Key Points
Selective inhibitor of nuclear export compounds are a novel class of XPO1 inhibitors.
Selinexor is safe and efficacious in patients with R/R NHL.
Abstract
Patients with relapsed or refractory (R/R) non-Hodgkin lymphoma (NHL) have a poor prognosis and limited treatment options. We evaluated selinexor, an orally bioavailable, first-in-class inhibitor of the nuclear export protein XPO1, in this phase 1 trial to assess safety and determine a recommended phase 2 dose (RP2D). Seventy-nine patients with various NHL histologies, including diffuse large B-cell lymphoma, Richter’s transformation, mantle cell lymphoma, follicular lymphoma, and chronic lymphocytic leukemia, were enrolled. In the dose-escalation phase, patients received 3 to 80 mg/m2 of selinexor in 3- or 4-week cycles and were assessed for toxicities, pharmacokinetics, and antitumor activity. In the dose-expansion phase, patients were treated with selinexor at 35 or 60 mg/m2. The most common grade 3 to 4 drug-related adverse events were thrombocytopenia (47%), neutropenia (32%), anemia (27%), leukopenia (16%), fatigue (11%), and hyponatremia (10%). Tumor biopsies showed decreases in cell-signaling pathways (Bcl-2, Bcl-6, c-Myc), reduced proliferation (Ki67), nuclear localization of XPO1 cargos (p53, PTEN), and increased apoptosis after treatment. Twenty-two (31%) of the 70 evaluable patients had an objective responses, including 4 complete responses and 18 partial responses, which were observed across a spectrum of NHL subtypes. A dose of 35 mg/m2 (60 mg) was identified as the RP2D. These findings suggest that inhibition of XPO1 with oral selinexor at 35 mg/m2 is a safe therapy with encouraging and durable anticancer activity in patients with R/R NHL. The trial was registered at www.clinicaltrials.gov as #NCT01607892.
Introduction
Despite significant progress in the treatment of lymphoma over the last 3 decades, many patients with non-Hodgkin lymphoma NHL, whether aggressive or indolent, will die as a result of their disease.1-3 The development of novel therapeutics that address this unmet medical need remains an important priority. Furthermore, genetic profiling of NHL has revealed diverse and high-risk subtypes that were previously unknown or underappreciated.4,5 With this degree of heterogeneity, improved outcomes over a broad range of lymphomas will require therapeutics that address fundamental mechanisms that are dysregulated in cancer.6
The export of tumor suppressor proteins (TSPs), including p53, p73, p21, p27, Rb, BRCA1/2, and IκB, out of the cell nucleus leads to their functional inactivation and inability to carry out their genome surveillance functions. Despite their heterogeneous structures, nearly all TSPs are exported from the nucleus to the cytoplasm by exportin 1 (XPO1/CRM1).7-10 In addition, XPO1 is centrally involved in regulating cytoplasmic levels of the messenger RNA transcripts for multiple oncoproteins such as c-Myc, Bcl-2, and Bcl-6. The capped messenger RNAs of these proteins bind to eukaryotic translation initiation factor 4E and are exported from the nucleus exclusively by XPO1.11-13 Increased XPO1 expression has been identified in nearly all malignancies and is correlated with aggressive, poor-prognosis disease.7,8
Selinexor is a first-in-class, oral therapeutic that forms a slowly reversible covalent bond with XPO1, leading to its inactivation. Inhibition of XPO1 with selinexor results in the accumulation and reactivation of TSPs in the cell nucleus; reduction in oncoproteins including c-Myc, Bcl-2, and Bcl-6; and induction of cell-cycle arrest and apoptosis.10 Selinexor is tumoricidal in preclinical models across a broad spectrum of cancer types.14-18 Verdinexor, an analog of selinexor, produced a 34% objective response (OR) rate in clinical trials in spontaneous canine lymphoma.14-19
On the basis of these findings, we initiated an international, multicenter phase 1 clinical trial to assess the effects of selinexor in patients with advanced hematologic malignancies. Here, we report on the safety and preliminary antitumor activity of selinexor in relapsed or refractory (R/R) NHL. Furthermore, we propose mechanistic explanations for the observed signals of selinexor clinical activity across different subtypes of NHL.
