Survival of cytoplasts.
Freshly prepared cytoplasts as well as cytoplasts cultured overnight without additions, with 500 ng/mL G-CSF or 400 μmol/L zVAD-fmk were stained with annexin-V and were analyzed by FASCscan. Cytoplasts without annexin-V staining were counted as viable cytoplasts (lower left quadrant on each plot). Survival was expressed as the percentage of viable cytoplasts in the total cytoplast population. (A) Almost all fresh cytoplasts were alive. (B) Untreated cytoplasts cultured overnight underwent spontaneous apoptosis. (C) Addition of G-CSF had no effect on cytoplast survival. (D) zVAD-fmk significantly increased cytoplast survival (*P < .05 versus untreated and G-CSF–treated cytoplasts). Values represent means ± SEM of 3 separate experiments.
Survival of cytoplasts.
Freshly prepared cytoplasts as well as cytoplasts cultured overnight without additions, with 500 ng/mL G-CSF or 400 μmol/L zVAD-fmk were stained with annexin-V and were analyzed by FASCscan. Cytoplasts without annexin-V staining were counted as viable cytoplasts (lower left quadrant on each plot). Survival was expressed as the percentage of viable cytoplasts in the total cytoplast population. (A) Almost all fresh cytoplasts were alive. (B) Untreated cytoplasts cultured overnight underwent spontaneous apoptosis. (C) Addition of G-CSF had no effect on cytoplast survival. (D) zVAD-fmk significantly increased cytoplast survival (*P < .05 versus untreated and G-CSF–treated cytoplasts). Values represent means ± SEM of 3 separate experiments.
Procaspase-3 cleavage in cytoplasts.
Cytoplasts were incubated as described in Figure 7. Then whole cytoplast lysates were subjected to SDS-PAGE, and Western blot was performed with an anticaspase-3 polyclonal antibody and an HRP-conjugated secondary antibody. (A) Positive control with procaspase-3 (Jurkat T-cells). (B) Freshly prepared cytoplasts demonstrated only 32-kd procaspase-3. (C) In cytoplasts incubated without stimuli procaspase-3 was cleaved and a 17-kd cleavage product appeared. (D) Addition of G-CSF did not prevent procaspase-3 cleavage and appearance of a 17-kd cleavage product. (E) zVAD-fmk completely inhibited procaspase-3 processing. Results are representative of 3 independent experiments.
Procaspase-3 cleavage in cytoplasts.
Cytoplasts were incubated as described in Figure 7. Then whole cytoplast lysates were subjected to SDS-PAGE, and Western blot was performed with an anticaspase-3 polyclonal antibody and an HRP-conjugated secondary antibody. (A) Positive control with procaspase-3 (Jurkat T-cells). (B) Freshly prepared cytoplasts demonstrated only 32-kd procaspase-3. (C) In cytoplasts incubated without stimuli procaspase-3 was cleaved and a 17-kd cleavage product appeared. (D) Addition of G-CSF did not prevent procaspase-3 cleavage and appearance of a 17-kd cleavage product. (E) zVAD-fmk completely inhibited procaspase-3 processing. Results are representative of 3 independent experiments.
Activation of caspase-3 in cytoplasts.
Cytoplasts were cultured as described in Figure 7. Then the cytoplasts were fixed, permeabilized, stained with mAb specific for active caspase-3, and analyzed by CLSM. (A) Fresh cytoplasts showed faint background staining. (B,C) Overnight-cultured untreated or G-CSF–treated cytoplasts, respectively, revealed bright punctate staining representing active caspase-3. (D) Cells treated with zVAD-fmk did not demonstrate active caspase-3. Bar is 5 μm. Results are representative of at least 3 independent experiments.
Activation of caspase-3 in cytoplasts.
Cytoplasts were cultured as described in Figure 7. Then the cytoplasts were fixed, permeabilized, stained with mAb specific for active caspase-3, and analyzed by CLSM. (A) Fresh cytoplasts showed faint background staining. (B,C) Overnight-cultured untreated or G-CSF–treated cytoplasts, respectively, revealed bright punctate staining representing active caspase-3. (D) Cells treated with zVAD-fmk did not demonstrate active caspase-3. Bar is 5 μm. Results are representative of at least 3 independent experiments.
