Key Points
Crosspriming of AAV capsid-specific CD8+ T cells requires cooperation between distinct subsets of DCs.
Innate immune sensing of the viral DNA genome induces cross-presentation of viral capsid in trans.
Abstract
Adeno-associated virus (AAV) is a replication-deficient parvovirus that is extensively used as a gene therapy vector. CD8+ T-cell responses against the AAV capsid protein can, however, affect therapeutic efficacy. Little is known about the in vivo mechanism that leads to the crosspriming of CD8+ T cells against the input viral capsid antigen. In this study, we report that the Toll-like receptor 9 (TLR9)–MyD88 pattern-recognition receptor pathway is uniquely capable of initiating this response. By contrast, the absence of TLR2, STING, or the addition of TLR4 agonist has no effect. Surprisingly, both conventional dendritic cells (cDCs) and plasmacytoid DCs (pDCs) are required for the crosspriming of capsid-specific CD8+ T cells, whereas other antigen-presenting cells are not involved. TLR9 signaling is specifically essential in pDCs but not in cDCs, indicating that sensing of the viral genome by pDCs activates cDCs in trans to cross-present capsid antigen during CD8+ T-cell activation. Cross-presentation and crosspriming depend not only on TLR9, but also on interferon type I signaling, and both mechanisms can be inhibited by administering specific molecules to prevent induction of capsid-specific CD8+ T cells. Thus, these outcomes directly point to therapeutic interventions and demonstrate that innate immune blockade can eliminate unwanted immune responses in gene therapy.
Introduction
The immune system has evolved exquisite mechanisms to sense molecular structures associated with pathogens such as viruses. It is critical to understand how such innate immune sensing is linked to activation of antigen-specific immune responses. Particularly puzzling has been the immune response to adeno-associated virus (AAV).1 This small, non-enveloped parvovirus that encapsidates a single-stranded DNA genome is naturally replication-deficient in the absence of a helper virus. Recombinant AAV vectors are devoid of viral genes and are widely used for human gene therapy.2-4 AAV elicits minimal innate immune responses and has been a favorite tool for therapeutic in vivo gene delivery, often resulting in long-term expression in animal models. Therefore, it came as a surprise that AAV administration in patients with hemophilia B caused a CD8+ T-cell response against the viral input capsid, which is not expressed by the recombinant genome. This T-cell response has been a major impediment for persistence of AAV vectors in human hepatocytes and has also caused inflammation in skeletal muscle.5-11
The antigen processing and presentation mechanism that leads to CD8+ T-cell priming against structural protein components of viral particles remains to be defined, prompting us to study how cross-presentation via major histocompatibility complex (MHC) class I occurs upon uptake of the exogenous viral capsid by antigen-presenting cells (APCs). In general, recognition of pathogen-associated molecular patterns by pattern recognition receptors (PRRs) such as the Toll-like receptors (TLRs) leads to the upregulation of co-stimulatory markers and cytokine production, which drive cellular and humoral immunity.12 Innate immune responses against AAV depend on TLR9, an endosomal DNA receptor that signals through its cytoplasmic adaptor MyD88 to induce the nuclear factor-κB (NF-κB) proinflammatory pathway and interferon type I (IFN I) expression.13,14 TLR9 signaling is enhanced for AAV vectors modified to package self-complementary genomes; these vectors induce enhanced CD8+ T-cell responses to their gene products.15,16 Other studies have implicated TLR2 in sensing the AAV capsid.17 Although antibody formation against the capsid or gene product of AAV occurs independently of TLR signaling, CD8+ T-cell responses against the transgene gene product require the TLR9-MyD88 pathway and can be ablated by depleting the AAV genome of immune stimulatory cytosine guanine dinucleotide motifs.18,19
Little is known about the role of professional APCs in CD8+ T-cell responses to AAV, and in vivo studies are entirely lacking. Most studies investigating the MHC I presentation of AAV capsid have focused on the target cells of gene transfer, which may become flagged for destruction by CD8+ T cells.20,21 Presumably, crosspriming of CD8+ T cells is dependent on cross-presentation of input capsid antigen by APCs. In vitro, plasmacytoid dendritic cells (pDCs) were the only cell type capable of producing IFN I in response to AAV.13 However, the in vivo mechanisms that lead to CD8+ T-cell responses against input capsid are largely unknown.
Here, we find that crosspriming of capsid-specific CD8+ T cells is dependent on (1) TLR9 sensing of the viral genome specifically in pDCs, (2) IFN I signaling, and (3) cross-presentation by conventional DCs (cDCs). Therefore, a complex immune response mechanism against the virus has evolved, in which 2 types of DCs need to cooperate to sense the viral particle and cross-present its protein capsid.
Materials and methods
Mouse strains and procedures
Wild-type (WT) C57BL/6, TLR2−/−, MyD88−/−, AP3−/−, μMT, OT-1, STINGgt/gt, CD11c-DTR, BDCA2-DTR, and WT BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). TLR9−/− mice were bred in-house.15 DC-MyD88−/− mice were generated as described in “Generation and genotyping of DC-MyD88−/− mice” in the supplemental Materials, available on the Blood Web site. All transgenic and knockout mice were on a C57BL/6 background and 6- to 8-weeks old, housed under specific pathogen-free conditions at the University of Florida, and treated under the Institutional Animal Care and Use Committee‐approved protocols. AAV vectors were produced by transfection of HEK-293 cells and purified by iodixanol gradient as published.18 Vectors were injected intramuscularly (IM) or IV (into the tail vein) as described.18 Plasma samples were collected by retro-orbital bleed into heparinized capillary tubes.18 Various reagents were injected into the mice as detailed in supplemental Materials.
Flow cytometry and enzyme-linked immunospot (ELISPOT)
Single-cell suspensions of splenocytes were prepared as published.18 Following pretreatment with FcR block (BD Biosciences, San Diego, CA), splenocytes or peripheral blood cells were stained with antibodies to CD8 (53-6.7), B220 (RA3-6B2), and iTAg MHC Tetramer (H2-Kb-SIINFEKL; Beckman Coulter, Brea, CA). SIINFEKL/H-2Kb complex on cell surfaces were detected with 25D1.16 antibody bound to phycoerythrin (BioLegend, San Diego, CA). DCs were identified with fluorochrome-labeled CD11c (N418), CD11c (HL3), PDCA-1 (129c1), and PDCA-1 (927, BioLegend). Data were collected on an LSR-II flow cytometer (BD Biosciences) and analyzed with FCS Express (De Novo Software, Glendale, CA). ELISPOT assays to quantitate IFN-γ+ responses against native AAV2 CD8+ T-cell epitopes in C57BL/6 and BALB/c mice were as published.15,22
Cell sorting and adoptive transfers
CD8+ T cells were negatively selected and magnetically purified from splenocytes of OT-1 mice using a CD8a+ T-cell Isolation Kit (Miltenyi Biotec, Auburn, CA). Cells were labeled with 4 μM CellTrace Violet (CTV) (Life Technologies, Carlsbad, CA) per the manufacturer’s instructions. Recipient mice received 5 × 106 labeled CD8+ T cells 1 or 2 days after vector injection. Spleens were harvested 3 to 7 days later to measure proliferation by flow cytometry. For cDC and pDC sorting, cells were magnetically enriched from splenocytes using Pan DC MicroBeads (Miltenyi Biotec). Cells were labeled with CD11c (N418) and PDCA-1 (ebio927) antibodies, and purified using a FACSAria II cell sorter (BD Biosciences). Post-sort purity was determined to be >92%, and cross-contamination by either cell type was 0.01% to 1.1%.