Methods
Study oversight and design
This phase 1 dose-escalation study was conducted across 12 sites in the United States, Canada, and Denmark to assess the effects of oral selinexor in patients with advanced hematologic malignancies. The primary objectives of the study were to determine the safety, tolerability, recommended phase 2 dose (RP2D), and maximum-tolerated dose (MTD) of selinexor. Secondary objectives were to assess tumor response, pharmacokinetics, and pharmacodynamics. The study enrolled heavily pretreated patients ≥18 years of age with no curative treatment options; documented disease progression at study start; Eastern Cooperative Oncology Group performance status ≤2; and adequate hematologic (platelets ≥30 × 109/L, hemoglobin ≥8 g/dL, absolute neutrophil count ≥0.8 × 109/L, and white blood cell count ≥1.5 × 109/L), hepatic (bilirubin <2 times the upper limit of normal [ULN], aspartate aminotransferase <2.5 times ULN, and alanine aminotransferase <2.5 times ULN), renal (glomerular filtration rate ≥ 30 mL/min), and cardiovascular function. Patients were excluded from the study if they were pregnant or breastfeeding, had received any form of anticancer therapy within 2 weeks before day 1 of cycle 1 (6 weeks for radioimmunotherapy), had HIV or hepatitis infection, had active graft-versus-host disease after allogeneic stem-cell transplantation (SCT), or were deemed medically or psychologically unfit by the treating physician. Patients were allowed to receive dexamethasone (4-8 mg per week) while on study as a supportive care agent for nausea and decreased appetite. The study protocol was approved by the institutional review board or independent ethics committee at each participating center and is in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice principles, and local laws. All patients provided written informed consent before enrollment. All authors discussed and interpreted the results, vouch for the accuracy of the data, and collaborated in the preparation of the manuscript.
Selinexor administration, dose escalation, and dose expansion
Selinexor was administered orally in 21- or 28-day cycles, with the first patient dosed at 3 mg/m2 based on preclinical toxicology studies in rats and monkeys. A dose escalation of 100% was used in cohorts 1 to 3, and a dose escalation of 30% to 40% was used for cohorts ≥4. Initially, 10 doses of selinexor were administered in each 4-week cycle with a schedule of 3 times weekly (days 1, 3, and 5) in weeks 1 and 3 and 2 times weekly (days 1 and 3) in weeks 2 and 4 (schedule A). As the safety profile of selinexor emerged through early clinical experience, a lead-in week of 3 doses of 12 mg/m2 every other day was tested in an attempt to acclimate patients to selinexor before receiving the target dose (schedule B). The lead-in week proved ineffective at reducing toxicities and was subsequently abandoned in favor of twice-weekly dosing. This was followed by investigation of several twice-weekly schedules (days 1 and 2, schedule C; days 1 and 3, schedule D; and days 1 and 4, schedule E) and a high-dose once-weekly schedule (schedule F) in later cohorts based on pharmacodynamic results. Lastly, a 3-week schedule of twice-weekly treatment (days 1 and 3) with a 1-week break was tested. Dose escalation continued until at least 2 patients among a cohort of 3 to 6 patients experienced a dose-limiting toxicity (DLT) during the first treatment cycle. DLTs were evaluable only in dose-escalation patients meeting inclusion/exclusion criteria at screening and were defined as any of the following occurring in the first 28 days (or 21 days for schedule G): frequently missed doses because of drug-related toxicity (≥3 doses for schedules A-E and ≥2 doses for schedules F and G), discontinuation because of drug-related toxicity, any-grade ≥3 nonhematologic adverse event (AE) except alopecia or electrolyte abnormalities while taking optimal supportive care agents, grade 4 neutropenia, febrile neutropenia, or grade ≥3 thrombocytopenia. After dose escalation, patients with diffuse large B-cell lymphoma (DLBCL) were enrolled in 2 expansion cohorts of 35 and 60 mg/m2 to further assess safety, tolerability, and efficacy.
Safety
Safety and tolerability were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). AEs were determined by the investigator to be unrelated or possibly, probably, or definitely related to selinexor.
