Key Points
Translocation t(11;14) confers a favorable prognosis in AL amyloidosis patients treated with HDM.
Abstract
Cytogenetic aberrations detected by interphase fluorescence in situ hybridization (iFISH) of plasma cells are routinely evaluated as prognostic markers in multiple myeloma. This long-term follow-up study aimed to assess the prognosis of systemic light chain amyloidosis (AL) patients treated with high-dose melphalan (HDM) chemotherapy and autologous stem cell transplantation, depending on iFISH results. Therefore, we analyzed a consecutive cohort of 123 AL patients recruited from 2003 to 2014. HDM was safe, with only 1 of 123 patients dying as a result of treatment-related mortality, and effective, with a complete remission (CR) rate of 34%. Translocation t(11;14) as the most prevalent aberration (59%) led to an improved CR rate after high-dose therapy (41.2% vs 20.0%; P = .02), translating into a prolonged hematologic event-free survival (hemEFS; median, 46.1 vs 28.1 months; P = .05) and a trend for better overall survival (median, not reached vs 93.7 months; P = .07). In multivariate analysis, t(11;14) was confirmed as a favorable prognostic factor regarding hemEFS along with lower values for the difference between involved and uninvolved free light chains. Conversely, deletion 13q14, gain of 1q21, and hyperdiploidy had no significant prognostic impact. The high-risk cytogenetic aberrations t(4;14), t(14;16), and del(17p13) conferred an unfavorable prognosis, although statistical significance was reached only for univariate CR analysis in this small group of 9 patients. Thus, t(11;14) positivity in HDM-treated AL patients conferred superior CR rates and hemEFS. In view of the reduced response of t(11;14) to bortezomib, this highlights the impact of therapy on the prognostic role of cytogenetic aberrations.
Introduction
Systemic light chain amyloidosis (AL) is caused by the toxic effects and deposition of misfolded light chains, which are produced by monoclonal bone marrow plasma cells. Therapeutic strategies aim to eradicate this underlying plasma cell dyscrasia.
After the efficacy and feasibility of high-dose melphalan (HDM) chemotherapy with subsequent autologous stem cell transplantation had been demonstrated for the related plasma cell dyscrasia multiple myeloma (MM), HDM was also established for AL by clinical trials performed in the early 1990s.1-4 These trials showed the efficacy of this therapy in AL. However, the treatment-related morbidity and mortality risk was higher than in MM as a result of the organ impairment inherent in AL.2,3 At the time, HDM was the only effective treatment to target the underlying plasma cell dyscrasia. The therapeutic options have now been increased by the establishment of the melphalan-dexamethasone protocol,5 by the introduction of proteasome inhibitors such as bortezomib6,7 and immunomodulatory drugs such as lenalidomide,8,9 and by their use in combination therapies.10-13 However, HDM offers the prospect of long-term remission in AL.14-16 Therefore, it remains a therapeutic option for carefully selected AL patients at specialized centers,15 although controversy regarding its pros and cons persists.17
In MM, cytogenetic aberrations are established as prognostic markers for risk stratification.18-20 It was the aim of this long-term follow-up study to identify cytogenetic AL patient subsets21-24 that benefit most from HDM. For this purpose, we assessed the impact of cytogenetic aberrations as detected by interphase fluorescence in situ hybridization (iFISH) in a large cohort of AL patients homogeneously treated with HDM.
Patients and methods
Patients
The Institutional Review Board of the University of Heidelberg approved the study. This retrospective study includes all AL patients initially deemed eligible for HDM at our amyloidosis center from February 2003 to May 2014. During this study period, a total of 173 patients were initially considered eligible for HDM. Among these 173 patients, iFISH cytogenetic testing results were available for 140 patients. Of this group, 123 patients ultimately proceeded to HDM and were used as the study cohort for statistical analysis. The patients in this group had not been included in any previous prognostic studies by our group. Among the remaining 33 AL patients without prior iFISH cytogenetic testing, 26 of 33 proceeded to HDM and were also assessed to rule out a bias by performance of cytogenetic testing. Patients with symptomatic MM or immunoglobulin M (IgM) gammopathy were not considered for this study because of their different biology.
Criteria that deemed patients eligible for HDM were age ≤70 years, New York Heart Association stage ≤2 and no symptomatic pleural effusion, a systolic blood pressure of >90 mmHg, and no threatening renal failure or preexisting dependency on dialysis. The study also included 10 patients with severe cardiac involvement who had received a heart transplant and subsequently proceeded to HDM.
Outcome assessment
Remission status was determined according to consensus criteria, which defined the categories of complete remission (CR), very good partial remission (VGPR), partial remission (PR), and no response.25 Accordingly, negativity of immunofixation in both serum and urine was required along with a normalized difference between involved and uninvolved free light chain (dFLC) ratio to meet the criteria for CR. After HDM, patients were typically followed up on a 3-month to 6-month basis, and the best remission was recorded. Overall survival (OS) and hematologic event-free survival (hemEFS) were calculated starting from the day of transplantation. For hemEFS, hematologic relapse, progression, initiation of a new therapy, or death (whichever came first) were defined as events. Treatment-related mortality (TRM) was defined as death resulting from adverse effects of treatment and was deemed unrelated to AL itself.
iFISH cytogenetic testing
FISH was performed as described previously.20,24 After plasma cell purification by auto-magnetic-activated cell sorting with CD138 immunobeads, iFISH was performed with commercial 2-color probe sets according to the manufacturer’s instructions (Kreatech, Amsterdam, The Netherlands, and MetaSystems, Altlussheim, Germany). The tested panel included IgH translocations t(11;14), t(4;14), and t(14;16) as well as probes for detecting numerical changes of the loci 1q21, 5p15, 5q35, 8p21, 9q34, 13q14, 15q22, 17p13, and 19q13. As in previous analyses, t(4;14), t(14;16), and deletion 17p13 were classified as high-risk aberrations in analogy to MM.18 Gains of 5p15/5q35, 9q34, and 15q22—whenever 2 of 3 were present—were categorized as hyperdiploidy according to the score by Wuilleme et al.26
Statistical analysis
Pairwise comparisons of clinical and hematologic factors with respect to chromosomal aberrations were performed by using Wilcoxon’s rank sum test for continuous factors and Fisher’s exact test for categorical factors. Remission rates for chromosomal subgroups were compared by using Fisher’s exact test.
Survival distributions for OS and hemEFS were estimated by using the Kaplan-Meier method and compared by using the log-rank test. When comparing survival curves, the P values of the corresponding log-rank tests were reported. Median follow-up time was estimated with the reverse Kaplan-Meier method.27 Additional univariate and multivariate analyses of OS and hemEFS were performed by using Cox proportional hazards regression models; additional univariate and multivariate analyses of remission were performed by using a logistic regression model. The log-transformed values of dFLC were used in all statistical analyses. Multivariate regression models were fit to the set of complete cases (n = 110 patients). All statistical tests were 2-sided and used a significance level of 5%. Hazard ratios and odds ratios were estimated with 95% confidence intervals (95% CIs). Statistical analysis was performed by using the statistical software environment R, version 3.1.3.
Results
Patients and treatment
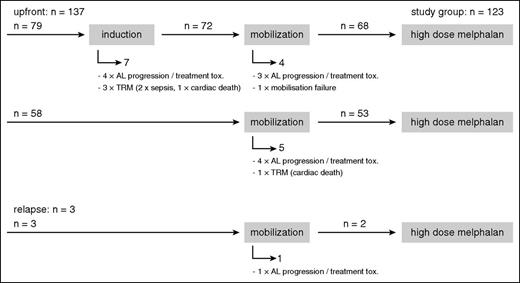
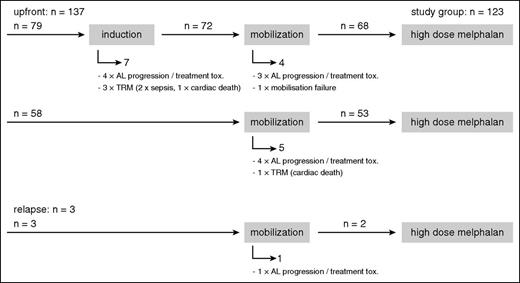
Most patients proceeded from induction to mobilization chemotherapy with subsequent stem cell harvest and finally to HDM.28 For the group with available iFISH results (n = 140), the CONSORT diagram (Figure 1) gives an overview of the number of patients in the respective therapy phases and the 17 patients who dropped out before HDM. In all, 12 patients were withdrawn from the HDM protocol because of AL progression or poor tolerability of induction or mobilization treatment, which led to the assessment that HDM had become too risky or that the patients no longer fulfilled the HDM eligibility criteria. One patient was unable to mobilize stem cells. These patients were switched to conventional chemotherapy protocols instead. Another 4 patients died from TRM, with 2 cases of sepsis and 2 cases of sudden cardiac death.
CONSORT diagram. Numbers of patients during the induction, mobilization, and HDM phase of the study for all patients with available iFISH results and reasons why patients dropped out during the induction and mobilization phase before HDM.
CONSORT diagram. Numbers of patients during the induction, mobilization, and HDM phase of the study for all patients with available iFISH results and reasons why patients dropped out during the induction and mobilization phase before HDM.
Of the 123 patients who ultimately received HDM, 121 received it as part of first-line treatment. Two patients had suffered hematologic progression after bortezomib-based therapy (within 12 months of first diagnosis) and were classified to receive HDM as relapse treatment.
In the cohort that received transplantation (n = 123), 70 patients received an induction therapy consisting of a median of 3 cycles (range, 1 to 7 cycles). The induction was steroid based in 38 of these patients, mostly dexamethasone monotherapy (n = 33), but also vincristine-doxorubicin-dexamethasone (VAD; n = 2), melphalan-dexamethasone (n = 2), or melphalan-prednisone (n = 1). In the other 32 patients, the induction was bortezomib based, mostly bortezomib-dexamethasone (n = 27), bortezomib-cyclophosphamide-dexamethasone (n = 4), or bortezomib-doxorubicin-dexamethasone (n = 1). The remaining 53 patients received no induction treatment. Obviously, the choice of induction strategy was heterogeneous over time, given that patients were recruited over a period of 11 years. The earliest patients were treated within the HD-AL-2 trial,29 which by protocol provided 3 induction cycles of dexamethasone. After completion of the HD-AL-2 trial in 2008, most patients received no induction. However, once the novel agent bortezomib became available in Germany in 2008, it was offered to patients with high dFLC or M-protein gradient at diagnosis.
Before stem cell harvest, the majority of patients were treated with a mobilization chemotherapy regimen to obtain a better stem cell yield. Thereby, either the CAD regimen (cyclophosphamide 1000 mg/m2, doxorubicin 4 × 15 mg/m2, and dexamethasone 4 × 40 mg [n = 85]) or the Cyclo-Dex regimen (cyclophosphamide 2 × 1000 mg/m2 and dexamethasone 2 × 20 mg [n = 16]) were used. In another 21 patients, including all patients who had already had a heart transplantation, stem cells were harvested after stimulation with granulocyte colony-stimulating factor alone. One patient received a transplant harvested from a syngeneic twin.
HDM chemotherapy was administered at 2 × 100 mg/m2 (standard dose) on 2 successive days. The dose was adjusted for impaired renal function in 33 patients. Only 7 patients received a reduced dose because of relevant cardiac involvement or age. Accordingly, adherence to full dosage was high. None of the patients received any kind of maintenance therapy.
The clinical characteristics of the HDM-treated study cohort with available iFISH cytogenetics at first diagnosis (n = 123) are shown in Table 1. They closely reflect the eligibility criteria for HDM. Considering general fitness and severity of organ involvement, the study cohort represents a favorable risk group. Mayo stages I and II prevailed, and only a few patients met Mayo stage III criteria.30 The median dFLC of 143.5 mg/L in the study group was below the published cutoff of 180 mg/L31 and well below the median value observed for non-transplant cohorts at our center (181 mg/L in the melphalan-dexamethasone cohort32 and 317 mg/L in the bortezomib study33 ).
Distribution of cytogenetic aberrations
Characteristically for AL, t(11;14) was by far the most prevalent cytogenetic aberration with 72 (59%) of 123 patients being positive, followed by del(13q14) in 36 (29%) of 123 and gain of 1q21 in 25 (22%) of 116 (Table 2). As expected, hyperdiploidy and high-risk aberrations were less prevalent with 16 (14%) of 111 and 9 (7%) of 122, respectively.
Table 2 also lists the cytogenetic aberrations of the 17 patients who did not reach HDM and dropped out during the induction or mobilization phase. The frequencies of cytogenetic aberrations, including the t(11;14) rate with 9 (53%) of 17, are comparable to those of the group who received a transplant.
The distribution of hematologic and clinical parameters between the t(11;14)-positive and t(11;14)-negative group is shown in supplemental Table 1 (available on the Blood Web site). The groups were well balanced with the exception of the association of t(11;14) with an earlier stage of gammopathy (AL only vs AL + MM I) previously reported by our group.24 However, with the newly implemented 10% plasmocytosis cutoff34 instead of the 30% cutoff,2 there was no longer a statistically significant imbalance.
Remission rates before HDM
Among the 70 patients who received induction therapy, 2 (3%) of 69 evaluable patients had attained CR at the time of HDM, and both belonged to the dexamethasone-based induction group. The overall VGPR or better rate was 7 (13%) of 53 evaluable patients (13% in the dexamethasone-based induction group and 13% in the obviously negatively selected bortezomib-based induction group with higher dFLC counts), and the rate for PR or better was 20 (38%) of 52 (30% and 45%, respectively). There were no statistically significant differences among cytogenetic groups. In detail, the t(11;14)-positive group had a CR rate of 0 (0%) of 39, a VGPR or better rate of 2 (6%) of 31, and a PR or better rate of 9 (29%) of 31. The t(11;14)-negative group had a CR rate of 2 (7%) of 30, a VGPR or better rate of 5 (23%) of 22, and a PR or better rate of 11 (52%) of 21. In the bortezomib induction subgroup (n = 32), the VGPR or better rate was 4 (13%) of 30, and the PR rate was 13 (45%) of 29. Again, the VGPR or better rate and the PR or better rate were lower in the t(11;14)-positive group with 2 (11%) of 19 and 7 (37%) of 19 compared with the t(11;14)-negative group with 2 (18%) of 11 and 6 (60%) of 10, respectively.
Remission rates after HDM
Given the decisive role of attained CR for prognosis in HDM-treated AL patients,14,16,35-38 remissions after HDM were analyzed on a CR vs non-CR basis. Two patients achieved CR after induction, 1 patient after mobilization chemotherapy, and 37 patients after HDM, which leads to an overall CR rate after HDM of 40 (34.2%) of 117 for evaluable patients. Patients who were t(11;14) positive had a higher CR rate in response to HDM than patients who were t(11;14) negative with 28 (41.2%) of 68 vs 9 (20.0%) of 46 (P = .02; supplemental Table 2). Other cytogenetic aberrations had no significant impact on the prospects of attaining CR, with CR rates of 7 (21.2%) of 33 for del(13q14) (P = .13) and 5 (21.7%) of 23 for gain of 1q21 (P = .32). No statistically significant effect was observed for hyperdiploidy, when the Wuilleme score was applied (4 [26.7%] of 15; P = .77) or when the individual probes 5p15/5q35, 9q34, 15q22, and 19q13 were assessed. The CR rate of 0 (0%) of 9 for high-risk patients was statistically significantly inferior to that of other patients in this study (P = .03).
HemEFS
With a median follow-up time of 68.5 months, median hemEFS for the overall study group was 32.1 months. Patients harboring t(11;14) had superior hemEFS (median, 46.1 vs 28.1 months; P = .05; Figure 2). This favorable effect of t(11;14) on hemEFS after HDM could also be discerned within the bortezomib induction subgroup (28.2 vs 14.4 months; P = .05). Median hemEFS for patients with vs without del(13q14) was 27.6 vs 33.7 months (P = .79), for patients with vs without gain of 1q21 was 31.1 vs 44.0 months (P = .13), and for patients with hyperdiploidy as defined by the Wuilleme score vs without hyperdiploidy was 31.1 vs 36.0 months (P = .37). No significant differences were detectable for the individual hyperdiploidy probes (data not shown). Patients with high-risk aberrations had a median hemEFS time of 28.1 months (P = .30) vs 32.4 months for patients without high-risk aberrations.
hemEFS and OS of the study cohort depending on iFISH results. (A,C,E,G,I) hemEFS curves and (B,D,F,H,J) OS curves for (A-B) t(11;14), (C-D) del(13q14), (E-F) gain of 1q21, (G-H) hyperdiploidy, and (I-J) high-risk aberrations. pos, positive; pts, patients.
hemEFS and OS of the study cohort depending on iFISH results. (A,C,E,G,I) hemEFS curves and (B,D,F,H,J) OS curves for (A-B) t(11;14), (C-D) del(13q14), (E-F) gain of 1q21, (G-H) hyperdiploidy, and (I-J) high-risk aberrations. pos, positive; pts, patients.
OS
With a median follow-up time of 69.2 months, median OS for the overall study group was 128.8 months. The overwhelming number of deaths was attributable to progression of AL (n = 24) or adverse effects of treatment for relapse (n = 4). Only 1 patient died as a result of HDM TRM when succumbing to pneumonia and sepsis on day +55 after HDM. This was also the only case of mortality by day +100 after HDM. Another 3 patients suffered death unrelated to progression of AL, with 1 case each of septic cholecystitis in CR after 16.4 months, heart transplant rejection after 3.9 months, and amyotrophic lateral sclerosis after 27.2 months.
Patients with t(11;14) had a trend for a longer OS (median, not reached vs 93.7 months; 5-year OS: 78.8% [95% CI, 67.7%-89.8%] vs 67.3% [95% CI, 53.0%-81.6%]; P = .07; Figure 2). Del(13q14), gain of 1q21, and hyperdiploidy had no significant impact on OS (del(13q14) vs no del(13q14): 128.8 months vs not reached; P = .10; gain of 1q21 vs no gain of 1q21: not reached vs 128.8 months; P = .93; and hyperdiploidy by Wuilleme score vs no hyperdiploidy: 90.6 vs 128.8 months; P = .84; individual hyperdiploidy probes: data not shown). Median OS was reduced to 47.4 months vs not reached in high-risk patients (5-year OS: 33.3% [95% CI, 0%-71.1%] vs 76.5% [95% CI, 67.7%-85.3%]), although statistical significance was not attained in this small subgroup (P = .06).
Multivariate analysis
For multivariate analysis, the cytogenetic aberrations t(11;14) and gain of 1q21 were tested along with age at transplantation, light chain restriction, dFLC, Mayo score, and modification of diet in renal disease as the relevant determinants of AL disease; effective reduction of melphalan dosage was introduced as the major therapy-related variable. The Mayo score was set at 1 for the 10 patients with prior heart transplant. Application of induction therapy was used as a stratifying variable to avoid selection bias. The univariate analysis of individual variables with respect to hemEFS and OS is provided in supplemental Table 3. As in univariate analysis, translocation t(11;14) showed a statistically significant favorable effect in multivariate analysis regarding hemEFS (P = .014) although not with respect to OS (P = .14; Table 3). The significance regarding hemEFS was retained in the bortezomib induction subgroup (hazard ratio, 0.12; 95% CI, 0.02-0.79; P = .03). As for the other variables, dFLC was a prognostic risk factor for both hemEFS and OS (P = .002 each). Effectively reduced melphalan dosage was associated with shortened hemEFS (P = .006) although not with OS. In addition, we performed Cox regression models with Firth correction, which implement penalization terms for the number of factors.39,40 This model yielded results very similar to the unpenalized regression models above, again confirming the significance of t(11;14) and effective melphalan dose reduction regarding hemEFS (P = .016 and P = .001, respectively) and of dFLC regarding both hemEFS and OS (P = .001 each).
In the multivariate analysis for CR after HDM, only lower dFLC was significantly associated with a higher CR rate (P = .03), whereas statistical significance was slightly missed for t(11;14) positivity (P = .08) and κ light chain restriction (P = .08) (supplemental Table 4).
Landmark analysis addressing the value of attaining CR after HDM
Landmark analyses were performed starting at 15 months after HDM. They showed a strongly improved prognosis for both hemEFS and OS for those patients who had attained CR by this time point (P < .001 and P = .007, respectively). The prognostic benefit of attaining CR was particularly strong in the t(11;14)-positive group (P = .002 and P = .046, respectively) but was also visible in the t(11;14)-negative group (P = .13 and P = .11, respectively).
Patients without available iFISH results
The prognoses for the 26 HDM-treated patients without available iFISH results were comparable to those of the study cohort with available iFISH results. There were no statistically significant differences between groups regarding CR rate (44.0% vs 35.9%; P = .50), hemEFS (median, 30.1 vs 32.1 months; P = .98), and OS (median, 83.2 vs 128.8 months; P = .19). Thus, patient selection by performed iFISH testing did not seem to produce a selection bias.
Discussion
The overall OS rates in this study are encouraging with a respectable proportion of long-term survivors in hematologic CR. We observed CR rates in the 30% to 40% range, similar to those previously reported by other groups.2,15,16,36,37,41,42 TRM after HDM was very low with a single death. Because eligibility criteria for HDM at our institution are comparable to those of other centers,15 our study is representative and corroborates the value of HDM as a treatment option for selected eligible patients at experienced centers, even in the era of novel agents. The majority of patients received induction therapy, on the basis of our experience with 2 successive HDM trials.29,43 The pretransplant TRM was at an acceptable level with 4 of 140 patients affected. However, 12 patients could not proceed to HDM because of AL progression or treatment toxicity during the induction or mobilization phase, although these patients could be switched to alternative therapies.
CR rates after HDM were 2 times higher in patients harboring t(11;14). As expected, given the repeatedly confirmed strong favorable prognostic impact of attaining CR,14-16,35-38 and as proven by a landmark analysis in this study, the higher CR rates in the t(11;14)-positive group also translated into statistically superior hemEFS. Notably, the favorable hemEFS of the t(11;14)-positive group was retained in the multivariate analyses when tested along with the relevant hematologic and clinical baseline variables. The choice of cofactors was guided by the comprehensive multivariate models developed by other AL centers.4,14,15,35-37,44 Statistical significance for OS was missed, likely because of access to bortezomib and lenalidomide in second-line therapy, which improved the prognosis of patients with an unresponsive or relapsing plasma cell dyscrasia after HDM.
We are convinced that the favorable prognosis of t(11;14) truly reflects the responsiveness of the t(11;14)-positive clone to HDM for several reasons. First, it lies in the very nature of the HDM group that it is selected for the clinically fittest patients. Thus, the severity of organ involvement plays a lesser role than in other AL patient cohorts. In the long run, the prognosis of the HDM group is mostly determined by the biology of the clonal plasma cells and only to a lesser degree by the severity of organ involvement. This is also corroborated by the multivariate analysis, in which plasma cell factors (iFISH cytogenetics and dFLC) prevail over risk factors of organ involvement such as cardiac biomarkers. Second, only patients who actually received HDM were considered for statistical analysis. Thus, the response assessment is not distorted by patients who did not reach HDM. Actually, the rate of t(11;14)-positive patients is slightly lower in the drop-out group with 9 (53%) of 17 as compared with the HDM-treated group with 72 (59%) of 123, which strongly argues against a selection bias in favor of t(11;14) brought in by the preceding induction and mobilization chemotherapy. Third, deaths as a result of HDM TRM or unrelated to AL progression were exceptionally rare with only 4 patients affected. Thus, the outcome in this study is not seriously distorted by adverse effects of HDM. Fourth, we strongly adhered to a full dosage of melphalan, which argues against a relevant bias by dose reductions well known to happen at the expense of efficacy.4,15,36,44 To summarize, the outcome of this HDM study largely reflects the biology of the plasma cell clone, its responsiveness to HDM, and its recurrence risk.
When viewing this HDM study in conjunction with our previously analyzed melphalan-dexamethasone cohort,32 which had received melphalan at standard dose of 16 mg/m2 intravenously per cycle,5,45 some parallels are obvious. In both studies, t(11;14) confers an intermediate to favorable prognosis (landmark analysis of melphalan-dexamethasone: OS, 49.9 vs 18.0 months; P = .06). However, the responsiveness of t(11;14) to the melphalan-based regimens contrasts with the adverse prognostic effect of t(11;14) in bortezomib-treated cohorts previously reported by our group.33 Nevertheless, we do not regard the 2 melphalan studies as contradictory to the bortezomib study. First, melphalan and bortezomib are distinct drugs with biologically different mechanisms of action. Second, in the bortezomib induction subgroup of this HDM study, the t(11;14)-positive patients had a poorer response to bortezomib induction in line with our previous bortezomib study, and this disadvantage of t(11;14)-positive patients was reversed only after HMD. Third, therapy assignment has introduced a selection, since the HDM and bortezomib cohorts represent opposite sides of the clinical spectrum in AL. Thus, the prognostic impact of cytogenetic aberrations in AL largely depends on the administered therapy. A Mayo clinic study had shown a negative prognostic effect of t(11;14),46 a finding later confirmed in a larger study cohort for the subgroup with bone marrow plasmocytosis at ≤10%.47 Because these 2 Mayo Clinic studies did not stratify for treatment groups, we do not deem them contradictory to our data.
In the HDM and the melphalan-dexamethasone cohorts, the cytogenetic high-risk features t(4;14), t(14;16), or del(17p13) conferred early hematologic progression and a shortened OS, although the small number of high-risk patients precluded definite conclusions in both respective cohorts. However, gain of 1q21, which proved to be an adverse risk factor in the melphalan-dexamethasone cohort, had no prognostic effect in the HDM cohort. It is unclear whether gain of 1q21 loses its adverse prognostic role in the setting of HDM or whether the lack of significance for hemEFS is a result of the lack of statistical power for these rarer abnormalities, a problem that also applies to del(13q14).
Although the gammopathies in MM and AL share common cytogenetic aberrations and patterns in the oncogenetic tree model,23,24,48 the plasma cell dyscrasia in AL is nevertheless biologically distinct from that in MM. First, the underlying gammopathy in AL is at an earlier stage, typically at the monoclonal gammopathy of undetermined significance level,2 because it is rather the amyloidogenic properties of the light chains (the unlucky protein) than the aggressiveness of the clonal plasma cells that leads to symptomatic disease.23,24,37 Second, the frequencies of the respective cytogenetic aberrations differ, with t(11;14) as the prevailing aberration detectable in about half of AL patients,21-23,46,47 whereas hyperdiploidy is rare, and high-risk aberrations such as t(4;14), t(14;16), and del(17p13) are scarce.23,46,47 Third, the different biology of the gammopathies in MM and AL is also highlighted by the discrepant prognosis. The long-term prognosis of AL patients who have received transplants was proven superior to that of their MM counterparts in a head-to-head comparison with higher CR rates of 40% vs 29% and longer OS of 113 vs 59.5 months.37 Thus, serologic CRs in AL appear to be more precious and of a more enduring quality than those in MM.16 In MM treated with HDM, t(11;14) has been consistently shown to confer an intermediate to favorable prognosis.49,50 Conversely, t(4;14), t(14;16), and del(17p13) were subsumed as high-risk aberrations because of their poor outcome after an HDM-containing regimen.18 Thus, this AL study recapitulates prognostic effects of cytogenetic aberrations known from HDM-treated MM patients. This result was not necessarily expected, given that the plasma cell dyscrasia in AL is biologically distinct and more vulnerable to HDM as discussed above.
In conclusion, in this long-term follow-up study in AL, patients harboring t(11;14) benefitted most from HDM. In agreement with our previously published melphalan-dexamethasone cohort,32 t(11;14) seems to be responsive to melphalan-based therapy in general. Pending confirmation by other groups, iFISH cytogenetics may thus help to refine first-line therapy recommendations in AL.
Presented at the Annual Meeting of the German Society of Hematology/Oncology, Hamburg, Germany, October 10-14, 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by grants from the Dietmar-Hopp foundation “Heidelberger Konzept zur Optimierung der Diagnostik und Therapie des Multiplen Myeloms” (H.G.) and the Federal Ministry of Education and Research (GERAMY) (A.J. and S.O.S.).
Authorship
Contribution: T.B., U.H., and S.O.S designed the research; U.H., S.O.S., T.B., C. Kimmich, H.G., P.D., and A.D.H. treated patients; T.B. and U.H. collected the data; T.B., U.H., C. Kimmich, P.D., A.D.H., and S.O.S. analyzed and interpreted data; A.S., D.H., and H.G. performed plasma cell enrichment; M.G. and A.J. performed iFISH cytogenetic testing; C. Kunz and A.B. performed the statistical analysis; T.B. and C. Kunz created the figures and tables; T.B., C. Kunz, U.H., and S.O.S. wrote the manuscript; and all authors revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: H.G. received research support from Celgene, Janssen, Chugai, Novartis, Bristol-Myers Squibb, and Millennium Pharmaceuticals; was on the advisory boards for Janssen, Celgene, Novartis, Onyx, Amgen Takeda, and Bristol-Myers Squibb; and received honoraria from Celgene, Janssen, Novartis, Chugai, Onyx, and Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Ute Hegenbart, University of Heidelberg, Medical Department V, Amyloidosis Center, Im Neuenheimer Feld 410, D-69120 Heidelberg, Germany; e-mail: ute.hegenbart@med.uni-heidelberg.de.
References
Author notes
T.B. and U.H. contributed equally to this study.