Statistics
Results are reported as means ± standard error of the mean (SEM). Significant differences between groups were determined with unpaired Student t test, Mann-Whitney U test, or two-way analysis of variance with Bonferroni posttests, as appropriate. P values of < .05 were considered significant. Analyses were performed using GraphPad Prism software (San Diego, CA). Differences are indicated as *P < .05, **P < .01, ***P < .001, and ns = not significant.
Results
The TLR9-MyD88 pathway is requisite for capsid-specific CD8+ T-cell responses
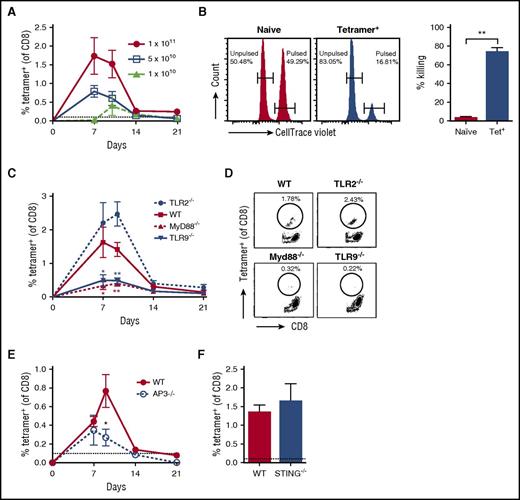
In order to track capsid-specific T cells, we generated a modified AAV serotype 2 vector that contains the peptide sequence SIINFEKL (the immunodominant CD8+ T-cell epitope of the model antigen ovalbumin) and is equally infective as AAV2 (supplemental Figure 1). To verify the ability of AAV vectors modified to package self-complementary genomes into AAV2-SIINFEKL capsid to induce anti-capsid CD8+ T cells, we IM injected WT C57BL/6 mice with 1 × 1011 vg/mouse. Using the H2-Kb-SIINFEKL tetramer, we detected capsid-specific CD8+ T cells in both spleen and peripheral blood 7 days post-injection (supplemental Figures 2 and 3). The response was vector dose-dependent, peaked between 7 to 10 days post-injection, and largely subsided by day 21 (Figure 1A). Induced cytotoxic T lymphocytes (CTLs) were functional in an in vivo killing assay (Figure 1B). No response was detected at ≤5 × 109 vg/mouse (data not shown). In contrast, TLR9−/− and MyD88−/− mice failed to respond to the AAV capsid (Figure 1C-D), whereas the response was only partially impaired in mice deficient in adaptor protein 3 (Figure 1E). This protein is thought to be required for IFN I production upon endosomal sensing of viral DNA by TLR9.23
Unique role of TLR9-MyD88 signaling in CD8+T-cell responses to the AAV capsid. (A) Dose response of AAV2-SIINFEKL in WT mice (n = 4/group) following IM injection. Anti-capsid CD8+ T-cell responses were assessed in peripheral blood on days 7, 10, 14, and 21 by flow cytometry with an H2-Kb–SIINFEKL tetramer. (B) In vivo cell killing assay to demonstrate functionality of anti-capsid CD8+ T cells. Representative histograms show preferential killing of SIINFEKL peptide pulsed, 3 μM CTV-labeled splenocytes adoptively transferred into mice positive for anti-capsid CD8+ T cells (tetramer+). Control unpulsed, 0.3 μM CTV-labeled splenocytes were not killed. No killing was observed in naïve mice that were negative for anti-capsid CD8+ T cells. Percentage killing (n = 2/group) of SIINFEKL peptide-pulsed splenocytes by tetramer+ or naïve mice is indicated. (C) WT, TLR2−/−, TLR9−/−, or MyD88−/− mice were IM injected with 1 × 1011 vector genomes (vg) of AAV2-SIINFEKL (n = 4/group). Anti-capsid CD8+ T-cell responses were assessed in peripheral blood by flow cytometry as a function of time. Significant differences compared with WT mice are indicated. (D) Examples of peak responses for individual mice. (E) WT or AP3−/− mice were IM injected with AAV2-SIINFEKL, and anti-capsid CD8+ T-cell responses were assessed in peripheral blood over time (n = 4/group). (F) WT or STING−/− mice were IM injected with AAV2-SIINFEKL, and anti-capsid CD8+ T-cell responses were assessed in peripheral blood 8 days post-injection (n = 4/group). The dotted line at 0.1% represents the limit of detection of capsid-specific CD8+ T cells using the tetramer. Data points are averages ± SEM. Statistically significant decreases compared with responses in WT mice are indicated. P values: (B) **P =.005; (C) day 7, *P =.03 for WT vs MyD88−/− and *P =.049 for WT vs TLR9−/−; (C) day 10, **P =.004 for WT vs MyD88−/− and **P =.006 for WT vs TLR9−/−; (E) *P =.045. IM, intramuscular(ly).
Unique role of TLR9-MyD88 signaling in CD8+T-cell responses to the AAV capsid. (A) Dose response of AAV2-SIINFEKL in WT mice (n = 4/group) following IM injection. Anti-capsid CD8+ T-cell responses were assessed in peripheral blood on days 7, 10, 14, and 21 by flow cytometry with an H2-Kb–SIINFEKL tetramer. (B) In vivo cell killing assay to demonstrate functionality of anti-capsid CD8+ T cells. Representative histograms show preferential killing of SIINFEKL peptide pulsed, 3 μM CTV-labeled splenocytes adoptively transferred into mice positive for anti-capsid CD8+ T cells (tetramer+). Control unpulsed, 0.3 μM CTV-labeled splenocytes were not killed. No killing was observed in naïve mice that were negative for anti-capsid CD8+ T cells. Percentage killing (n = 2/group) of SIINFEKL peptide-pulsed splenocytes by tetramer+ or naïve mice is indicated. (C) WT, TLR2−/−, TLR9−/−, or MyD88−/− mice were IM injected with 1 × 1011 vector genomes (vg) of AAV2-SIINFEKL (n = 4/group). Anti-capsid CD8+ T-cell responses were assessed in peripheral blood by flow cytometry as a function of time. Significant differences compared with WT mice are indicated. (D) Examples of peak responses for individual mice. (E) WT or AP3−/− mice were IM injected with AAV2-SIINFEKL, and anti-capsid CD8+ T-cell responses were assessed in peripheral blood over time (n = 4/group). (F) WT or STING−/− mice were IM injected with AAV2-SIINFEKL, and anti-capsid CD8+ T-cell responses were assessed in peripheral blood 8 days post-injection (n = 4/group). The dotted line at 0.1% represents the limit of detection of capsid-specific CD8+ T cells using the tetramer. Data points are averages ± SEM. Statistically significant decreases compared with responses in WT mice are indicated. P values: (B) **P =.005; (C) day 7, *P =.03 for WT vs MyD88−/− and *P =.049 for WT vs TLR9−/−; (C) day 10, **P =.004 for WT vs MyD88−/− and **P =.006 for WT vs TLR9−/−; (E) *P =.045. IM, intramuscular(ly).