Pharmacokinetic assessment
In cycle 1, serial blood samples were obtained predose and at 0.5, 1, 2, 4, 8, 24, and 48 hours postdose on day 1 and at 0, 1, 2, 4, and 8 hours on days 15 or 17. Plasma concentrations of selinexor were measured at Tandem Labs (Durham, NC) using a validated liquid chromatography–tandem mass spectrometry method (Sciex API 4000). Pharmacokinetic parameters were calculated by standard noncompartmental analysis using PK Solutions Software (Summit Research Services, Montrose, CO).
Pharmacodynamics
Immunohistochemical (IHC) analyses were performed by CGI Laboratories (Rutherford, NH). Paired tumor biopsies collected at baseline and in week 1 of cycle 2 were stained with hematoxylin and eosin (Richard-Allan Scientific) or antibodies against Ki67 (Cell Marque), apoptag (Millipore), cleaved caspase 3 (Cell Signaling), p53 (Santa Cruz Biotechnology), PTEN (ThermoFisher Scientific), STAT3 (pS727; Abcam), c-Myc (Abcam), Bcl-2 (DAKO), or Bcl-6 (Abcam) using the Benchmark Ultra Stainer per the manufacturers’ instructions. Slides were scanned with the Aperio ScanScope AT Turbo at ×10 magnification. Subclassification of DLBCL was based on the Hans and Tally algorithms using IHC methods previously described.20,21 When available, data for c-Myc, Bcl-2, and Bcl-6 translocations in patients with DLBCL were provided by the institutions, or fluorescence in situ hybridization was performed on tumor biopsies at CGI Laboratories according to standard methods. In brief, specimens were sectioned at 4 μm, mounted on slides, and baked overnight at 60°C. The slides were deparaffinized with Citrisolv (Fisher, MA) followed by treatment with 0.2 N hydrochloric acid pretreatment reagent (Abbott Molecular, Downers Grove, IL) for 30 minutes at 80°C and protease solution (Abbott Molecular) for 30 minutes at 37°C. The slides were then dehydrated in 70%, 85%, and 100% ethanol; 10 μL of probe was applied to the area of interest and denatured for 5 minutes at 72°C. Probe sets were designed for specific translocations or chromosomal breaks. The translocation probes were obtained from Vysis (Downers Grove, IL) and included spectrum orange–labeled Bcl-2 (18q21), spectrum orange–labeled c-Myc (8q24), spectrum aqua–labeled centromeric 8 (8q11-8p11), and spectrum green–labeled IGH (14q32). The break-apart probes included spectrum orange– and spectrum green–labeled c-Myc (8q24) obtained from Rainbow Scientific (Windsor, CT) and spectrum orange– and spectrum green–labeled Bcl-6 (3q27) obtained from Vysis (Downers Grove, IL). The slides were incubated for 14 to 18 hours in a humidified chamber at 37°C. Posthybridization washes were performed with ×0.2 SSC/0.3% NP-40 solution at 72°C for 2 minutes and ×2 SSC/0.3% NP-40 solution at room temperature for 1 minute. Slides were then counterstained with DAPI antifading medium. The slides were visualized with a fluorescence microscope equipped with MetaSystems ISIS software. A certified hematopathologist scored 200 interphase cells. Scoring was restricted to tumor cells based on the identification of areas of tumor on adjacent hematoxylin and eosin–stained sections.
Efficacy
Disease response was measured according to the International Working Group Response Criteria for NHL, the International Workshop on Chronic Lymphocytic Leukemia (CLL), and response criteria for cutaneous T-cell lymphoma.22-24 Patients were required to complete 1 cycle (4 weeks) of selinexor or have evidence of disease progression before completing cycle 1 to be considered evaluable for response. For sites with available positron emission tomography (PET) imaging, tumor volume was quantified by PET computed tomography (CT) or in rare cases PET magnetic resonance imaging, and for sites where PET was not available, CT or magnetic resonance imaging was used. Tumor measurements were made at baseline and after the first or second cycle of selinexor treatment. Patients underwent imaging every other cycle until disease progression or withdrawal of consent.
Statistics
Dose proportionality (F test) and Student t tests were used to analyze percentage of body weight lost after 2 cycles and days on study for patients treated with ≤40 and >40 mg/m2 selinexor, respectively.
