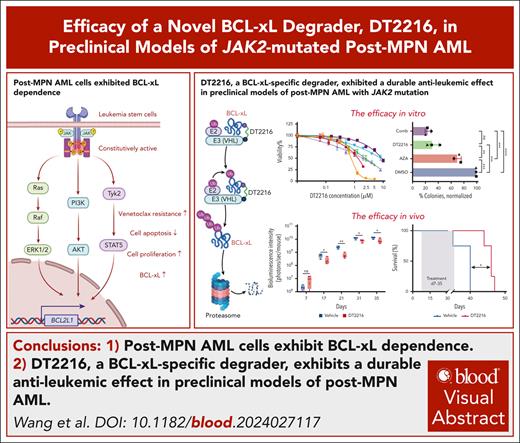
Key Points
Post-MPN AML cells exhibit BCL-xL dependence.
DT2216, a BCL-xL–specific degrader, exhibits a durable antileukemic effect in preclinical models of post-MPN AML.
Visual Abstract
Acute myeloid leukemia (AML) that evolves from myeloproliferative neoplasm (MPN) is known as post-MPN AML. Current treatments do not significantly extend survival beyond 12 months. B-cell lymphoma-extra large (BCL-xL) has been found to be overexpressed in leucocytes from patients with MPN, making it a potential therapeutic target. We investigated the role of BCL-xL in post-MPN AML and tested the efficacy of DT2216, a platelet-sparing BCL-xL proteolysis-targeting chimera, in preclinical models of post-MPN AML. We found that BCL2L1, the gene encoding BCL-xL, is expressed at higher levels in patients with post-MPN AML than in those with de novo AML. Single-cell multiomics analysis revealed that leukemia cells harboring both MPN-driver and TP53 mutations exhibited higher BCL2L1 expression and elevated scores for leukemia stem cell, megakaryocyte development, and erythroid progenitor than wild-type cells. BH3 profiling confirmed a strong dependence on BCL-xL in post-MPN AML cells. DT2216 alone, or in combination with standard AML/MPN therapies, effectively degraded BCL-xL, reduced the apoptotic threshold, and induced apoptosis in post-MPN AML cells. DT2216 effectively eliminated viable cells in JAK2-mutant AML cell lines, induced pluripotent stem cell–derived hematopoietic progenitor cells, primary samples, and reduced tumor burden in cell line–derived xenograft model in vivo by degrading BCL-xL. DT2216, either as a single agent or in combination with azacytidine, effectively inhibited the clonogenic potential of CD34+ leukemia cells from patients with post-MPN AML. In summary, our data indicate that the survival of post-MPN AML is BCL-xL dependent, and DT2216 may offer therapeutic advantage in this high-risk leukemia subset with limited treatment options.
Introduction
Acute myeloid leukemia (AML) that develops from myeloproliferative neoplasm (MPN), also known as post-MPN AML, affects 0.7% to 3.8% of patients with essential thrombocytosis,1-4 2.3% to 6.8% of patients with polycythemia vera,4,5 and 3.9% to 20.6% of patients with primary myelofibrosis (PMF).4,6-8 Among these, PMF is the most likely to transform to AML and does so in the shortest latency period. Patients with post-MPN AML are typically older,6 require transfusions,9 and have received previous treatments.7
Most patients with post-MPN AML retain mutations in JAK2, CALR, or MPL after blast transformation.10 Conversely, some patients lose MPN-driver mutations during the transformation.11 Post-MPN AML serves a model of genetic evolution, in which specific genes (eg, TP53, RUNX1, ASXL1, TET2, IDH1/2, PTPN11, and SRSF2) play a role in the transformation process.10,12-15 This is supported by recent data in the genetically engineered murine Jak2V617F/+ model, whereby biallelic alteration of Trp53 (murine analog of human TP53 gene; Jak2V617F/+Tp53−/− and Jak2V617F/+Tp53R172H/−) results in lethal acute erythroleukemic transformation.16
Patients with post-MPN AML are less responsive to currently available therapies for either AML or MPN, including chemotherapy, hypomethylating agents, B-cell lymphoma 2 (BCL-2) inhibitors, or JAK2 inhibitors. The median overall survival for these patients is <1 year.17 MPN-driver mutations are known to constitutively activate the cytokine receptor18-20/JAK-STAT21,22/MAPK23/PI3K-AKT24 pathways. BCL2L1 was progressively overexpressed in leucocytes from patients with MPN,25 making the inhibition of B-cell lymphoma-extra large (BCL-xL) a critical therapeutic target for these patients. The clinical trial of ABT-263 (navitoclax), a dual BCL-2/BCL-xL inhibitor, in combination with ruxolitinib, demonstrated promising results in patients with PMF, resulting in ≥35% spleen volume reduction in 41% of patients, 1 to 2 grade bone marrow fibrosis improvement in 33% of patients, and increased hemoglobin in 63% of patients.26 Compared with hematopoietic stem and progenitor cells (HSPCs) from PMF, those from post-MPN AML exhibit further upregulation of the JAK-STAT and PI3K-AKT-mTOR pathways.27BCL2L1 is one of the direct transcriptional targets of STAT5 and NF-κB,28 and the resultant increase in BCL-xL may provide a survival advantage to these malignant cells while cooperating with the oncogenic signaling to maintain the diseased state.29,30 These findings suggest that targeting BCL-xL may represent a potential treatment strategy for post-MPN AML. However, given that platelets highly rely on BCL-xL for the survival,31 thrombocytopenia prompted navitoclax dose reduction or interruption in 56% of patients,26 limiting its clinical utility. Thus, the development of novel agents selectively targeting BCL-xL in malignant cells is urgently needed.
DT2216, a von Hippel-Lindau (VHL)–recruiting BCL-xL proteolysis-targeting chimera (PROTAC), has been shown to induce potent degradation of BCL-xL in leukemia cells. VHL is highly expressed in leukemia cells but minimally expressed in platelets. This differential expression allows for targeting of BCL-xL in leukemia cells while sparing platelets.32 In this study, we evaluated the preclinical efficacy of DT2216 alone and in combination with conventional drugs in post-MPN AML models.
Materials and methods
Data collection and preprocessing
We used genome-wide CRISPR screen data and RNA-sequencing (RNA-seq) data for AML cell lines from the DepMap portal (https://depmap.org/portal/download/all/). RNA-seq data for 491 patients with AML were sourced from the Beat AML 2.0 cohort (https://registry.opendata.aws/beataml), and RNA-seq data of paired patients were obtained from GSE210253 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE210253). In addition, RNA-seq data for Jak2 and Trp53 mutant mouse models in the MPN and post-AML stages focusing on megakaryocyte-erythroid progenitors (MEPs) were accessed via GSE180851 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE180851).