G-CSF effect on survival and caspase-3 activation in cytoplasts
Because G-CSF needs to bind to a specific surface receptor to mediate its effects in PMNs,38 we investigated the expression of the G-CSF receptor on cytoplasts by flow cytometry. The MFI of bound anti-G-CSF receptor mAb was 193 ± 22 in PMNs, and 88 ± 10 in cytoplasts (data represent mean ± SEM of the MFI from 3 separate experiments). The difference in MFI between PMNs and cytoplasts is due to the fact that the cell surface area of PMNs is 2 to 3 times larger than that of cytoplasts.31 Moreover, the number of G-CSF receptors on the cell surface is not critical for the impact of G-CSF on cells, because the ligation of only a small fraction of the receptors is already sufficient to mediate the maximal biologic response.39 Although the expression of the G-CSF receptor on cytoplasts can be considered to be adequate, addition of G-CSF to the culture medium had no effect on cytoplast apoptosis (35.7% ± 9.0% annexin-V− cells), in contrast to the delay of PMN apoptosis by G-CSF (compare Figure 7C and Figure 1C). Furthermore, in cytoplasts, G-CSF prevented neither caspase-3 processing (Figure 8, lane D) nor the appearance of active caspase-3 (Figure 9C), whereas G-CSF prevented caspase-3 processing and activation in PMNs (Figure 3, lane D, and Figure 4C). At the same time, zVAD-fmk (Figure 7D) reduced cytoplast apoptosis (91.5% ± 6.1% annexin-V− cytoplasts) and inhibited caspase-3 activation (Figure 8, lane E, and Figure 9D). Similar effects of zVAD-fmk were found in PMNs (Figure 1D, Figure 3, lane E, and Figure4D). Bax remained punctate under either treatment (not shown). We suppose that the G-CSF prosurvival effect and caspase-3 inhibiting action in PMNs are transcription-dependent events.
CHX abolishes the antiapoptotic effect of G-CSF in intact PMNs
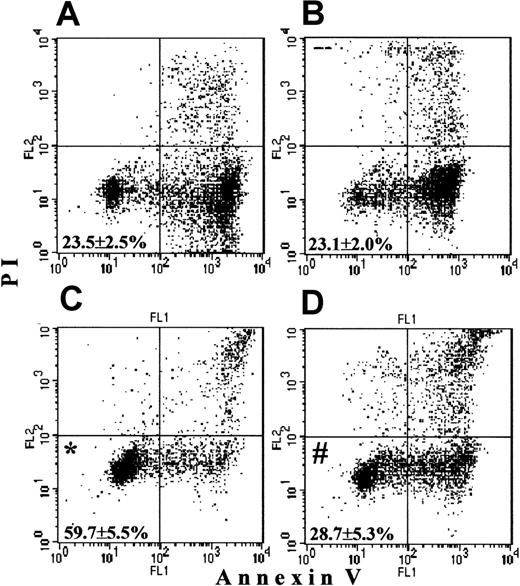
To support a protein synthesis–dependent character of the G-CSF prosurvival effect, we used CHX, a well-known inhibitor of protein synthesis. The overnight survival of untreated PMNs, which was 23.5% ± 2.5% in this set of experiments (Figure10A), was not disturbed by addition of the small dose of CHX (5 μg/mL; Figure 10B; 23.1% ± 2.0% surviving PMNs). G-CSF protected 59.7% ± 5.5% PMNs from apoptosis (Figure 10C). At the same time, when coincubated overnight, CHX completely abrogated the prosurvival effect of G-CSF, with 28.7% ± 5.3% surviving cells (Figure 10D). Hence, this provides additional evidence that the G-CSF prosurvival effect is a strictly protein synthesis-dependent event in PMNs.
Inhibition of the prosurvival effect of G-CSF in PMNs by CHX.