Results
Patient demographics
Between July 2012 and December 2014, 79 patients with NHL were enrolled, including 47 patients in dose-escalation (NHL, 40 and CLL, 7) and 32 in dose-expansion cohorts (DLBCL, 28; Richter’s transformation, 3; and grade 3b follicular lymphoma [FL], 1). Patients had received a median of 4 prior therapies. Of the 43 patients with DLBCL, 12 had disease refractory to their last therapy, and 30% had undergone autologous SCT. A full summary of patient baseline characteristics can be found in Table 1.
Safety
Selinexor was tested at 13 dose levels (3-80 mg/m2) using 7 different schedules (Table 2). There was 1 DLT reported in the initial dose-escalation cohorts in which a patient dosed at 23 mg/m2 experienced grade 4 thrombocytopenia for >5 days without bleeding. The patient remained on therapy for 84 days with stable disease at the request of the treating physician and received platelet support according to institutional guidelines before disease progression.
The median duration of therapy for all patients was 49 days (range, 4-1071+ days) with 18% continuing treatment >6 months and 8% >1 year. In the dose-expansion cohorts, the median duration of therapy was 66 days (range, 23-602 days) for patients treated at 35 mg/m2 and 32 days (range, 11-314 days) for patients treated at 60 mg/m2. Treatment-related AEs as determined by the investigator that were reported in ≥10% of patients are shown in Table 2. The toxicity profile of selinexor was similar between the NHL histological subgroups (supplemental Table 1, available on the Blood Web site). The most frequent nonhematologic AEs were nausea (66%), fatigue (61%), anorexia (57%), vomiting (37%), and diarrhea (34%), which were primarily grade 1 and 2 and improved with typical supportive care for anorexia and nausea including 5-HT3 antagonists, D2 antagonists, and antiemetic doses of dexamethasone, megestrol, and/or olanzapine. The most common grade 3 or 4 toxicities were hematologic, including thrombocytopenia (47%), neutropenia (32%), and anemia (27%); however, there were significant baseline cytopenias; 15% had platelet count <75 × 109/L, and 13% had an absolute neutrophil count <1.5 × 109/L before starting therapy. Twenty-three patients experienced at least 1 selinexor-related, nonhematologic grade 3 AE, and 1 patient had a grade 4 AE (cataract). The most common nonhematologic grade 3 AEs were fatigue (11%) and hyponatremia (10%).
A total of 50 serious AEs (SAEs) of any causality were reported. Eleven of the SAEs were assessed by the investigator as being at least potentially drug related and occurred at doses between 30 and 70 mg/m2. Nine of these SAEs were grade 3 and included 2 episodes of dehydration in the same patient, 2 episodes of asymptomatic hyponatremia, and 1 each of encephalitis, febrile neutropenia, decreased ejection fraction, confusion, and increased serum amylase. All patients with grade 3 SAEs recovered with supportive care or drug interruption. The 2 grade 4 SAEs included anemia, which resolved in 1 day after a blood transfusion, and 1 case of a worsening cataract that did not resolve in a patient with a preexisting grade 1 cataract before selinexor treatment who received concomitant dexamethasone and hydrocortisone while on study. Onset of the grade 4 cataract occurred 49 days after study treatment. A full summary of drug-related SAEs can be found in supplemental Table 2.
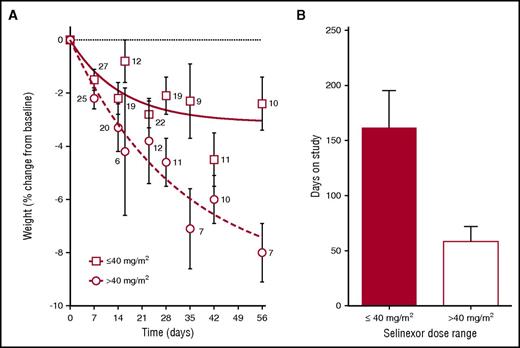
Twelve of the 79 patients underwent dose reductions from a median of 35 mg/m2 (range, 30-70 mg/m2) to 23 mg/m2 (range, 20-60 mg/m2). Of the 28 patients who withdrew consent, 15 did so because of the incidence or severity of AEs. When comparing doses of ≤40 and >40 mg/m2, weight loss after 2 cycles (−2.4% vs −8.0%, respectively; P < .001) and time on therapy (161 ± 33 days vs 58 ± 14 days, respectively; P = .01) were significant differences (Figure 1). On the basis of these key observations, the RP2D was determined to be 35 mg/m2 (∼60 mg flat dose).