Processed single-cell multiomics data for patients with MPN and post-MPN AML, along with the scripts associated with Figure 2 and supplemental Figures 2-4 (available on the Blood website), were downloaded from Zenodo (https://zenodo.org/records/8060602) and GitHub (https://github.com/albarmeira/p53-transformation). For the study, we analyzed 14 patients with post-MPN AML and 9 with MPN, 5 of whom had paired samples. Among 14 patients with post-MPN AML, 13 harbored concurrent MPN-driver and TP53 mutations. To integrate these transcriptomes, we used the SingCellaR package’s runHarmony, runDiffusionMap function. Signature scores were calculated using a custom function, “plot_diffusionmap_label_by_gene_set,” referencing gene sets from supplemental Table 2. To establish a normal hematopoietic reference, we used a previously published human hematopoietic atlas,33 accessible at https://github.com/GreenleafLab/MPAL-Single-Cell-2019. This reference served as a basis for projecting single-cell transcriptomes from de novo AML,34 MPN, and post-MPN AML samples using latent semantic index projection to plot Figure 2A-B and supplemental Figure 2F (supplemental Methods).
Cell lines and culture
Leukemia cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA) or German Collection of Microorganisms and Cell Cultures (DSMZ; Braunschweig, Germany). HEL, SET2, and UKE-1 ruxolitinib-resistant (Ruxo-Re) cells were generated as previously described.35 Detailed protocols for cell line culture can be found in the supplemental Methods.
Clinical samples
The primary post-MPN AML samples were obtained from patients with informed consent, according to the requirements of the MD Anderson Cancer Center Institutional Review Board (LAB02-652) and the Declaration of Helsinki. Mononuclear cells were purified by Ficoll-Hypaque density centrifugation (supplemental Methods). The clinical features of the patients are listed in supplemental Table 1.
CRISPR-Cas9–mediated knockout of BCL2L1 and TP53
The CRISPR ribonucleoprotein complex was used to target BCL2L1 and TP53. Cell growth was monitored every 24 hours using the CellTiter-Glo assay from Promega (Madison, WI; supplemental Methods).
Quantitative reverse transcription PCR
Total RNA was extracted using the RNeasy Mini kit (Qiagen, 74104). A reverse transcription reaction was performed using the iScript complementary DNA synthesis kit (Bio-Rad, 1708891). For the determination of BCL2L1, BCL2, and MCL1 messenger RNA (mRNA) expression, quantitative reverse transcription–polymerase chain reaction (PCR) was conducted using PowerUp SYBR Green Master Mix (Applied Biosystems, A25742) and the Bio-Rad CFX96 Touch Real-Time PCR Detection System (supplemental Methods).
Drug treatment and 50% inhibitory concentration (IC50) measurement
DT2216 was obtained from Dialectic Therapeutics. Detailed information regarding the drugs used in this study can be found in the supplemental Methods. We conducted the CellTiter-Glo assay using JAK2-mutant (JAK2-mut) AML cell lines and primary leukemia samples to assess the efficacy of DT2216-based treatments. For combination treatments (DT2216 plus azacytidine [AZA]/ruxolitinib/venetoclax/S63845), the 2 drugs were mixed at a fixed ratio and serially diluted. The combination index (CI) values were calculated using CalcuSyn software (BioSoft; supplemental Methods).
Generation of MPN-iPSCs through patient cell reprogramming
BH3 profiling
BH3 profiling was conducted as previously reported.38 In brief, cells from different cell lines, iPSC lines, or primary samples were permeabilized with digitonin and exposed to BH3 peptides (BIM, HRK, and NOXA; synthesized by New England Peptide), S63845, ABT-199, ABT-263, or DT2216. The mitochondrial transmembrane potential loss was monitored using cytochrome c.
Colony-forming unit assay
Colony-forming unit assays were performed to assess the antileukemic effects on the colony formation of CD34+ cells sorted from patients with post-MPN AML (supplemental Methods).
Western blotting
Cells were lysed in protein lysis buffer. Equal amounts of protein samples were resolved sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to nitrocellulose membrane. Immunoblotting was performed with primary antibodies: BCL-2 (Dako Omnis), myeloid leukemia-1 (MCL-1; Santa Cruz Biotechnology), p53, poly(ADP-ribose) polymerase (PARP), cleaved PARP, cleaved caspase 3, BCL-xL (the latter 3 from Cell Signaling Technology), β-actin, and α-tubulin (the latter 2 from Abcam). The samples were then incubated in secondary antibodies for 1 hour and washed 3 times in 1× phosphate-buffered saline with Tween 20. Blots were scanned with an Odyssey Infrared Imaging System (LI-COR Biosciences; supplemental Methods).
Animal experiments
For studies of efficacy of DT2216 in vivo, cell line–derived xenograft (CDX) models were created as follows. First, 1 × 106 luciferase-expressing SET2 cells were intravenously injected into NCG (NOD-Prkdcem26Cd52Il2rgem26Cd22/NjuCrl) mice from Charles River Laboratories, and engraftment was confirmed on day 7 using bioluminescence imaging (BLI) with an Ami HT in vivo imaging system (Spectral Instruments Imaging). Mice were randomized into treatment groups (4 mice per group) and treated with DT2216 (15 mg/kg intraperitoneally every 4 days) or vehicle from day 7 to day 35, then reassessed weekly by BLI. Another cohort was established by the same method for pharmacodynamics of BCL-xL degradation (supplemental Methods).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 10 or R (version 4.0.5) software (supplemental Methods).
Results
JAK2-mut AML cell lines and samples from patients with post-MPN AML exhibit BCL-xL dependence
Typically, leukemia clones arising from a JAK2/CALR/MPL-mutated cell population exhibit rapid clonal expansion, chromosomal instability, and blast transformation after acquiring somatic genetic (eg, TP53) and/or epigenetic remodeling events (eg, TET2, ASXL1).17 Our previous data39 demonstrated higher BCL-xL expression at protein level in JAK2-mut AML cell lines (supplemental Figure 1A). Analysis of the Cancer Cell Line Encyclopedia database revealed significantly upregulated BCL2L1 gene expression in JAK2-mut AML cell lines compared with other AML cell lines (Figure 1A-B; P = .02). Additionally, using the Public Avana 21Q2 data set, we found that the JAK2-mut cell lines had lower BCL2L1 CRISPR gene scores than other AML cell lines, indicating stronger dependence on BCL-xL (Figure 1C-D; P = .02). This trend was more pronounced in AML cell lines with comutations of JAK2 and TP53 (JAK2_TP53 mut, red dots). We validated this observation by knocking out the BCL2L1 gene using CRISPR-Cas9, which halted proliferation of JAK2-mut AML cells (SET2 and HEL), regardless of whether the cells were sensitive or resistant to ruxolitinib, whereas BCL2L1 silencing had no impact on non–JAK2-mut AML cell lines (Figure 1E-F; supplemental Figure 1B-D). BCL2L1 mRNA overexpression was validated in post-MPN AML primary samples compared with de novo AML samples in the Beat AML 2.0 cohort (Figure 1G; P = .0026).40
BCL-xL plays a critical role in the survival of JAK2-mut AML cell lines and patients with post-MPN AML. (A) BCL2L1 mRNA expression in AML cell lines in the Cancer Cell Line Encyclopedia database. (B) When the cell lines shown in panel A were grouped, JAK2-mut cell lines had significantly higher BCL2L1 expression than other AML cell lines. ∗P < .05. (C) BCL2L1 CRISPR gene knockout (KO) effect in AML cell lines in the Public Avana 21Q2 (DepMap) data set. More negative value corelate a stronger dependence. (D) When the cell lines shown in panel C were grouped, JAK2-mut cell lines had significantly lower BCL2L1 CRISPR gene scores than other AML cell lines. More negative values indicate stronger dependence. ∗P < .05. (E) Proliferation curves of JAK2-mut cell lines (SET2 and HEL) after BCL2L1 KO. (F) Proliferation curves of JAK2-WT AML cell lines (THP1 and OCI-AML3) after BCL2L1 gene KO. (G) RNA-seq analysis of BCL2L1 in patient data in the Beat AML 2.0 cohort. Cases were stratified according to AML with history of previous MPN (n = 28), prior MDS/MPN overlap (n = 24), prior MDS (n = 63), de novo AML (n = 323), or therapy-related AML (n = 53). Patients with prior MPN showed significantly higher BCL2L1 expression than patients with de novo AML. ∗∗P < .01. RLU, relative light unit; RPKM, reads per kilobase million; TPM, transcripts per million.