We next assessed whether TLR9 was requisite for in vivo cross-presentation of AAV capsid in a widely used and highly sensitive indirect assay, which is based on the proliferation of adoptively transferred transgenic OT-1 CD8+ T cells (Figure 2A; supplemental Figure 4). Although OT-1 cells proliferated upon transfer into WT mice that had received AAV2-SIINFEKL, proliferation was substantially reduced when OT-1 cells were adoptively transferred into TLR9−/− recipients (Figure 2B-C). Proliferation of OT-1 cells is specifically dependent on administering AAV2-SIINFEKL, because no response was obtained with unmodified AAV2 lacking the SIINFEKL motif (Figure 2B-C). Thus, this indicated that TLR9 is also required for optimal cross-presentation of capsid antigen by MHC I.
TLR9 is required for proliferation of transgenic capsid-specific CD8+T cells. WT or TLR9−/− mice were injected with AAV2-SIINFEKL or unmodified AAV2 (n = 3/group). Two days later, CD8+ T cells were magnetically isolated from OT-1 mice, labeled with CTV, and adoptively transferred at 5 × 106 cells/mouse. (A) Timeline. (B) Representative plots of proliferation of adoptively transferred OT-1 cells. (C) Quantification of the percent proliferation, percent divided (percent of original cells that divided at least once), and the division index (the average number of cells that a dividing cell became). Data points are averages ± SEM. P values: (C) percent proliferation, ***P < .0001 for WT vs TLR9−/−, ***P =.0002 for WT vs WT(AAV2), **P =.007 for TLR9−/− vs WT(AAV2); percent divided, *P =.02 for WT vs TLR9−/−, **P =.007 for WT vs WT(AAV2); division index, *P =.02 for WT vs TLR9−/−, **P =.006 for WT vs WT(AAV2). ns, not significant.
TLR9 is required for proliferation of transgenic capsid-specific CD8+T cells. WT or TLR9−/− mice were injected with AAV2-SIINFEKL or unmodified AAV2 (n = 3/group). Two days later, CD8+ T cells were magnetically isolated from OT-1 mice, labeled with CTV, and adoptively transferred at 5 × 106 cells/mouse. (A) Timeline. (B) Representative plots of proliferation of adoptively transferred OT-1 cells. (C) Quantification of the percent proliferation, percent divided (percent of original cells that divided at least once), and the division index (the average number of cells that a dividing cell became). Data points are averages ± SEM. P values: (C) percent proliferation, ***P < .0001 for WT vs TLR9−/−, ***P =.0002 for WT vs WT(AAV2), **P =.007 for TLR9−/− vs WT(AAV2); percent divided, *P =.02 for WT vs TLR9−/−, **P =.007 for WT vs WT(AAV2); division index, *P =.02 for WT vs TLR9−/−, **P =.006 for WT vs WT(AAV2). ns, not significant.
Other potentially relevant receptors were also evaluated. No reduction in the CD8+ T-cell response was observed in TLR2−/− mice, in line with our previous observations that this receptor has limited impact on the adaptive response to AAV (Figure 1C-D).18 Because AAV is a DNA virus, we also determined responses in STING−/− mice, in which signaling through several cytoplasmic sensors of DNA is impaired.24 However, responses were identical to those in WT mice (Figure 1F). Finally, to determine whether an alternative source of inflammation could alter the response, WT or TLR9−/− mice were injected with AAV2-SIINFEKL in conjunction with 4 daily intraperitoneal injections of the TLR4 activator lipopolysaccharide, starting at day 0. Lipopolysaccharide injections neither enhanced the response in WT mice nor rescued it in TLR9−/− mice 10 days post-injection (supplemental Figure 5). Together, these data confirm a critical and unique role of TLR9-MyD88 signaling in CD8+ T-cell responses to the AAV capsid.
DCs are the essential APCs for anti-capsid CTL responses
We next set out to determine which APCs (DCs, macrophages, or B cells) would be required for the CD8+ T-cell response to AAV capsid. Transient depletion of DCs in CD11c-DTR mice significantly reduced the CD8+ T-cell response 7 days post-administration of AAV2-SIINFEKL (Figure 3A-B; supplemental Figures 6B-C and 7). The subsequent recovery of the response was likely due to repopulation of DCs, which is largely complete within <1 week.25 In contrast, the administration of GdCl3, which inactivates Kupffer cells and M1 macrophages, did not reduce CD8+ T-cell responses to capsid upon muscle- or liver-directed gene transfer (Figure 3A-B; supplemental Figure 7; and data not shown). This is in contrast to MHC II presentation of the transgene product, which is blocked by GdCl3 in lymph nodes and spleen.26
Conventional DCs, but not macrophages or B cells, are necessary for anti-capsid CD8+T-cell responses. (A) Experimental timeline of WT, CD11c-DTR, gadolinium chloride (GdCl3)-treated, or μMT mice that were IM injected with AAV2-SIINFEKL (n = 4/group). CD11c-DTR mice received diphtheria toxin (DT) on days −1 and 0. Mice received GdCl3 on days −1 and 0. (B) Quantification of capsid-specific CD8+ T-cell response in peripheral blood of APC-depleted mice as a function of time. (C) Time-course of anti-CD20 depletion experiment. WT mice received phosphate-buffered saline (PBS) or anti-CD20 in 2 doses 3 weeks apart. One day after the second injection, mice were IM injected with AAV2-SIINFEKL, and capsid-specific CD8+ T-cell responses were assessed 7 days later (n = 4/group). (D) Quantification of capsid-specific CD8+ T-cell response. The dotted line at 0.1% represents the limit of detection of capsid-specific CD8+ T cells using the tetramer. Data points are averages ± SEM. (E) Time-course of DC depletion with chlodronate liposomes, and (F) quantification of capsid-specific CD8+ T-cell response. (G) Time-course of AAV2 administration and DC depletion in CD11c-DTR mice using DT or WT BALB/c mice using chlodronate liposomes, followed by measurement of CD8+ T-cell frequency of response to native capsid epitopes. (H) Quantitation of IFN-γ ELISPOT assays (n = 4/group; data are averages ± SEM for epitope-stimulated minus mock-stimulated cultures). P values: (B) *P =.02 for WT vs CD11c-DTR; (F) **P =.005 for day 7 and *P =.02 for day 10; (H) *P =.03 for both experiments. ns, not significant.