Patient weight over the first 2 cycles and days on study as functions of selinexor dose. (A) Average patient weight ± standard error of the mean (SEM) at various time points over the first 2 cycles is depicted for patients treated with ≤40 mg/m2 (open squares, solid fit line) and >40 mg/m2 (open circles, dashed fit line). Curves were fit with GraphPad software using a 1-phase decay equation. The curves differ significantly (P < .001) by dose proportionality (F test). The number of patients with available body weight measurements at each time point (day) is located next to each open circle or open square. (B) Average days on study ± SEM for patients treated with ≤40 mg/m2 (red bar; n = 46) and >40 mg/m2 (white bar; n = 33). The bars differ significantly (P = .005) by Student t test.
Patient weight over the first 2 cycles and days on study as functions of selinexor dose. (A) Average patient weight ± standard error of the mean (SEM) at various time points over the first 2 cycles is depicted for patients treated with ≤40 mg/m2 (open squares, solid fit line) and >40 mg/m2 (open circles, dashed fit line). Curves were fit with GraphPad software using a 1-phase decay equation. The curves differ significantly (P < .001) by dose proportionality (F test). The number of patients with available body weight measurements at each time point (day) is located next to each open circle or open square. (B) Average days on study ± SEM for patients treated with ≤40 mg/m2 (red bar; n = 46) and >40 mg/m2 (white bar; n = 33). The bars differ significantly (P = .005) by Student t test.
Pharmacokinetics and pharmacodynamics
Selinexor pharmacokinetics were assessed in a subset of patients over a dose range of 3 to 60 mg/m2 after dose 1 and dose 5, 7, or 8. Peak serum concentration and area under the curve were dose dependent (P < .0001) with no evidence of drug accumulation after multiple doses (supplemental Figure 1; supplemental Table 3). Peak plasma concentrations of selinexor were reached within 2 to 4 hours post-treatment, and a terminal half-life of approximately 5 to 7 hours was observed across the tested dose levels. Pharmacokinetic parameters showed comparable correlations and minimal variability when drug levels were assessed independent of body surface area, suggesting fixed milligram doses could be used in future studies. IHC analysis was performed on a subset of paired pre- and on-treatment tumor biopsies from responding patients (n = 3). In patients with DLBCL or FL, a marked decrease in proliferation and increase in apoptosis were observed in samples after 5 weeks of selinexor treatment as compared with baseline (supplemental Figure 2). Tumor sections also showed nuclear accumulation of XPO1 cargo proteins p53 and PTEN, as well as decreased staining of key growth and survival factors, such as c-Myc, Bcl-2, Bcl-6, and pSTAT.
Efficacy
Of the 79 patients with NHL enrolled, 9 were not evaluable for response based on withdrawal of consent before disease assessment (n = 6), death unrelated to selinexor (n = 2; pneumonia and heart attack), and lack of adequate baseline imaging (n = 1). Twenty-two (31%) of the 70 evaluable patients had ORs, including 4 complete responses (CRs; 6%) and 18 partial responses (PRs; 26%), which were observed across the spectrum of B- and T-cell NHL subtypes. Twenty-one patients (30%) had stable disease (SD; 5 for >4 months), for a disease control rate of 61%. OR was most frequent at a dose range of 30 to 35 mg/m2. DLBCL was the most common NHL subtype represented in this study (41 evaluable patients), in which a 32% OR rate (4 CRs and 9 PRs) and 51% disease control rate were observed (Table 3). In addition, 5 of 8 patients with Richter’s transformation were evaluable, with 2 achieving PRs and 2 SD (Table 4).
Across all doses, 46 patients had quantitative, on-study assessments of tumor lesion size, of whom 34 showed a reduction in tumor burden after selinexor treatment (Figure 2). The remaining 24 patients considered evaluable for response did not have quantitative tumor measurements while on study but were assessed as having disease progression based on clinical symptoms (20 patients) or SD (4 patients) based on being on study >1 cycle without evidence of disease progression before withdrawing consent.