BCL-xL plays a critical role in the survival of JAK2-mut AML cell lines and patients with post-MPN AML. (A) BCL2L1 mRNA expression in AML cell lines in the Cancer Cell Line Encyclopedia database. (B) When the cell lines shown in panel A were grouped, JAK2-mut cell lines had significantly higher BCL2L1 expression than other AML cell lines. ∗P < .05. (C) BCL2L1 CRISPR gene knockout (KO) effect in AML cell lines in the Public Avana 21Q2 (DepMap) data set. More negative value corelate a stronger dependence. (D) When the cell lines shown in panel C were grouped, JAK2-mut cell lines had significantly lower BCL2L1 CRISPR gene scores than other AML cell lines. More negative values indicate stronger dependence. ∗P < .05. (E) Proliferation curves of JAK2-mut cell lines (SET2 and HEL) after BCL2L1 KO. (F) Proliferation curves of JAK2-WT AML cell lines (THP1 and OCI-AML3) after BCL2L1 gene KO. (G) RNA-seq analysis of BCL2L1 in patient data in the Beat AML 2.0 cohort. Cases were stratified according to AML with history of previous MPN (n = 28), prior MDS/MPN overlap (n = 24), prior MDS (n = 63), de novo AML (n = 323), or therapy-related AML (n = 53). Patients with prior MPN showed significantly higher BCL2L1 expression than patients with de novo AML. ∗∗P < .01. RLU, relative light unit; RPKM, reads per kilobase million; TPM, transcripts per million.
To investigate the molecular drivers of leukemic transformation, we analyzed 9709 Lin−CD34+ HSPCs from 13 patients with post-MPN AML harboring both TP53 and MPN-driver mutations, using targeted and genotyping RNA expression technique-sequencing (TARGET)-seq41 multiomics data.42 Notably, JAK2/CALR mutant TP53 WT (JAK2/CALR mut_TP53 wt) cells were the predominant cell population in the chronic phase of MPN, whereas JAK2/CALR_TP53 mutant (JAK2/CALR_TP53 mut) cells dominated during the acute transformation phase. JAK2/CALR_TP53 mut HSPCs displayed marked upregulation of the JAK-STAT, KRAS signaling, and PI3K-AKT genes compared with both JAK2/CALR_TP53 wt and JAK2/CALR mut_TP53 wt HSPCs (supplemental Figure 2A-D). Because BCL2L1 is a downstream transcriptional target of these pathways, we hypothesized that post-MPN AML may maintain or upregulate the BCL2L1 overexpression (a hallmark of MPN25), sustaining BCL-xL–dependent survival driven by amplified oncogenic signaling. To address this, we integrated single-cell RNA-seq data42 of 12 527 HSPCs from 9 patients with MPN (4020 HSPCs) and 14 patients with post-MPN AML (8507 HSPCs) and projected them onto a healthy-donor hematopoietic hierarchy (supplemental Figure 2E).33 This projection revealed hematopoietic stem cell (HSC)/erythroid progenitor enrichment in MPN, further amplified in post-MPN AML (Figure 2A-B, blue dots). BCL2L1 remained highly expressed and showed a trend toward upregulation in HSPCs of post-MPN AML (Figure 2A; P = .052), and BCL2 expression was increased (Figure 2B; P = .0049). In contrast, de novo AML HSPCs were enriched in myeloid signatures (supplemental Figure 2F).34 Further analysis of data from 5 paired patients with MPN and post-MPN AML in our cohort and in an independent data set (bulk RNA-seq)43 revealed sustained BCL2L1 expression at both the single-cell level (2312 MPN HSPCs vs 4307 post-MPN AML HSPCs; P = .83; supplemental Figure 3A-B) and at the bulk RNA level (n = 11; P = .07; GSE210253; supplemental Figure 3C), demonstrating persistent high BCL2L1 expression in chronic MPN and post-MPN AML.
Molecular and functional analysis of LSCs in patients with post-MPN AML. (A) Latent semantic index projection of 4020 cells from 9 patients with MPN and 8507 cells from 14 patients with post-MPN AML, and the expression of BCL2L1 onto the healthy donor (HD) hematopoietic hierarchy atlas shown in supplemental Figure 2E. (B) Latent semantic index projection of the same cell shown in panel A and the expression of BCL2 onto the HD hematopoietic hierarchy atlas shown in supplemental Figure 2E. (C) Three-dimensional diffusion map of 8000 Lin−CD34+ cells from 13 patients with post-MPN AML colored by genotype (left). (D-E) Three-dimensional diffusion map of the same cells from panel C colored by score of megakaryocyte development (D) and erythroid progenitor (E). (F-G) Three-dimensional diffusion map of the same cell shown in panel C colored by BCL2L1 expression (F) and BCL2 expression (G). (H) The expression of BCL2L1 in the leukemic MEPs (Jak2V617F/+Trp53–/– and Jak2V617F/+Trp53R172H/–) compared with MEP derived from mice at the chronic MPN stage. ∗∗∗∗P < .0001; ∗∗∗P < .001. DC, diffusion component; UMAP, uniform manifold approximation and projection.
Molecular and functional analysis of LSCs in patients with post-MPN AML. (A) Latent semantic index projection of 4020 cells from 9 patients with MPN and 8507 cells from 14 patients with post-MPN AML, and the expression of BCL2L1 onto the healthy donor (HD) hematopoietic hierarchy atlas shown in supplemental Figure 2E. (B) Latent semantic index projection of the same cell shown in panel A and the expression of BCL2 onto the HD hematopoietic hierarchy atlas shown in supplemental Figure 2E. (C) Three-dimensional diffusion map of 8000 Lin−CD34+ cells from 13 patients with post-MPN AML colored by genotype (left). (D-E) Three-dimensional diffusion map of the same cells from panel C colored by score of megakaryocyte development (D) and erythroid progenitor (E). (F-G) Three-dimensional diffusion map of the same cell shown in panel C colored by BCL2L1 expression (F) and BCL2 expression (G). (H) The expression of BCL2L1 in the leukemic MEPs (Jak2V617F/+Trp53–/– and Jak2V617F/+Trp53R172H/–) compared with MEP derived from mice at the chronic MPN stage. ∗∗∗∗P < .0001; ∗∗∗P < .001. DC, diffusion component; UMAP, uniform manifold approximation and projection.