Conventional DCs, but not macrophages or B cells, are necessary for anti-capsid CD8+T-cell responses. (A) Experimental timeline of WT, CD11c-DTR, gadolinium chloride (GdCl3)-treated, or μMT mice that were IM injected with AAV2-SIINFEKL (n = 4/group). CD11c-DTR mice received diphtheria toxin (DT) on days −1 and 0. Mice received GdCl3 on days −1 and 0. (B) Quantification of capsid-specific CD8+ T-cell response in peripheral blood of APC-depleted mice as a function of time. (C) Time-course of anti-CD20 depletion experiment. WT mice received phosphate-buffered saline (PBS) or anti-CD20 in 2 doses 3 weeks apart. One day after the second injection, mice were IM injected with AAV2-SIINFEKL, and capsid-specific CD8+ T-cell responses were assessed 7 days later (n = 4/group). (D) Quantification of capsid-specific CD8+ T-cell response. The dotted line at 0.1% represents the limit of detection of capsid-specific CD8+ T cells using the tetramer. Data points are averages ± SEM. (E) Time-course of DC depletion with chlodronate liposomes, and (F) quantification of capsid-specific CD8+ T-cell response. (G) Time-course of AAV2 administration and DC depletion in CD11c-DTR mice using DT or WT BALB/c mice using chlodronate liposomes, followed by measurement of CD8+ T-cell frequency of response to native capsid epitopes. (H) Quantitation of IFN-γ ELISPOT assays (n = 4/group; data are averages ± SEM for epitope-stimulated minus mock-stimulated cultures). P values: (B) *P =.02 for WT vs CD11c-DTR; (F) **P =.005 for day 7 and *P =.02 for day 10; (H) *P =.03 for both experiments. ns, not significant.
Given that anti-capsid CD8+ T cells were not detected in a patient who received monoclonal anti-CD20 prior to AAV gene transfer, we also investigated the effect of B-cell depletion.27 WT mice received 2 doses of α-mCD20, and AAV2-SIINFEKL was delivered at the nadir of B-cell frequency (Figure 3C).28 Near-complete depletion of B cells was observed in peripheral blood by flow cytometry at the time of vector administration (supplemental Figure 6A). No significant alteration in the frequency of tetramer-positive CD8+ T cells was observed in this model (Figure 3D) or in B-cell–deficient μMT mice (Figure 3A-B; supplemental Figure 7). IV injection of 200 μL clodronate liposomes substantially depleted DCs (supplemental Figure 8), which also resulted in significantly diminished anti-capsid responses against AAV2-SIINFEKL and native AAV2 epitopes (Figure 3E-H). At this dose, only minor depletion of macrophages was observed (supplemental Figure 8). Thus, we conclude that DCs are the primary cell type that is involved in crosspriming.
CD8+ T-cell responses to AAV capsid require cDCs but not cDC-intrinsic TLR9-MyD88
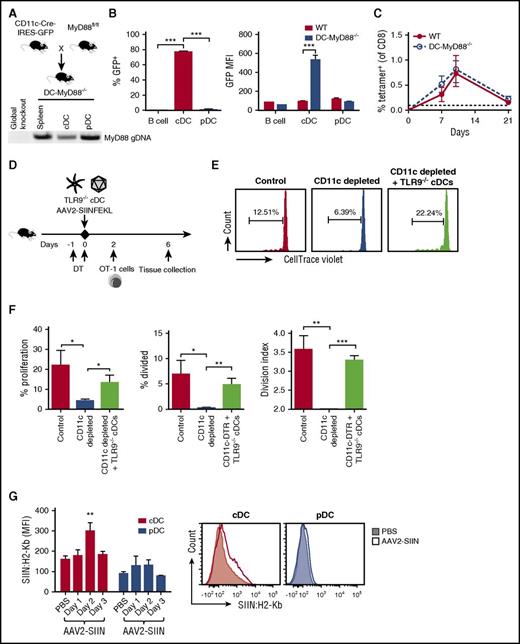
In an effort to link the observations that TLR9-MyD88 pathway and DCs were required for T-cell activation, we generated mice specifically lacking MyD88 expression in DCs (DC-MyD88−/− mice) by crossing CD11c-Cre mice with MyD88fl/fl mice (Figure 4A). Surprisingly, these mice responded to AAV2-SIINFEKL in a similar fashion as the WT mice (Figure 4C). Further analysis revealed that Cre expression in DC-MyD88−/− mice (which can be detected via a co-expressed GFP reporter) occurred in cDCs but not pDCs (Figure 4A-B; supplemental Figure 9). To further examine the activity of Cre in these cell populations, cDCs and pDCs were flow sorted, and followed by genomic DNA isolation for polymerase chain reaction analysis (Figure 4A). In good correlation with GFP expression, the MyD88 gene was reduced in cDCs but not pDCs relative to total splenocytes (Figure 4A).
AAV capsid is MHC I presented by cDCs, which do not require intrinsic TLR9-MyD88 signaling. (A) Transgenic mice expressing Cre recombinase under the control of a CD11c promoter were crossed with mice containing a MyD88 gene flanked by loxP sites. The resulting animals were termed DC-MyD88−/− mice. Genomic DNA was isolated and polymerase chain reaction analysis of MyD88 was performed (100 ng DNA/sample), along with genomic DNA from a global MyD88−/− mouse (that had been generated by others by Cre excision from the same MyD88fl/fl strain) to confirm that detection is lost after Cre-mediated excision. (B) Spleens were harvested from WT or DC-MyD88−/− mice (n = 4-5/group) and analyzed for Cre expression using the GFP transgene in B cells (CD19+), cDCs (CD11c+ PDCA-1−), and pDCs (CD11cint PDCA-1+). Percent GFP+ was calculated via Overton subtraction using WT mice as a control, and GFP mean fluorescence intensity in each cell population was quantified. (C) WT or DC-MyD88−/− mice were IM injected with AAV2-SIINFEKL and tetramer-positive cells were quantified over time (n = 4/group). The dotted line at 0.1% represents the limit of detection of capsid-specific CD8+ T cells using the tetramer. (D) Experimental outline of TLR9−/− cDC adoptive transfers. CD11c-DTR mice received 100 ng DT in order to deplete endogenous cDC. Mice then either received both AAV2-SIINFEKL (IM) and 3 to 4 × 106 cDC from TLR9−/− mice or AAV2-SIINFEKL only. Another group of mice that received AAV2-SIINFEKL but no DT served as positive control for the immune response, and is labeled as “Control.” Mice were injected with 5 × 106 CTV-labeled OT-1 cells 2 days later. (E) Representative plots of proliferation of adoptively transferred OT-1 cells. (F) Quantification of the percent proliferation, percent divided (percent of original cells that divided at least once), and the division index (the average number of cells that a dividing cell became). (G) Detection of capsid antigen presentation in inguinal lymph node by staining with a phycoerythrin-labeled antibody specific to SIINFEKL/H-2Kb as a function of time after IM injection of 1 × 1011 vg of AAV2-SIINFEKL vector in WT C57BL/6 mice. Shown are average mean fluorescence intensity ± SEM for cDCs (CD11chi, PDCA-1−) and pDCs (CD11cmid, PDCA-1+) 1, 2, or 3 days after vector injection (n = 4, 4, or 2, respectively). Cells from PBS-injected mice, analyzed in parallel, serve for baseline level of staining (n = 4). Also shown is a representative histogram for a PBS- vs vector-injected (2-day time point) mouse. P values: (B) ***P < .0001 for differences in % GFP, **P =.003 for MFI; (F) percent proliferation, *P =.025 for control vs depleted and *P =.048 for depleted vs “+ TLR9−/− cDCs”; percent divided, *P =.025 for control vs depleted and **P =.003 for depleted vs “+ TLR9−/− cDCs”; division index, **P =.0025 for control vs depleted and ***P < .0001 for depleted vs “+ TLR9−/− cDCs”; (G) **P < .01 by ANOVA. GFP, green fluorescent protein.
AAV capsid is MHC I presented by cDCs, which do not require intrinsic TLR9-MyD88 signaling. (A) Transgenic mice expressing Cre recombinase under the control of a CD11c promoter were crossed with mice containing a MyD88 gene flanked by loxP sites. The resulting animals were termed DC-MyD88−/− mice. Genomic DNA was isolated and polymerase chain reaction analysis of MyD88 was performed (100 ng DNA/sample), along with genomic DNA from a global MyD88−/− mouse (that had been generated by others by Cre excision from the same MyD88fl/fl strain) to confirm that detection is lost after Cre-mediated excision. (B) Spleens were harvested from WT or DC-MyD88−/− mice (n = 4-5/group) and analyzed for Cre expression using the GFP transgene in B cells (CD19+), cDCs (CD11c+ PDCA-1−), and pDCs (CD11cint PDCA-1+). Percent GFP+ was calculated via Overton subtraction using WT mice as a control, and GFP mean fluorescence intensity in each cell population was quantified. (C) WT or DC-MyD88−/− mice were IM injected with AAV2-SIINFEKL and tetramer-positive cells were quantified over time (n = 4/group). The dotted line at 0.1% represents the limit of detection of capsid-specific CD8+ T cells using the tetramer. (D) Experimental outline of TLR9−/− cDC adoptive transfers. CD11c-DTR mice received 100 ng DT in order to deplete endogenous cDC. Mice then either received both AAV2-SIINFEKL (IM) and 3 to 4 × 106 cDC from TLR9−/− mice or AAV2-SIINFEKL only. Another group of mice that received AAV2-SIINFEKL but no DT served as positive control for the immune response, and is labeled as “Control.” Mice were injected with 5 × 106 CTV-labeled OT-1 cells 2 days later. (E) Representative plots of proliferation of adoptively transferred OT-1 cells. (F) Quantification of the percent proliferation, percent divided (percent of original cells that divided at least once), and the division index (the average number of cells that a dividing cell became). (G) Detection of capsid antigen presentation in inguinal lymph node by staining with a phycoerythrin-labeled antibody specific to SIINFEKL/H-2Kb as a function of time after IM injection of 1 × 1011 vg of AAV2-SIINFEKL vector in WT C57BL/6 mice. Shown are average mean fluorescence intensity ± SEM for cDCs (CD11chi, PDCA-1−) and pDCs (CD11cmid, PDCA-1+) 1, 2, or 3 days after vector injection (n = 4, 4, or 2, respectively). Cells from PBS-injected mice, analyzed in parallel, serve for baseline level of staining (n = 4). Also shown is a representative histogram for a PBS- vs vector-injected (2-day time point) mouse. P values: (B) ***P < .0001 for differences in % GFP, **P =.003 for MFI; (F) percent proliferation, *P =.025 for control vs depleted and *P =.048 for depleted vs “+ TLR9−/− cDCs”; percent divided, *P =.025 for control vs depleted and **P =.003 for depleted vs “+ TLR9−/− cDCs”; division index, **P =.0025 for control vs depleted and ***P < .0001 for depleted vs “+ TLR9−/− cDCs”; (G) **P < .01 by ANOVA. GFP, green fluorescent protein.
Taken together, the results from Figures 1-4 implied that cDCs were needed for the response to capsid, whereas TLR9-MyD88 signaling in a different cell type (rather than cDC intrinsic signaling) provided immune activation. To further address this point, we performed adoptive transfers of TLR9−/− cDCs and labeled OT-1 cells into CD11c-DTR mice (Figure 4E-F). Proliferation of OT-1 cells in response to AAV2-SIINFEKL was abrogated by depletion of CD11chi cells (Figure 4E-F). Adoptive transfer of flow-sorted TLR9−/− cDCs restored OT-1 proliferation in CD11c-DTR mice, in which endogenous cDCs had been depleted by DT injection (Figure 4D-F). Finally, IM injection of this vector results in MHC I presentation of SIINFEKL in cDCs in the draining lymph node by 48 hours (Figure 4G). Together, these data reaffirm a crucial role of cDCs in cross-presentation, even though they are not the source of the required TLR9 signaling.
pDCs with intact TLR9 promote crosspriming of capsid-specific CD8+ T cells
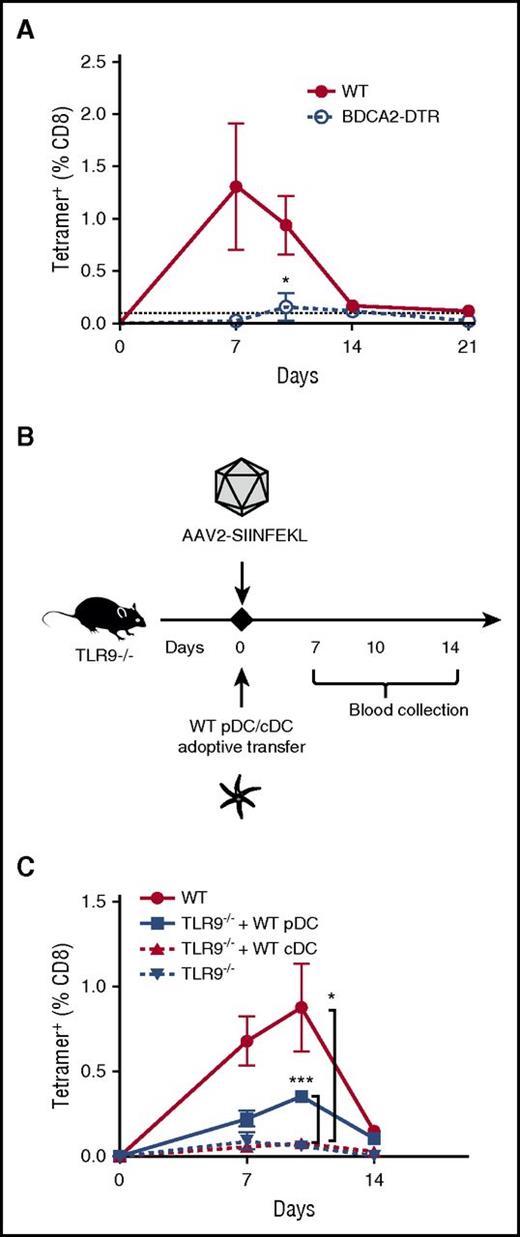
Next, we sought to determine the cell type that provides the critical TLR9 signal. Because TLR9−/− cDCs restored capsid antigen presentation upon depletion of endogenous TLR9+/+ cDCs in CD11c-DTR mice, and because endogenous pDCs are spared following DT or chlodronate liposome administration (supplemental Figures 6B and 8A-B), we hypothesized that pDCs could fulfill this sensing role. Injection of AAV2-SIINFEKL into BDCA2-DTR mice (which allow specific ablation of pDCs; supplemental Figure 6D-E) showed that pDCs were indeed requisite for crosspriming of anti-capsid CD8+ T cells (Figure 5A).29 Depletion of pDCs with DT also resulted in a modest reduction in OT-1 cell proliferation upon transfer to BDCA2-DTR mice (supplemental Figure 10), suggesting a lesser requirement of pDC in cross-presentation compared with crosspriming.