Waterfall plot by NHL subtype of change in target lesions. Quantitative target lesion assessment was not available for 24 evaluable patients. These included 20 patients with progressive disease based solely on clinical symptoms and 4 patients with stable disease who remained on study >1 cycle without evidence of disease progression before withdrawing consent. Black diagonal lines indicate PET-CT–confirmed CR. MCL, mantle cell lymphoma.
Waterfall plot by NHL subtype of change in target lesions. Quantitative target lesion assessment was not available for 24 evaluable patients. These included 20 patients with progressive disease based solely on clinical symptoms and 4 patients with stable disease who remained on study >1 cycle without evidence of disease progression before withdrawing consent. Black diagonal lines indicate PET-CT–confirmed CR. MCL, mantle cell lymphoma.
Responses were observed at a median selinexor dose of 35 mg/m2 (interquartile range, 21.75 mg/m2), with a median time to best response of 54 days (interquartile range, 118 days). Fifty-nine percent of responses were identified on the first postdrug CT scan, and the median duration of response was >7 months. Three patients withdrew consent to undergo SCT.
A total of 38 patients received at least 1 dose of dexamethasone as a supportive care agent while on study. Of the 22 patients who responded to treatment, 13 received concomitant dexamethasone (median dose, 8 mg per week; range, 4-8 mg), 4 of whom received dexamethasone after they had achieved their best response. The remaining 9 patients who responded were treated with dexamethasone as a prophylactic supportive care agent at the time of study initiation, which continued throughout treatment.
Among the patients with DLBCL, similar response rates were observed in germinal center B-cell–like (GCB) and non-GCB subtypes (Table 4). Six patients with DLBCL had FISH-based identification of c-Myc, Bcl-2, and/or Bcl-6 translocations, qualifying as having poor-prognosis double-hit or triple-hit lymphomas. Three of these 6 patients responded (1 CR and 2 PRs), and these 3 patients remained on study for 19, 7, and 3 months; the third patient withdrew consent to undergo SCT.
Of the 4 patients who achieved CR, 3 had de novo and 1 had transformed DLBCL, and all were heavily pretreated, with 2 to 6 prior therapies. The range of selinexor starting doses was 23 to 35 mg/m2 among the 4 patients, and PET-CT confirmed CR was declared after 6 to 18 cycles. As of the close of study, all patients with DLBCL who achieved CR were alive, having received treatment for 35, 28, 20, and 16 months, and 3 were continuing on study (Figure 3). PET-CT scans demonstrating CR in a patient with DLBCL are shown in supplemental Figure 3.
Time on study for patients who responded to selinexor treatment. Swimmers plot of time on study for patients achieving PR or better. Patients achieving CR are shown in green and PR in blue. Red lines indicate time at which best response was confirmed. *Indicates first PET-CT scan at cycle 16 confirming CR. Arrows indicate the patient is still on study as of the date of data cutoff. CTCL, cutaneous T-cell lymphoma; FLG3b, follicular lymphoma grade 3b; MCL, mantle cell lymphoma; RT, Richter’s transformation; WC–AE, withdrawal of consent because of AEs; WC-transplant, withdrawal of consent to undergo SCT; X, progressive disease.
Time on study for patients who responded to selinexor treatment. Swimmers plot of time on study for patients achieving PR or better. Patients achieving CR are shown in green and PR in blue. Red lines indicate time at which best response was confirmed. *Indicates first PET-CT scan at cycle 16 confirming CR. Arrows indicate the patient is still on study as of the date of data cutoff. CTCL, cutaneous T-cell lymphoma; FLG3b, follicular lymphoma grade 3b; MCL, mantle cell lymphoma; RT, Richter’s transformation; WC–AE, withdrawal of consent because of AEs; WC-transplant, withdrawal of consent to undergo SCT; X, progressive disease.