Single-cell molecular signatures of post-MPN AML
Next, we investigated the intracellular heterogeneity and transcriptional characteristics of HSPCs in patients with post-MPN AML at the single-cell level. The single-cell multiomics analysis of 13 patients with post-MPN AML from the same cohort with concurrent MPN-driver and TP53 mutations revealed that JAK2/CALR_TP53 mut HSPCs formed distinct clusters from JAK2/CALR_TP53 wt and JAK2/CALR mut_TP53 wt HSPCs (Figure 2C). We investigated lineage features in post-MPN AML. JAK2/CALR_TP53-mut HSPCs exhibited enhanced megakaryocyte development (P < 2.2 × 10–16; Figure 2D; supplemental Figure 3D; supplemental Table 2) and erythroid progenitor (P = 8.42 × 10–14; compared with JAK2/CALR_TP53 wt HSPCs; Figure 2E; supplemental Figure 3E; supplemental Table 2) alongside diminished HSC score (P = 3.01 × 10−10) and highest leukemic stem cell (LSC) score (P = .003; supplemental Figure 3F-G; supplemental Table 2). Given the known association of erythro-megakaryocytic lineage bias with JAK2,16,44CALR,45 and TP53,42 mutations and elevated BCL2L1 expression in erythroid/megakaryocytic AML,46 we explored BCL2 family expression in genomically distinct HSPC clones. JAK2/CALR_TP53 mut HSPCs exhibited higher expression of BCL2L1 (P = .008; compared with JAK2/CALR_TP53 wt HSPCs; Figure 2F; supplemental Figure 4A-B), BCL2 (P = .021; compared with JAK2/CALR mut_TP53 wt HSPCs; Figure 2G; supplemental Figure 4A-B), and BCL2L2 (P = .003; supplemental Figure 4A-B), with lower MCL1 expression (P = .042; supplemental Figure 4A-B). In addition, JAK2/CALR_TP53 mut HSPCs exhibited upregulated proapoptotic genes (eg, BAK1, BAX, BCL2L11, BID, PMAIP1, BAD, and BIK; supplemental Figure 4A-C). These transcriptional changes reflect the requirement for compensatory overexpression of antiapoptotic proteins such as BCL-xL and BCL-2 in sustaining survival46-48 under persistent oncogenic stress.49,50
Because we observed higher BCL2L1 levels in post-MPN AML clones harboring TP53-mut HSPCs, we next silenced TP53 in TP53-wt JAK2-mut UKE-1 cells using CRISPR-induced knockout and did not observe significant differences in BCL2L1 RNA or BCL-xL protein levels (supplemental Figure 5A-C). Given limitations of short-term in vitro experiments upon acute silencing of TP53, we further explored expression of Bcl2l1 gene levels in stem/progenitor compartment of post-MPN AML murine model of Jak2V617F/+ and biallelic alteration of Trp53 characterized by the expansion of the bipotent MEPs and lethal erythroleukemic transformation.16 The transcriptomic analysis of sorted progenitor cells demonstrated that Bcl2l1 is significantly upregulated in the leukemic MEPs (Jak2V617F/+Trp53–/– and Jak2V617F/+Trp53R172H/–; P = 9.86 × 10–6 and P = .000938; Figure 2H) compared with MEPs from mice with the same genetic background at the chronic MPN stage.
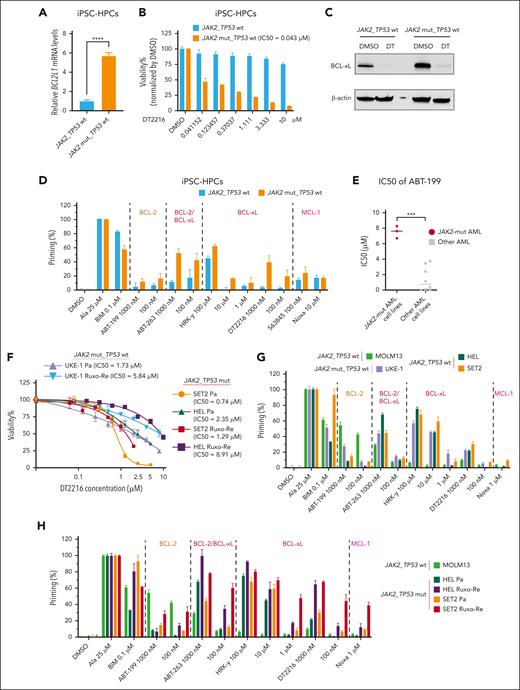
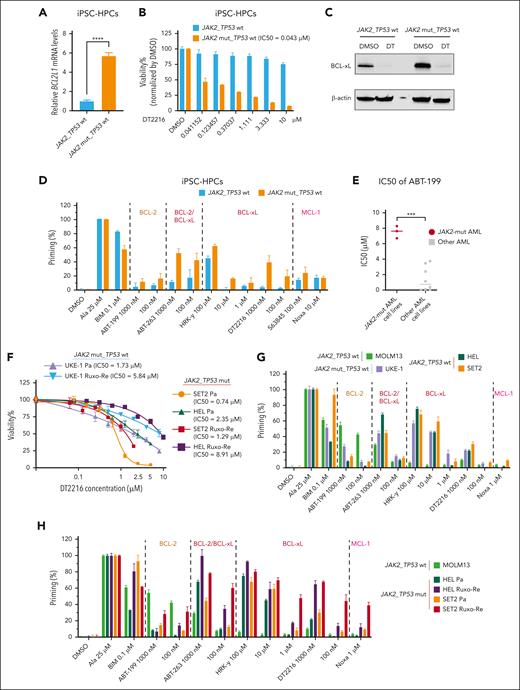
JAK2V617Fmutation enhancesBCL2L1expression iniPSC-HPCs,and DT2216 as a single agent significantly reduces cell viability inJAK2-mut AML cell lines and iPSC-HSPCs
IPSCs, in addition to established preclinical in vitro and in vivo models using cell lines and patient samples, provide an opportunity to further explore targeted therapies for leukemia-specific mutations. Thus, we compared BCL2L1 expression in iPSC-HPCs with JAK2V617F mutation with WT iPSC-HPCs from the same patients with MPN using qPCR. JAK2V617F-mutant iPSC-HPCs exhibited significantly higher BCL2L1 expression (P < .0001; Figure 3A). DT2216 treatment led to dose-dependent inhibition of cell viability in JAK2V617F iPSC-HPCs (IC50, 43 nM), whereas WT iPSC-HPCs were unaffected (Figure 3B), despite complete BCL-xL degradation in both cell types (Figure 3C). BH3 profiling indicated that, compared with the WT iPSC-HPCs, the JAK2V617F iPSC-HPCs released more cytochrome c after treatment with the BCL-xL–specific peptide HRK-Y, BCL-xL degrader DT2216, and dual BCL-xL/BCL-2 inhibitor ABT-263 (Figure 3D).