TLR9 is specifically required in pDCs for capsid-specific CD8+T-cell responses. (A) WT or BDCA2-DTR mice were IM injected with AAV2-SIINFEKL, and tetramer-positive cells were quantified over time (n = 4/group). BDCA2-DTR mice received DT intraperitoneally 3×/week. Statistically significant decrease compared with the response in WT mice is indicated. (B) Timeline of WT DC adoptive-transfer experiments. TLR9−/− mice (n = 4-5/group) were recipients of adoptively transferred WT pDC (3 × 106 cells) or WT cDC (5 × 106 cells), or nothing. On the same day, all mice were IM injected with AAV2-SIINFEKL. WT C57BL/6 mice served as a positive control. (C) Quantification of capsid-specific CD8+ T-cell responses over time in TLR9−/− mice that received either WT pDC, WT cDC, or nothing. The dotted line at 0.1% represents the limit of detection of capsid-specific CD8+ T cells using the tetramer. Data points are averages ± SEM. Statistically significant increases compared with responses in TLR9−/− mice that did not receive cell transfer are indicated. P values: (A) *P =.046; (C) ***P =.0002 for WT vs “TLR9−/− + WT pDC”; *P =.049 for TLR9−/− vs TLR9−/−.
TLR9 is specifically required in pDCs for capsid-specific CD8+T-cell responses. (A) WT or BDCA2-DTR mice were IM injected with AAV2-SIINFEKL, and tetramer-positive cells were quantified over time (n = 4/group). BDCA2-DTR mice received DT intraperitoneally 3×/week. Statistically significant decrease compared with the response in WT mice is indicated. (B) Timeline of WT DC adoptive-transfer experiments. TLR9−/− mice (n = 4-5/group) were recipients of adoptively transferred WT pDC (3 × 106 cells) or WT cDC (5 × 106 cells), or nothing. On the same day, all mice were IM injected with AAV2-SIINFEKL. WT C57BL/6 mice served as a positive control. (C) Quantification of capsid-specific CD8+ T-cell responses over time in TLR9−/− mice that received either WT pDC, WT cDC, or nothing. The dotted line at 0.1% represents the limit of detection of capsid-specific CD8+ T cells using the tetramer. Data points are averages ± SEM. Statistically significant increases compared with responses in TLR9−/− mice that did not receive cell transfer are indicated. P values: (A) *P =.046; (C) ***P =.0002 for WT vs “TLR9−/− + WT pDC”; *P =.049 for TLR9−/− vs TLR9−/−.
To establish a specific role of the TLR9-MyD88 pathway in pDCs, we flow-sorted cDCs and pDCs from WT mice and adoptively transferred either population to TLR9−/− mice (Figure 5B; supplemental Figure 11). Following injection of AAV2-SIINFEKL, the CD8+ T-cell response to capsid was rescued in mice receiving WT pDCs, but not in TLR9−/− mice that received WT cDCs (Figure 5C). Taken together, our results lead us to conclude that both pDCs and cDCs are required for the CTL response to capsid. TLR9 activity in pDCs rather than cDCs provides the activation signal required to generate the capsid-specific CD8+ T-cell response, while cDCs are primarily responsible for antigen presentation. Consistent with this role, flow analysis of splenocytes harvested 1 hour after administration of fluorescently labeled AAV2 viral particles showed a more substantial uptake by cDCs than by pDCs (supplemental Figure 12).
Cross-presentation of AAV capsid depends on IFN I signaling
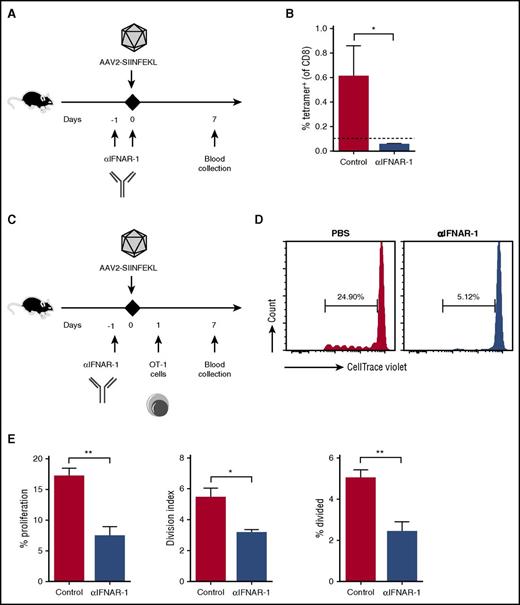
One characteristic of pDCs is the ability to produce IFN I in response to TLR signaling. Thus, to identify a potential link between the genome sensing in pDCs and presentation by cDCs, we investigated a requirement for IFN I signaling. WT mice were injected with a monoclonal antibody that blocks IFN-α/β signaling through the IFN I receptor (αIFNAR-1),30 which resulted in abrogation of the anti-capsid CD8+ T-cell response (Figure 6A-B). To test whether cross-presentation of capsid depended on IFN I signaling, αIFNAR-1 was injected into WT mice that in addition to AAV2-SIINFEKL received adoptively transferred OT-1 cells. Blocking the IFN I receptor substantially reduced proliferation of OT-1 cells in response to capsid (Figure 6C-E) but not to SIINFEKL peptide antigen (supplemental Figure 13).
Blocking the IFN I receptor eliminates capsid-specific CD8+T-cell responses. (A) Experimental timeline. WT mice received PBS or αIFNAR-1 antibody 1 day before IM injection of AAV2-SIINFEKL (n = 4/group). Capsid-specific CD8+ T-cell responses were assessed 7 days later. (B) Quantification of tetramer-positive cells from peripheral blood of control or treated mice. The dotted line at 0.1% represents the limit of detection of capsid-specific CD8+ T cells using the tetramer. (C) Experimental timeline of OT-1 adoptive-transfer experiments. WT mice received PBS or αIFNAR-1 antibody 1 day before IM injection of AAV2-SIINFEKL (n = 4/group). Mice were injected with 5 × 106 CTV-labeled OT-1 cells 1 day later. (D) Representative plots of proliferation of adoptively transferred OT-1 cells. (E) Quantification of the percent proliferation, percent divided (percent of original cells that divided at least once), and the division index (the average number of cells that a dividing cell became). Data points are averages ± SEM. P values: (B) *P =.04; (E) **P =.004 for percent proliferation, *P =.014 for division index, **P =.007 for percent divided.
Blocking the IFN I receptor eliminates capsid-specific CD8+T-cell responses. (A) Experimental timeline. WT mice received PBS or αIFNAR-1 antibody 1 day before IM injection of AAV2-SIINFEKL (n = 4/group). Capsid-specific CD8+ T-cell responses were assessed 7 days later. (B) Quantification of tetramer-positive cells from peripheral blood of control or treated mice. The dotted line at 0.1% represents the limit of detection of capsid-specific CD8+ T cells using the tetramer. (C) Experimental timeline of OT-1 adoptive-transfer experiments. WT mice received PBS or αIFNAR-1 antibody 1 day before IM injection of AAV2-SIINFEKL (n = 4/group). Mice were injected with 5 × 106 CTV-labeled OT-1 cells 1 day later. (D) Representative plots of proliferation of adoptively transferred OT-1 cells. (E) Quantification of the percent proliferation, percent divided (percent of original cells that divided at least once), and the division index (the average number of cells that a dividing cell became). Data points are averages ± SEM. P values: (B) *P =.04; (E) **P =.004 for percent proliferation, *P =.014 for division index, **P =.007 for percent divided.
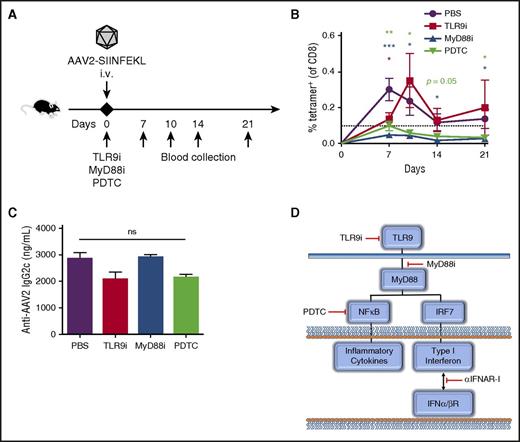
Blockade of innate immune pathways can prevent capsid-specific CTL responses
In contrast to skeletal muscle, capsid-specific CD8+ T cells often cause a complete loss of therapy in liver-directed gene transfer, as has been documented in gene therapy for hemophilia, unless immune suppression is applied.31 Therefore, we wanted to determine whether inhibition of specific points in the innate immunity pathways could prevent the response to IV vector administration (Figure 7A,D). Co-injection of an oligonucleotide that inhibits TLR9 (TLR9i) with AAV2-SIINFEKL transiently inhibited the formation of anti-capsid CD8+ T cells (Figure 7B). However, a peptide that blocks TLR-MyD88 interaction (MyD88i) had a more lasting effect (Figure 7B). To test for the effect of blocking signal transduction downstream of TLR9-MyD88, we investigated the inhibition of NF-κB signaling. Co-administration of pyrrolidine dithiocarbamate, a small molecule inhibitor of NF-κB, was similarly able to prevent the formation of tetramer-positive cells through 21 days post-injection (Figure 7B).32 Interestingly, none of these inhibitors were able to suppress antibody formation against capsid, further confirming independence of the humoral response from TLR signaling (Figure 7C).18 Together, these results show that specific inhibition of different steps in the innate response pathways could be of therapeutic value to prevent CD8+ T-cell activation (Figure 7D).
Inhibitors of TLR9 or its downstream signaling block the capsid-specific CD8+T-cell response. (A) Experimental timeline. TLR9i, MyD88i, pyrrolidine dithiocarbamate, or PBS were IV co-injected with 1 × 1011 vg AAV2-SIINFEKL into WT C57BL/6 mice. (B) Tetramer-positive CD8+ T cells were quantified over time in peripheral blood (n = 4/group). (C) Anti-AAV2 immunoglobulin 2c antibodies were quantified by enzyme-linked immunosorbent assay 2 months post-injection. (D) Cartoon indicating specific targets of the TLR9 signaling pathway blocked by the small molecule inhibitors and antibody used in this study. Data points are averages ± SEM. Statistical comparisons are made relative to mice that received only vector in PBS. ns, not significant; PDTC, pyrrolidine dithiocarbamate.
Inhibitors of TLR9 or its downstream signaling block the capsid-specific CD8+T-cell response. (A) Experimental timeline. TLR9i, MyD88i, pyrrolidine dithiocarbamate, or PBS were IV co-injected with 1 × 1011 vg AAV2-SIINFEKL into WT C57BL/6 mice. (B) Tetramer-positive CD8+ T cells were quantified over time in peripheral blood (n = 4/group). (C) Anti-AAV2 immunoglobulin 2c antibodies were quantified by enzyme-linked immunosorbent assay 2 months post-injection. (D) Cartoon indicating specific targets of the TLR9 signaling pathway blocked by the small molecule inhibitors and antibody used in this study. Data points are averages ± SEM. Statistical comparisons are made relative to mice that received only vector in PBS. ns, not significant; PDTC, pyrrolidine dithiocarbamate.
Discussion
Pattern recognition of AAV vector genome shapes CD8+ T-cell responses against capsid
The crosspriming mechanism that generates CD8+ T-cell responses against viral capsid involves innate immune sensing of the viral genome specifically by 1 subset of DCs (pDCs), thereby providing the signal that induces presentation by another subset (cDCs). Hence, pDCs and cDCs need to cooperate to generate an MHC I-restricted T-cell response against the structural protein component of the viral particle (supplemental Figure 14). TLR9 signaling in general is more connected to activation of CD8+ T cells than to B-cell responses against AAV.13,15,18,19 This pathway appears to be uniquely responsible for inducing capsid-specific CD8+ T cells, whereas an alternative activation signal via TLR4 cannot compensate for the lack of TLR9. The requirement for a specific PRR is in contrast to redundant sensing mechanisms that can shape the response to several more complex viruses such as influenza, adenovirus, or lentivirus.33-35
Cooperation between pDCs and cDCs achieves crosspriming of CD8+ T cells
Others have shown that TLR activation in DCs upon phagocytosis of bacteria leads to enhanced cross-presentation of bacterial antigens by MHC I as a result of increased recruitment of MHC I from the endosomal recycling compartment.36 Through this mechanism, sensing of the invading pathogen is directly linked to optimal presentation of pathogen-derived antigens within the same DC. Here, we show that a more complex mechanism has evolved in the antiviral response, in which 2 cell types need to cooperate to sense the viral particle via a TLR, present the viral antigen, and activate a CD8+ T-cell response. Recently, Brewitz et al found that optimal priming of the antiviral CD8+ T-cell response against an antigen expressed from vaccinia virus (an enveloped DNA virus of the poxvirus family) upon cross-presentation by cDCs also required activation via pDCs.37 Initial interactions between cDCs and CD8+ T cells attracted pDCs by inducing their migration through stimulation of their CCR5 receptor with chemokines. We find that, in addition, the pDCs themselves need to be activated via PRR stimulation. Intact TLR9-MyD88 pathway in cDCs could not compensate for a lack of this pathway in pDCs, which implies that cDCs are unable to sense the AAV particle and/or provide an activation signal. Conversely, pDCs with TLR9-MyD88 were sufficient to rescue the ability of TLR9−/− mice to prime capsid-specific CD8+ T cells.