PMNs were cultured overnight without additions, with 5 μg/mL CHX, with 500 ng/mL G-CSF, or with the combination of CHX and G-CSF. Then the cells were stained and analyzed by FACScan as described in Figure1.(A) Untreated cell cultures overnight demonstrated a basal level of survival. (B) A low dose of CHX had no effect on basal survival. (C) G-CSF significantly increased survival (*P < .05 versus untreated cells). (D) The presence of CXH in the G-CSF culture abrogated the prosurvival effect of G-CSF (#P > .05 versus untreated cells). Values represent means ± SEM of 3 separate experiments.
Inhibition of the prosurvival effect of G-CSF in PMNs by CHX.
PMNs were cultured overnight without additions, with 5 μg/mL CHX, with 500 ng/mL G-CSF, or with the combination of CHX and G-CSF. Then the cells were stained and analyzed by FACScan as described in Figure1.(A) Untreated cell cultures overnight demonstrated a basal level of survival. (B) A low dose of CHX had no effect on basal survival. (C) G-CSF significantly increased survival (*P < .05 versus untreated cells). (D) The presence of CXH in the G-CSF culture abrogated the prosurvival effect of G-CSF (#P > .05 versus untreated cells). Values represent means ± SEM of 3 separate experiments.
Discussion
In our study, we examined the mechanisms of apoptosis in PMNs. Using a specific fluorescent mitochondrial stain and a mAb against a mitochondrial cytochrome c oxidase component, we found that PMNs do contain mitochondria. One of the possible routes of apoptosis in PMNs is mitochondria dependent and includes Bax-to-mitochondria translocation with subsequent executioner caspase activation. Our findings demonstrate that the G-CSF prosurvival effect coincides with prevention of mitochondrial clustering and Bax translocation, with concomitant inhibition of caspase-3 activity and retardation of apoptosis. This might be a possible route of G-CSF prosurvival action. At the same time, the general caspase inhibitor zVAD-fmk was found to inhibit caspase-3 and to delay PMN apoptosis without blocking the Bax-to-mitochondria translocation. In previous work with a cell-free apoptosis system40 and human embryonic kidney cells,41 it has also been shown that zVAD-fmk, despite caspase inhibition and prevention of apoptosis, was not able to prevent mitochondrial dysfunction and cytochrome c release. Apparently, Bax redistribution and mitochondrial dysfunction may be required but are not sufficient to induce apoptosis. Only if Bax translocation results in caspase-3 activation, as in untreated, overnight-cultured PMNs, does apoptosis of PMNs occur. This statement is supported by the finding that neither mitochondria nor Bax individually induce proteolytic processing and activation of caspases.42 Thus, our results support a dominant role of the executioner caspases in the downstream events of the apoptotic process in PMNs.
Experiments with PMN cytoplasts revealed additional features of the PMN apoptotic machinery and the G-CSF antiapoptotic effect in PMN. In the absence of a nucleus and mitochondria, cytoplasts underwent apoptotic changes, that is, phosphatidylserine exposure and caspase-3 activation, reminiscent of intact PMNs. Furthermore, even in “apoptotic” cytoplasts, Bax preserved a punctate distribution, showing no clustering as seen in PMNs. This underscores that in PMNs mitochondria serve as “docking sites” for Bax, and Bax movement to mitochondria is an important step in the activation of the mitochondria-dependent route of apoptosis in PMNs. In addition, there must be a second, mitochondria-independent route of PMN apoptosis, which was evident in cytoplasts. Activation of this alternative route may explain the observation that G-CSF is unable to completely prevent apoptosis in intact PMNs.
In cytoplasts, G-CSF did not prevent caspase-3 activation and phosphatidylserine “flip-flop,” and thus it showed no prosurvival effect. In PMNs, where the protein synthesis apparatus is intact, G-CSF had a prominent antiapoptotic effect. This indicates that agents like G-CSF, in line with previous findings, require de novo protein synthesis to produce a prosurvival effect.26 27Experiments with CHX, in which this inhibitor of protein synthesis prevented the prosurvival effect of G-CSF, gave additional support for this idea. On the other hand, considering the absence of mitochondria in PMN-derived cytoplasts, these data also indicate a mitochondria-dependent character of the G-CSF antiapoptotic action in intact PMNs.