DT2216 as a single agent significantly reduced cell viability in iPSC-HPCs with JAK2V617F mutation and JAK2-mut AML cell lines. (A) BCL2L1 mRNA expression in iPSC-HPCs with or without JAK2V617F mutation from a patient with MPN. ∗∗∗∗P < .0001. (B) Cell viability of iPSC-HPCs with the indicated concentrations of DT2216 for 72 hours. (C) Western blots for iPSC-HPCs with or without JAK2V617F mutation treated with 1 μM DT2216 for 72 hours. β-Actin was used as the loading control for the western blot. (D) BH3 profiling of iPSC-HPCs with or without JAK2V617F mutation. The BH3 peptides and drugs were categorized by their target molecules. (E) IC50 values of venetoclax at 24 hours in AML cell lines. ∗∗∗P < .001. (F) Cell viability of JAK2-mut parental (Pa) and Ruxo-Re AML cell lines (HEL, SET2, and UKE-1) with the indicated concentrations of DT2216 for 72 hours. (G) BH3 profiling of JAK2-mut AML cell lines and the MOLM13 AML cell line (BCL-2 dependent). (H) BH3 profiling of paired SET2 and HEL Pa and Ruxo-Re cell lines and the MOLM3 cell line.
DT2216 as a single agent significantly reduced cell viability in iPSC-HPCs with JAK2V617F mutation and JAK2-mut AML cell lines. (A) BCL2L1 mRNA expression in iPSC-HPCs with or without JAK2V617F mutation from a patient with MPN. ∗∗∗∗P < .0001. (B) Cell viability of iPSC-HPCs with the indicated concentrations of DT2216 for 72 hours. (C) Western blots for iPSC-HPCs with or without JAK2V617F mutation treated with 1 μM DT2216 for 72 hours. β-Actin was used as the loading control for the western blot. (D) BH3 profiling of iPSC-HPCs with or without JAK2V617F mutation. The BH3 peptides and drugs were categorized by their target molecules. (E) IC50 values of venetoclax at 24 hours in AML cell lines. ∗∗∗P < .001. (F) Cell viability of JAK2-mut parental (Pa) and Ruxo-Re AML cell lines (HEL, SET2, and UKE-1) with the indicated concentrations of DT2216 for 72 hours. (G) BH3 profiling of JAK2-mut AML cell lines and the MOLM13 AML cell line (BCL-2 dependent). (H) BH3 profiling of paired SET2 and HEL Pa and Ruxo-Re cell lines and the MOLM3 cell line.
Next, we also determined the responses of JAK2-mut AML cell lines to the BCL-2 inhibitor venetoclax (ABT-199). The median IC50 value of venetoclax in JAK2-mut AML cells was higher than in non-JAK2-mut AML cell lines (7.54 ± 0.79 vs 1.41 ± 1.58 μM; P < .0001; Figure 3E). In several clinical reports, patients with post-MPN AML had poor response to venetoclax-based regimens, both in newly diagnosed and relapsed/refractory setting.51
On the contrary, DT2216 significantly reduced cell viability of JAK2-mut AML cells (average IC50, 1.61 ± 0.81 μM in parental lines and 5.35 ± 3.84 μM in Ruxo-Re lines at 72 hours; Figure 3F). BH3 profiling showed that, compared with BCL-2–dependent MOLM13 cells,52JAK2-mut cell lines (HEL, SET2, and UKE-1) released more cytochrome c after exposure to BCL-xL–selective peptide HRK-Y, BCL-xL degrader DT2216, and BCL-xL/BCL-2 inhibitor ABT-263, and less in response to BCL-2 selective inhibitor venetoclax (Figure 3G). In addition, compared with parental cells, Ruxo-Re cells showed increased BCL-xL dependence by BH3 profiling (Figure 3H).
DT2216 combined with AZA, ruxolitinib, or venetoclax reduce viability of JAK2-mut AML cell lines
To explore the therapeutic potential of targeting BCL-xL in post-MPN AML, we combined DT2216 with drugs used clinically in AML/MPN practice (ruxolitinib, AZA, and venetoclax), and with MCL-1 inhibitor (S63845).
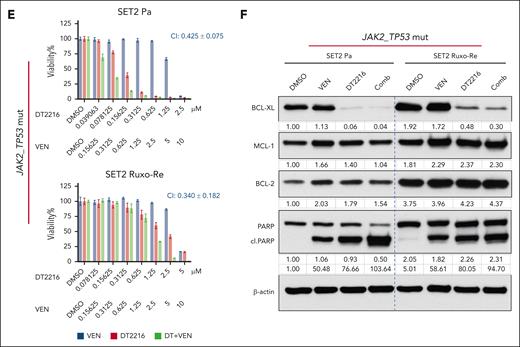
DT2216 and AZA demonstrated potent efficacy and effective combinatorial activity in SET2 cells at 72 hours (CI: parental, 0.217 ± 0.100; Ruxo-Re, 0.206 ± 0.099; Figure 4A) and UKE-1 cells (parental, 0.132 ± 0.140; Ruxo-Re, 0.063 ± 0.063; supplemental Figure 6A), with a mean CI of 0.154 ± 0.072 across models (Figure 4A; supplemental Figure 6A). Western blot demonstrated efficient BCL-xL degradation and apoptosis (cleaved PARP) with DT2216 alone or in combination (Figure 4B).
The combination of DT2216 with ruxolitinib inhibited growth of Ruxo-Re SET2 cells (CI, 0.383 ± 0.136 vs 0.369 ± 0.124 in parental; Figure 4C). Furthermore, we validated the efficacy of this approach in other JAK2-mut AML cell lines (HEL and UKE-1; supplemental Figure 6B), and the average CI was 0.465 ± 0.211 in 3 paired JAK2-mut AML cell lines. Western blot further confirmed the on-target degradation of BCL-xL by combination of DT2216 with ruxolitinib (Figure 4D; supplemental Figure 6C). Of note, baseline expression of BCL-xL and BCL-2 were higher in Ruxo-Re SET2 cells (Figure 4B,D,F). The combination of DT2216 with either azacitidine or ruxolitinib further reduced BCL-2 and MCL-1 expression, with this trend being more pronounced in SET2 parental cells (Figure 4B,D). Use of a pan-caspase inhibitor quinoline-val-asp-difluorophenoxymethylketone (QVD) reversed the reduction in MCL-1 and BCL-2 levels, indicating caspase-dependent MCL-1 and BCL-2 degradation as reported in other models (supplemental Figure 6D).53,54
The combination of DT2216 with venetoclax similarly showed activity in SET2 cells, with CIs of 0.425 ± 0.075 in parental cells and 0.340 ± 0.182 in Ruxo-Re cells at 72 hours (Figure 4E-F). In JAK2-mut HEL parental and Ruxo-Re variants the average CI was 0.607 ± 0.054 (supplemental Figure 6E).
Finally, we evaluated the combination of DT2216 with MCL-1 inhibitor S63845. This combination showed synergistic effects, with an average CI of 0.130 ± 0.043 in SET2 and HEL cells (supplemental Figure 6F). Altogether, combinations with AZA and MCL-1 inhibitor showed the best synergy especially in Ruxo-Re cells.
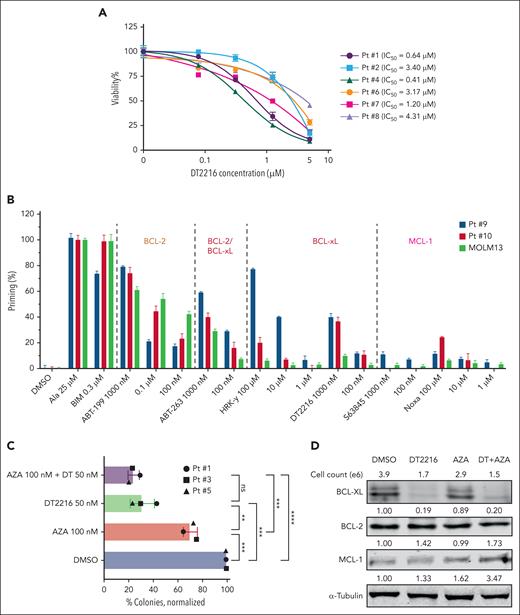
DT2216 alone or in combination with AZA effectively reduced cell viability and colony-formation potential of post-MPN AML CD34+ primary cells
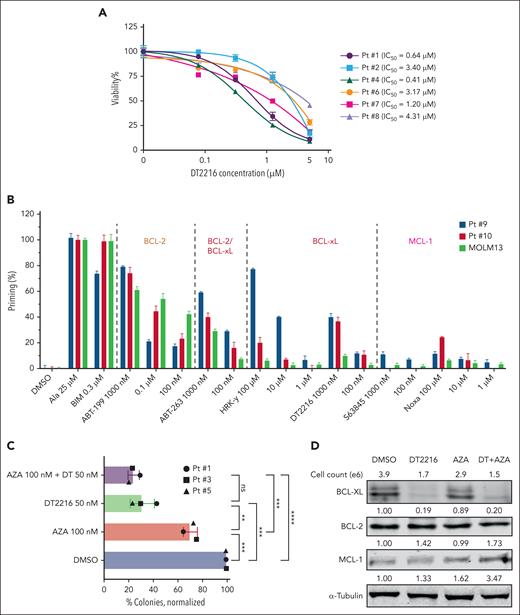
Next, we investigated the antileukemic activity of DT2216 in primary samples from patients with post-MPN AML. Notably, DT2216 as a single agent markedly reduced viability of CD34+ primary AML cells (n = 6), with an average IC50 value of 2.19 ± 1.64 μM (range, 0.41-4.31 μM) at 24 hours (Figure 5A). We obtained RNA from 4 patients and performed qPCR to measure the expression levels of the BCL2, BCL2L1, and MCL1 genes, using the SET2 cell line as a control. We observed a negative correlation between the BCL2L1/(BCL2 + BCL2L1+ MCL1) relative expression and IC50 (r = −0.9033; P = .0484; supplemental Figure 7A-B), indicating that the relative abundance of BCL2L1 compared with other antiapoptotic proteins may dictate responsiveness. No correlation was found between the relative expression of BCL2/(BCL2 + BCL2L1 + MCL1) or MCL1/(BCL2 + BCL2L1 + MCL1) and IC50. BH3 profiling showed that, compared with MOLM13 cells, 2 post-MPN AML samples tested released more cytochrome c after treatment with the HRK-Y, DT2216, and ABT-263 (Figure 5B), consistent with BCL-xL dependence.
DT2216 alone or in combination with AZA can effectively reduce the colony-formation ability of JAK2-mut AML CD34+ primary leukemia cells. (A) Viability of CD34+ AML cells isolated from patients with post-MPN AML treated with the indicated concentrations of DT2216 for 24 hours. Note: Patient 7’s samples included total leukemia cells. (B) BH3 profiling of cells from 2 patients with post-MPN AML and MOLM13 cells (as a control). (C) Colony formation for CD34+ AML cells isolated from patients with JAK2-mut AML and treated with DT2216 (50 nM) and/or AZA (100 nM) in methylcellulose for 10 to 14 days. Colonies were counted using the EVOS imaging system (Thermo Fisher Scientific, Bothell, WA) and significance determined by t test. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (D) Western blot analysis confirmed that DT2216 alone or in combination with AZA can effectively target and degrade BCL-xL in cells collected from methylcellulose. α-tubulin was used as the loading control for the western blot. ns, nonsignificant; Pt, patient.
DT2216 alone or in combination with AZA can effectively reduce the colony-formation ability of JAK2-mut AML CD34+ primary leukemia cells. (A) Viability of CD34+ AML cells isolated from patients with post-MPN AML treated with the indicated concentrations of DT2216 for 24 hours. Note: Patient 7’s samples included total leukemia cells. (B) BH3 profiling of cells from 2 patients with post-MPN AML and MOLM13 cells (as a control). (C) Colony formation for CD34+ AML cells isolated from patients with JAK2-mut AML and treated with DT2216 (50 nM) and/or AZA (100 nM) in methylcellulose for 10 to 14 days. Colonies were counted using the EVOS imaging system (Thermo Fisher Scientific, Bothell, WA) and significance determined by t test. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (D) Western blot analysis confirmed that DT2216 alone or in combination with AZA can effectively target and degrade BCL-xL in cells collected from methylcellulose. α-tubulin was used as the loading control for the western blot. ns, nonsignificant; Pt, patient.
Considering the CI of different combinations, the side effects of the drugs,55,56 and their accessibility, we chose the combined DT2216/AZA regimen for further studies.
Clonogenic assays in 3 primary JAK2-mut AML samples demonstrated a 30% (range, 25.7%-36.4%) reduction of colony count when treated with AZA (100 nM) alone (P = .0008; vs dimethyl sulfoxide [DMSO]), a 69% (range, 58.3%-77.5%) reduction when treated with DT2216 (50 nM) alone (P = .0003; vs DMSO), and a 76% (range, 70.9%-79.8%) reduction when treated with the combination of DT2216 (50 nM) and AZA (100 nM; P < .0001; vs DMSO; Figure 5C; supplemental Figure 7E). DT2216 not only effectively inhibited the colony-formation ability but also significantly reduced the size of the colonies compared with DMSO (supplemental Figure 7F).
After counting patient 5’s colonies, we collected cells after each treatment and analyzed by western blotting. As shown in Figure 5D, DT2216 alone and in combination with AZA efficiently degraded BCL-xL. In the combination treatment group, although the cell count was the lowest, remaining cells expressed elevated level of MCL-1, suggesting a possible mechanism of cell survival under DT2216 pressure. Together, these results showed that DT2216 alone or in combination with AZA led to significant antileukemic effects at nanomolar drug concentrations in post-MPN AML HSPCs.
DT2216 suppressed SET2 cell growth in vivo
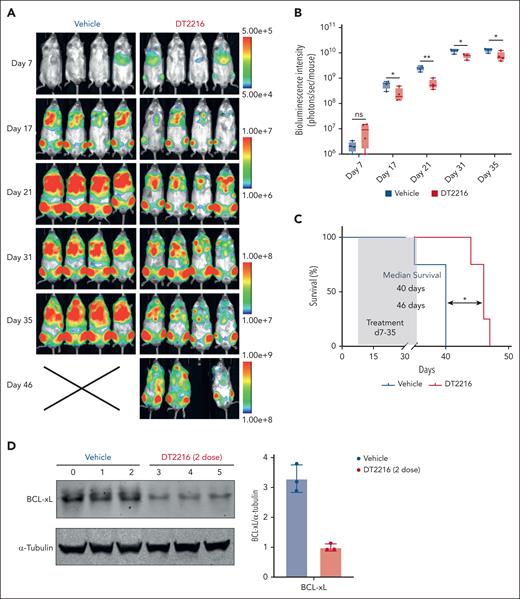
Our in vitro experiments highlighted BCL-xL as a promising target in post-MPN AML cell lines and patient-derived primary cells. To track SET2 cells in vivo, we labeled them with luciferase and green fluorescent protein. Subsequently, we intravenously injected these cells into NCG mice. After confirming leukemic engraftment through BLI, the mice were randomized into 2 groups to receive either vehicle or DT2216 (15 mg/kg intraperitoneally every 4 days) for 4 weeks. Mice tolerated DT2216 therapy well with no significant changes in body weight (supplemental Figure 8A). Compared with the vehicle group, DT2216 as a single agent significantly delayed SET2 AML cells progression in vivo, as shown by reduced bioluminescent signal intensity (Figure 6A-B) and prolonged survival (Figure 6C; P = .01). We also tested another cohort of mice to confirm the on-target degradation in vivo after 2 doses of DT2216 treatment. The engraftment of SET2 cells in the bone marrow and spleen was analyzed using flow cytometry (supplemental Figure 8B-C). Both BLI and flow cytometry confirmed that SET2 cells primarily engrafted in the bone marrow. Western blotting showed over 60% of BCL-xL being degraded in leukemic cells after only 2 doses of DT2216 (Figure 6D).
DT2216 leads to cell growth suppression in an SET2 AML model in vivo. (A) Bioluminescent images of mice transplanted with SET2 leukemia cells. Mice were administered vehicle or DT2216 twice a week from day 7 to day 35 after transplantation. The same mice are depicted at each time point (n = 4 mice per group). (B) Quantification of bioluminescence emitted from the whole body of each mouse described in panel A at the indicated time points. The P values were determined using the t test (∗P < .05; ∗∗P < .01). (C) Kaplan-Meier survival curves of the SET2 cell recipient mice described in panel A. The P value was determined using the log-rank Mantel-Cox test (∗P < .05). (D) Western blots showing the pharmacodynamics of BCL-xL degradation by DT2216 and densitometric quantification of BCL-xL in vehicle and DT2216 groups in a second cohort of mice (n = 3 mice per group). Starting at day 28 after SET2 cell transplantation, the mice were administered 2 doses, 4 days apart, of vehicle or DT2216 (15 mg/kg). α-Tubulin was used as the loading control for the western blot.
DT2216 leads to cell growth suppression in an SET2 AML model in vivo. (A) Bioluminescent images of mice transplanted with SET2 leukemia cells. Mice were administered vehicle or DT2216 twice a week from day 7 to day 35 after transplantation. The same mice are depicted at each time point (n = 4 mice per group). (B) Quantification of bioluminescence emitted from the whole body of each mouse described in panel A at the indicated time points. The P values were determined using the t test (∗P < .05; ∗∗P < .01). (C) Kaplan-Meier survival curves of the SET2 cell recipient mice described in panel A. The P value was determined using the log-rank Mantel-Cox test (∗P < .05). (D) Western blots showing the pharmacodynamics of BCL-xL degradation by DT2216 and densitometric quantification of BCL-xL in vehicle and DT2216 groups in a second cohort of mice (n = 3 mice per group). Starting at day 28 after SET2 cell transplantation, the mice were administered 2 doses, 4 days apart, of vehicle or DT2216 (15 mg/kg). α-Tubulin was used as the loading control for the western blot.
Discussion
MPNs are thought to be initiated at the level of the HSPCs because of the acquisition of specific driver mutations, which lead to persistent activation of JAK/STAT signaling,57,58 elaboration of a broad array of inflammatory cytokines,59-61 uncontrolled myeloproliferation,62 and an increasing risk of transformation to AML over time.4,7,8 When MPNs progress to AML, the prognosis is dismal, with resistance to conventional treatments,8,63 including venetoclax-based regimens, supporting an urgent need to explore new treatment strategies. Our study shed light on the critical role of BCL-xL in post-MPN AML. Notably, we found that this subtype of AML maintains high levels of BCL-xL. BH3 profiling revealed that JAK2-mut AML cell lines, iPSC-HSPCs, and post-MPN AML primary samples exhibited BCL-xL dependence. This dependency may underlie the resistance of patients with post-MPN AML to venetoclax-based regimens.51 In patient-derived iPSC-HPCs, we observed that JAK2V617F mutation significantly increased BCL2L1 mRNA expression. Additionally, Ruxo-Re cells displayed further increase in BCL-xL expression and dependency than parental cells, providing a rationale for targeting BCL-xL in patients with Ruxo-Re MPN. Taken together, our findings highlight that JAK2 mutation–induced BCL-xL upregulation, along with differentiation state(s), may contribute to elevated BCL-xL dependency in post-AML MPN, establishing BCL-xL as a potential therapeutic target.
Mutational inactivation and loss of TP53 are common somatic events in post-MPN AML but not in chronic MPNs,12,16 and a loss of TP53 combined with the JAK2V617F mutation has been shown to lead to AML in a mouse model.12,16 Mining single-cell multiomics data,42 we observed that HSPCs with only MPN-driver mutation, which are prevalent among patients with chronic-phase MPN, clustered together with WT HSPCs, with enriched HSC score, suggesting that these cells partially retain multilineage differentiation potential. HSPCs with the MPN-driver mutation alone are regarded as pre-LSCs.64 However, a distinct population of HSPCs harboring both JAK2/CALR and TP53 mutations clusters separately from WT HSPCs and MPN-driver mutation–alone HSPCs. Of importance, these JAK2/CALR_TP53-mutant HSPCs had higher levels of BCL2L1 and BCL2 expression. Importantly, these HSPCs constituted the dominant cell population in patients with post-MPN AML, exhibiting features typical of LSCs. Mutations in TP53 may therefore contribute to the complete conversion of pre-LSCs (JAK2/CALR mutant HSPCs) into LSCs.