Discussion
Although several new agents have been approved for the treatment of lymphoma, the development of novel therapies for the treatment of aggressive NHL has proven challenging.25 Curative therapy is possible in the R/R setting with salvage therapy and autologous SCT; however, outcomes have not improved over the past 20 years.26,27 Many agents have been evaluated in the palliative setting, but activity has been less than impressive in aggressive lymphoma.28-36
One of the primary end points of this study was to characterize the safety of the various selinexor dosing schedules. There was no evidence of cumulative toxicity with repeat dosing, and long-term tolerability was demonstrated, with 18% of patients receiving treatment for at least 6 months. The most frequently reported gastrointestinal and constitutional AEs were nausea, vomiting, anorexia, and fatigue, which could be managed with antiemetics and appetite stimulators employed in cancer treatment. Prophylactic treatment of patients with 5-HT3 antagonists, such as ondansetron, is strongly recommended to control nausea and vomiting. Dexamethasone at 4 to 8 mg with each dose of selinexor can provide further nausea control, improve appetite, and may reduce fatigue. Megestrol acetate 400 to 800 mg per day reduces anorexia and weight loss. Low-dose olanzapine (2.5-5 mg orally at night) reduces nausea and improves appetite, as does dronabinol. It is without doubt that treatment with selinexor in this phase 1 study, and other studies in solid tumors, does lead to an unfamiliar toxicity paradigm and constellation of symptoms for most oncologists.37,38 Experience with managing patients treated with selinexor and aggressive use of supportive care tools can mitigate most nonhematologic toxicities. Grade 3 or 4 AEs were largely hematologic in nature, including thrombocytopenia, neutropenia, and anemia, and could be managed through drug holidays, dose reductions, and/or growth factor support, such as eltrombopag for thrombocytopenia and filgrastim for neutropenia. It should be noted that given the permissive inclusion criteria of the study, many patients presented at baseline with significant cytopenias, and therefore, high incidences of grade 3 or 4 hematologic toxicities are not unexpected in this patient population. Less permissive hematologic criteria may have led to lower incidence of grade 3 or 4 cytopenias. Although an MTD was not reached in this trial, a parallel phase 1 study in patients with solid tumors (#NCT01607905) established an MTD in that population of 65 mg/m2, and 35 mg/m2 twice weekly was identified as the optimal dose and schedule. Similarly, on the basis of the improved tolerability and similar efficacy at doses below and above 40 mg/m2 in this trial, 35 mg/m2 (∼60 mg) is the proposed RP2D for treating NHL.
The OR rate of selinexor over the entire study population was 31%, with responses observed in multiple NHL subtypes, including DLBCL, FL, Richter’s transformation, mantle cell lymphoma, T-cell lymphoma, and CLL. Unlike in Hodgkin lymphoma, where response rates of 70% to 80% have been seen with the antibody-drug conjugate brentuximab vedotin and the immune checkpoint inhibitor nivolumab, broadly successful agents in NHL after rituximab have not been commonly identified.39-42 Compared with other targeted therapies studied across a spectrum of R/R NHL, selinexor showed comparable or favorable response rates with durable clinical benefit. Although there is potential for synergistic antilymphoma effects in patients who received an antiemetic dose of dexamethasone as a supportive care agent, more than half of the responses occurred in the absence of concomitant dexamethasone use, demonstrating the single-agent activity of selinexor in NHL.
Selinexor for the treatment of patients with DLBCL warrants further development, because this is the most common NHL in the Western world, and this population has a high unmet medical need given the inferior overall survival of patients for whom primary rituximab-based therapy fails.1,2,43 ORs were seen in 32% of heavily pretreated patients with DLBCL, and unlike other targeted agents such as the BTK inhibitor ibrutinib or lenalidomide, selinexor seems to have comparable efficacy in both GCB and non-GCB subtypes as well as in de novo and transformed DLBCL. Similarly, early signs of activity were seen in patients harboring c-Myc and dual translocations of Bcl-2 and c-Myc, 2 patient populations with a poor outcome in need of innovative therapies.44,45 On the basis of the encouraging response rates, a randomized phase 2 trial, SADAL (#NCT02227251), will test 2 dose levels of selinexor (60 and 100 mg) to specifically define efficacy and toxicity in R/R DLBCL.
In conclusion, selective inhibition of nuclear export is a promising treatment strategy. Selinexor has demonstrated a manageable safety profile and preliminary evidence of single-agent anticancer activity in multiple subtypes of R/R NHL. The reported study has led to the initiation of several phase 2 studies testing selinexor in patients with R/R hematologic malignancies. Efforts are also under way to identify biomarkers that enable stratification of patients and predict response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this trial and their families and the coinvestigators, nurses, and study coordinators at each of the sites. The authors also thank John Byrd for his contributions to the trial and development of selinexor.
This work was supported by Karyopharm Therapeutics. R.G. was supported by a Scholar in Clinical Research award from the Leukemia & Lymphoma Society.
Authorship
Contribution: J.K. performed research, analyzed data, and wrote the paper; M.S. performed research, analyzed data, and wrote the paper; R.B., P.M.M.-S., N.G., R.G., R.S., M.W., L.S., P.M., I.F., and M.J. performed research and reviewed the paper; T.J.U. analyzed data and wrote the paper; J.-R.S.-M. analyzed data and reviewed the paper; T.R. designed the study and reviewed the paper; S.F. performed research; R.C. analyzed data and wrote the paper; M.K. and S.S. designed the study and reviewed the paper; and M.G. performed research and wrote the paper. J.K. and S.S. had full access to all of the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. All authors discussed and interpreted the results, vouch for the accuracy of the data and collaborated in the preparation of the manuscript.
Conflict-of-interest disclosure: J.K. receives honoraria from Roche, Gilead, Janssen, Bristol-Myers Squibb, Merck, AbbVie, Celgene, Lundbeck, and Seattle Genetics; has a consulting/advisory role at Bristol-Myers Squibb, AbbVie, Janssen, Gilead, and Roche; and receives research funding from Roche. M.S. owns stock in Karyopharm Therapeutics; has a consulting/advisory role at Amgen, CTI, Karyopharm Therapeutics, ARIAD, Gilead, and Celgene; and receives research funding from Astex, Sunesis, Takeda, and TG Therapeutics. R.B. has a consulting/advisory role at Celgene and receives research funding from Celgene, Karyopharm Therapeutics, Merck, Bristol-Myers Squibb, and Millennium/Takeda. P.M.M.-S. receives an honorarium and a travel stipend from Karyopharm Therapeutics. N.G. receives honoraria from Heron, Sanofi, and Taiho and is a consultant/advisor at Heron. R.G. received a travel stipend from Karyopharm Therapeutics. R.S. receives honoraria from Amgen, Arog, Agios, Celfor, Celgene, Sunesis, Bristol-Myers Squibb, Pfizer, Novartis, Karyopharm Therapeutics, Fujifilm, AbbVie, Roche, Genentech, and Merck and receives research funding from Novartis. M.W. receives an honorarium from Janssen; is a consultant/advisor at Acerta Pharmaceuticals and Celltrion; and receives research funding from Janssen, Celgene, Pharmacyclics, and Onyx. L.S. has a consulting/advisory role at Novartis, Bristol-Myers Squibb, Pfizer, Celgene, and JAZZ. P.M. receives an honorarium from Janssen and is a consultant/advisor at Gilead, Celgene, Janssen, and Acerta. I.F. holds stock in Raintree and receives grant funding from Acerta, Agios, Calithera, Celgene, Constellation, Genentech, Gilead, ImmunoGen, Incyte, Infinity, Janssen, Karyopharm, KITE, Medivation, Millennium/Takeda, Novartis, OncoMed, Pharmacyclics, Portola, Roche, Seattle Genetics, TG Therapeutics, and Trillium. M.J. has a consulting/advisory role at Novo Nordisk and Endpoint and receives funding from Sunesis. T.J.U., J.-R.S.-M., T.R., S.F., R.C., M.K., and S.S. are employees and stockholders of Karyopharm Therapeutics. S.S. is the chief scientific officer of Karyopharm Therapeutics. M.K. is the chief executive officer of Karyopharm Therapeutics and has leadership/consulting roles at Verastem and Metamark. M.G. owns stock in COTA; has a consulting/advisory role at Bayer and Lilly; is on the speakers’ bureau at Bristol-Myers Squibb and Merck; and receives research funding from Novartis, AbbVie, MedImmune, Bristol-Myers Squibb, Rexahn Pharmaceuticals, Esanex, Karyopharm Therapeutics, TG Therapeutics, Daiichi Sankyo, EMD Serono, Lilly, Acceleron Pharma, Gilead, and Incyte.
Correspondence: John Kuruvilla, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, Room 5-221, 610 University Ave, Toronto, ON M5G 2M9, Canada; e-mail: john.kuruvilla@uhn.ca.