Crosspriming of CD8+ T cells entails co-stimulation in addition to antigen presentation following uptake and processing of viral particles. These events require activation signals, a process also known as “DC licensing.” The sum of our data suggests that IFN I production in response to TLR9 signaling in pDCs is a major component of the mechanism that leads to licensing of cDCs by AAV. pDCs produce large amounts of IFN I upon activation of their endosomal TLR7 or TLR9 sensors of nucleic acids, but also have important immune regulatory functions.29 Evidence for crosstalk between pDCs and cDCs has been obtained in other experimental models.38-42 Based on results with other viruses, we hypothesize that XCR1+/CD8α+ cDCs cross-present capsid antigen, whereas XCR1− cDCs may initially capture AAV particles.37
TLR9-dependent cross-presentation of capsid antigen
Classical MHC I presentation of endogenous peptides takes place after proteosomal degradation of proteins synthesized and ubiquitinated in a cell. Cross-presentation occurs when exogenous antigen, initially taken up into phagosomes or endosomes, is translocated to the cytoplasm and shunted into the proteasomal degradation pathway.43,44 In order to infect its target cells following receptor-mediated entry, AAV has to escape the endosome, which is facilitated by a phospholipase A2 activity in the viral protein 1 capsid protein that is exposed upon acidification in the endosome.45 Endosomal escape is required for cross-presentation of capsid antigen by hepatocytes.21,46,47 Removal of phosphorylation sites on the capsid reduces ubiquitination and thus MHC I presentation, thereby reducing the likelihood of transduced hepatocytes being targeted by CD8+ T cells.20,48-50 However, such engineered capsids still activate CD8+ T cells.20 Therefore, cross-presentation by cDCs is less influenced by capsid phosphorylation but rather by innate activation signals. The data suggest a lesser requirement of pDCs for cross-presentation than for crosspriming (whereas TLR9 and IFN I are required for both). Depletion of pDCs is not absolute in all compartments in the DT-induced model. However, we cannot rule out some contribution by other cell types to TLR9 signaling and IFN I production. A possible scenario is a basal level of initial cross-presentation by cDCs, which leads to recruitment of pDCs that, upon activation via TLR9, upregulate cross-presentation and co-stimulation by cDCs to achieve CD8+ T-cell priming.
Critical role for IFN I
We find that both cross-presentation of AAV capsid and priming of capsid-specific CD8+ T cells can be effectively blocked with α-IFNα/βR. The antibody had no effect on T-cell proliferation to soluble peptide antigen and therefore did not directly inhibit CD8+ T cells. We propose that cDCs process AAV capsid antigen in response to IFN I (triggered by TLR9 activation in pDCs). In trans activation may be direct or indirect, possibly involving additional cell types, such as NK cells. The literature has been controversial about the effects of IFN I, which some studies suggest merely promotes crosspriming (by causing upregulation of co-stimulatory molecules), whereas others suggest that IFN I can also induce cross-presentation.51-53 However, this may be dependent on the nature of the antigen, such as soluble protein vs particulate antigens. The role of IFN I in promoting cross-presentation of AAV capsid antigen and crosspriming of CD8+ T cells is likely localized to areas of DC–T-cell interactions, because we found no evidence for systemic increases in IFNα/β levels (data not shown). Interestingly, Nathwani et al successfully suppressed CD8+ T-cell responses to capsid in humans using steroids.6,7 This may not merely reflect a direct effect on the T cells, but also reduced cross-presentation and/or priming due to inhibited IFN I production.54
Implications for gene therapy
Because mice do not show the cytolytic response and related hepatotoxicity seen in humans, it has been underappreciated that AAV gene transfer in mice nonetheless results in crosspriming of capsid-specific CD8+ T cells (for both IM and IV routes). Therefore, we were able to use mouse models to study the crosspriming mechanism. Thus far, investigations have focused mostly on AAV2, although other capsids are also cross-presented.13,15,19,22 Interestingly, native AAV8 epitopes are not presented, at least in some mouse strains. However, inclusion of SIINFEKL showed that mice do process the antigen and can in principle present AAV8-derived epitopes.55 AAV-transduced mouse hepatocytes can be killed by adoptive transfer of ex vivo expanded capsid-specific CD8+ T cells.20 These experiments suggested that limited cross-presentation of capsid by hepatocytes is a reason why AAV-primed CD8+ T cells normally fail to target the liver in mice, although in situ negative regulatory pathways may also contribute.56 Further development of the model introduced here, perhaps in combination with gene transfer in PD-L1–deficient mice may overcome these limitations.
It has been difficult to reconcile the perceived ineffectiveness of AAV vectors to activate DCs with the T-cell responses documented in patients. Our data show vector dose-dependent crosspriming of capsid-specific CD8+ T cells through the coordinated activation of 2 DC subsets upon innate sensing of the viral genome. Although AAV elicits only weak innate inflammation, it nonetheless activates a mechanism that appears common to diverse antiviral responses.37 Conversely, because innate activation signals are less diverse than for more complex viruses, blockage of a single set in the innate pathway can prevent T-cell activation. In particular, use of MyD88i to inhibit the relay of the TLR signal to its cytoplasmic adapter and blockage of downstream pathways (IFN I and classical NF-κB pathways) were effective.15,57 Such more targeted interventions may eventually replace current general immune suppression.6,7,11,58 Direct antagonists of nucleic acid-sensing TLRs are in clinical trails for autoimmune diseases.59 However, oligodeoxynucleotides still have limited stability and half-life, which may explain why we observed only transient suppression.60 Repeat administration or using MyD88i instead, may be required. One should also consider that in humans, CD8+ T-cell responses against AAV capsid tend to emerge later than in mice and may also reflect memory responses, which may have differential requirements for activation (but still require some of these pathways).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases grant R01 AI51390 (R.W.H.), and National Institutes of Health, National Heart, Lung, and Blood Institute grants R01 HL097088 (R.W.H. and A. Srivastava) and R01 HL131093 (R.W.H. and C.T.). G.L.R. was supported as a fellow on training grant T32 AI 007110 from the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Authorship
Contribution: G.L.R., J.L.S., I.Z., S.R.P.K., A. Sherman, G.Q.P., E.B.-T., and M.B. performed experiments; G.L.R., A. Srivastava, M.A.W., C.T., M.B., and R.W.H. designed experiments; G.L.R., J.L.S., B.E.H., M.A.W., C.T., M.B., and R.W.H. analyzed and interpreted data; G.L.R., J.L.S., M.A.W., C.T., M.B., and R.W.H. wrote the manuscript; and R.W.H. supervised the study.
Conflict-of-interest disclosure: R.W.H. received royalty payments from Spark Therapeutics for license of AAV-FIX technology. A. Srivastava holds issued patents related to AAV gene therapy that have been licensed to Applied Genetic Technology Corporation. The remaining authors declare no completing financial interests.
Correspondence: Roland W. Herzog, Department of Pediatrics, Division of Cellular and Molecular Therapy, University of Florida, 2033 Mowry Rd, CGRC, Room 203, Gainesville, FL 32610; e-mail: rherzog@ufl.edu; and Moanaro Biswas, Cancer and Genetics Research Complex, University of Florida, 2033 Mowry Rd, Gainesville, FL 32610; e-mail: narobiswas@ufl.edu.