Furthermore, the elevated scores of erythroid progenitor and megakaryocyte development in JAK2/CALR_TP53-mutant HSPCs indicate that post-MPN AML exhibits signatures of erythroid and megakaryocytic differentiation. These findings aligned with previous reports of higher incidence of erythroblastic and megakaryoblastic morphology in post-MPN AML.5,8,15,65 Kuusanmäki et al found that AML cells with erythroid or megakaryocytic differentiation confer BCL-xL, rather than BCL-2 dependence,46 consistent with our findings in transformed AML post-MPN. It remains to be determined if high levels of BCL2L1 observed here by single-cell analysis in JAK2/TP53-mutated HSPCs is a direct consequence of TP53 mutations or altered differentiation state, possibly invoked by mutant TP53. Our preliminary experiments showed no immediate changes in BCL-xL mRNA or protein upon acute silencing of TP53. However, in the (Jak2V617F/+Trp53R172H/–) murine model, Bcl2l1 expression was significantly elevated in MEPs upon acute leukemic transformation, possibly as a result of long-term cellular adaptation or microenvironmental cues. Future long-term in vivo experiments using genetically engineered human or murine cells harboring distinct TP53 mutations coupled with single-cell multiomic analyses are warranted to address this important question.
Although our data strongly indicate BCL-xL priming in JAK2-mut post-MPN AML, survival dependencies may vary among distinct genomic subsets. For instance, patients who have experienced transformation without MPN-driver mutations are likely to have a leukemic clone originating from a coexisting population of cells that lack MPN-driver mutations.12,66 In addition, some may transform from triple-negative MPN. The biological characteristics and prosurvival dependencies of these subsets remain elusive and merit further investigation, highlighting the limitation of this study.
To mitigate thrombocytopenia resulting from on-target BCL-xL inhibition,67 we conducted preclinical research using DT2216, a VHL-based PROTAC specifically targeting BCL-xL. DT2216 has demonstrated safety in preclinical animal studies32 and is in a phase 1 clinical trial in patients with refractory and relapsed malignancies (ClinicalTrials.gov identifier: NCT04886622). It holds the promise of the first-in-class platelet-sparing BCL-xL–targeted therapeutic. As a single agent, DT2216 exhibited potent antileukemic efficacy in JAK2-mut AML cell lines (including Ruxo-Re cell lines), JAK2-mut iPSCs, and CD34+ primary cells from patients with post-MPN AML. Western blotting confirmed that this effect was achieved through on-target degradation of BCL-xL, without off-target effects.
To gain improved antileukemic efficacy, we combined DT2216 with commonly used agents. Synergistic effects were observed between DT2216 and AZA, and combinations with ruxolitinib and venetoclax showed additive growth inhibitory effects. Our previous work39 indicated that monotherapy with DT2216 or the second generation PROTAC 753B may increase MCL-1 expression, conferring protection against apoptosis. However, when DT2216 was combined with AZA or ruxolitinib, it reduced MCL-1, in part, through caspase-induced cleavage, achieving better antileukemic effects in SET2 cells. Although combination of DT2216 with MCL-1 inhibitor is highly potent, clinical use of MCL-1 inhibitors is halted because of on-target cardiac toxicities,56,68 and alternative strategies are needed. Our study revealed that DT2216 significantly inhibited the colony-forming ability of CD34+ leukemia cells from patients with post-MPN AML at nanomolar concentrations. Efficient elimination of LSCs is crucial to prevent AML relapse.69 In the in vivo experiments, administering DT2216 once every 4 days was sufficient enough to maintain effective concentration yielding BCL-xL degradation.32 This approach reduces dosing frequency, making it more manageable for patients. The SET2 CDX model validated the safety and efficacy of DT2216 monotherapy with no weight loss observed in mice. DT2216 reduced the leukemic burden and extended the survival of the mice, correlating with the degradation of BCL-xL. Future combination studies with azacitidine in patient-derived xenograft models are warranted.
Taken together, our findings underscore the dependence of post-MPN AML on BCL-xL. We postulate that safe targeting of BCL-xL with agents such as DT2216 represents a potential therapeutic approach for this challenging subtype AML. The promising efficacy of DT2216 in post-MPN AML was evidenced by reduced cell viability; on-target degradation of BCL-xL; synergistic antileukemic effects when combined with AZA, ruxolitinib, or venetoclax in vitro; and in vivo activity in CDX mouse model. These results provide valuable insights into future therapeutic strategies for the treatment of JAK2-mut post-MPN AML.
Acknowledgments
Sunita Patterson, a senior scientific editor in the Research Medical Library at MD Anderson Cancer Center, provided editorial assistance. DT2216 is exclusively licensed by Dialectic Therapeutics, which provided the drug for use in this study.
This work was supported by the National Institutes of Health/National Cancer Institute grant (R01 CA241191 [G.Z., M.K., and D.Z.]). H.A.A. was supported by an MD Anderson Physician Scientist award.
Authorship
Contribution: Z.W., A. Skwarska, Q.Z.T., and M.K. conceived and designed the study; Z.W., A.R.-M., P.S., A. Skwarska, V.G., and G.P. analyzed the data; Z.W. prepared the manuscript; Z.W., A. Skwarska, G.P., S.C., E.O., Y.J., and C.L.R. performed experiments; G.Z., D.Z., R.K.R., and H.A.A. participated in study design; A. Schurer, K.G., and K.B. generated ruxolitinib-resistant JAK2-mut cell lines; W.F., E.P.P., K.B., N.P., J.W.T., A.J.M., and M.K. provided clinical samples and information or sequencing data; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: G.Z. and D.Z. are cofounders of, and have equity in, Dialectic Therapeutics, which develops BCL-xL PROTAC to treat cancer. H.A.A. received Honoraria from Illumina and Alamar Biotechnology, in-kind support from Illumina, research support from Genentech, Enzyme-By-Design, GlaxoSmithKline, Blueprint Medicines, Ascentage and Illumina; served on advisory board for Cogent Biosciences and Consultant to Molecular Partners. The remaining authors declare no competing financial interests.
Correspondence: Marina Konopleva, Department of Medicine (Oncology), Montefiore Einstein Comprehensive Cancer Center, Albert Einstein College of Medicine, 1300 Morris Park Ave, Ullmann Building, Room 915, Bronx, NY 10461; email: marina.konopleva@einsteinmed.edu; and Qi Zhang Tatarata, Leukemia Department, MD Anderson Cancer Center, 1515 Holcombe Blvd, Room T6.3805, Houston, TX 77030; email: qizhang.tatarata@gmail.com.
References
Author notes
Presented in abstract form at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 11 December 2023.70
All data generated and analyzed during this study are included in this article and the supplemental Data. Additional data are available on request from the corresponding author, Marina Konopleva (marina.konopleva@einsteinmed.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.