Which protein(s) are needed for the antiapoptotic effect of G-CSF remain to be determined. A logical suggestion would be that an antiapoptotic Bcl-2 family member is involved, especially because Bcl-2 itself has been shown to prevent Bax-to-mitochondria translocation.11,14 But PMNs, either when freshly isolated or apoptotic, do not express Bcl-2,23,24,43 and the antiapoptotic Bcl-2–related proteins tested in our study, Mcl-1 and Bcl-XL, were found not to change their expression levels on execution of apoptosis. Probably, G-CSF is responsible for the synthesis of another Bcl-2–related candidate, or some different protein, for example, the upstream caspase-8 inhibitor FLIP, which has been shown in several recent publications to be up-regulated in endothelial cells,44,45macrophages,46 T cells,47 and B cells48 under prosurvival conditions. The other apoptosis route, as observed in PMN-derived cytoplasts, does not require mitochondria and Bax translocation. Nevertheless, caspase-3 activation is also involved in this route, being a final executioner of cell fate. Evidently, G-CSF has no effect on this mitochondria-independent pathway of apoptosis.
In conclusion, PMNs share with other cell types the ability to translocate Bax to mitochondria during apoptosis. However, we have demonstrated that apoptosis in PMNs is not strictly dependent on Bax translocation, as is supported by studies in Bax−/−mice.49,50 Although not formally studied in CCP32−/− models,15 51 the role for caspase-3 in neutrophil apoptosis may also be not as exclusive and absolute as suggested. G-CSF is a clinically relevant growth factor with a strong effect on promoting the life span of neutrophils in vitro and in vivo. Apart from the possible existence of additional and as yet uncharacterized routes in apoptosis, our data have now clearly demonstrated the mechanism of action as well as the mitochondria-dependent character of the G-CSF survival effect in intact PMNs.
Supported by a grant for doctoral student from the President of the Russian Federation (N.A.M.). T.W.K. is a research fellow of the Royal Dutch Academy of Sciences.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Taco W. Kuijpers, Central Laboratory of the Netherlands Blood Transfusion Service, Dept of Experimental Immunohematology, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: t_kuijpers@clb.nl.




![Fig. 6. Relocalization of Bax to the mitochondria in PMNs. / PMNs were cultured as described in Figure 1. Then the cells were stained with 1 μM MitoTracker Green FM, fixed, permeabilized, stained with antibody specific for Bax, and were analyzed with CLSM. (A) In fresh PMN Bax and the mitochondria were localized separately. (B) Overnight-cultured untreated cells demonstrated fusion of Bax and mitochondria into large aggregates (right panel in yellow). (C) Addition of G-CSF preserved the separate localization of Bax and mitochondria. (D) zVAD-fmk did not prevent aggregate formation and colocalization of Bax and mitochondria. (Comment: due to the fixation and permeabilization procedures, the mitochondrial staining [green] showed a more diffuse cytoplasmic pattern than the tubular structures shown in Figure 2, panels A and C). Bar is 5 μm. This figure is representative of at least 4 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.672/6/m_h80222003006.jpeg?Expires=1767697680&Signature=3hhe47DflJOmX7AhHMeIzoj5P0i9z0vha1DixJn5rQ7-aqgF7rkoQr5X2Zl1vWLM0q3py4~-KQ~uKnSrL9t7q~EbHVW89h096MrWq5-zyT7XD-IGVI5i40IjJywWV0QjJKlWXAyoqnBB3XqqXcgJsQhaYk1LGzHQM0hOFkL05RJIfKAZ3W87FCb7pbB6PxnZaYoqG0rVZ8lbbJNc02hO2PjoPNLG9DHrf8AY2nfQVHNf5Apayo9YzdBwDbcMKjsCdodxtdkZ1pyeAKa-~i2rv-5igfdKHDFBaeylPCp5SpUz2NLKI6enPruaKqDfPfZsKpBV1jF-FG7QCuHda3JrbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal