Key Points
Blood-induced joint damage is fully prevented by blocking IL-1β with a monoclonal antibody or receptor antagonist, not by TNFα blockade.
IL-1β blockade prevents release of IL-6 but not TNFα from monocyte/macrophages, whereas TNFα blockade does not affect IL-1β or IL-6 release.
Abstract
Joint bleeding after (sports) trauma, after major joint surgery, or as seen in hemophilia in general leads to arthropathy. Joint degeneration is considered to result from the direct effects of blood components on cartilage and indirectly from synovial inflammation. Blood-provided proinflammatory cytokines trigger chondrocytes and induce the production of cartilage-degrading proteases. In the presence of erythrocyte-derived iron, cytokines stimulate radical formation in the vicinity of chondrocytes inducing apoptosis. To unravel the role of interleukin (IL) 1β and tumor necrosis factor (TNF) α in the pathogenesis of this blood-induced cartilage damage, the effect of antagonizing these cytokines was examined in human in vitro cultures. Addition of recombinant human IL-1β monoclonal antibody or IL-1 receptor antagonist resulted in a dose- and time-dependent protection of cartilage from blood-induced damage. In higher concentrations, almost complete normalization of cartilage matrix proteoglycan turnover was achieved. This was accompanied by a reduction in IL-1β and IL-6 production in whole blood cultures, whereas TNFα production remained unaffected. Interestingly, addition of a TNFα monoclonal antibody, although demonstrated to inhibit the direct (transient) effects of TNFα on cartilage, exhibited no effect on blood-induced (prolonged) cartilage damage. It is demonstrated that IL-1β is crucial in the development of blood-induced joint damage, whereas TNFα is not. This hierarchical position of IL-1β in blood-induced joint damage warrants studies on targeting IL-1β to potentially prevent joint degeneration after a joint bleed.
Introduction
A bleed inside a joint after major joint surgery, after (sports)trauma, or as seen in hemophilia can lead to irreversible joint damage.1-3 Over the years, evidence has been gathered that even a single exposure of blood to a joint has profound damaging effects on joint cartilage.4-6 This devastating effect is not primarily the result of synovial inflammation induced by blood components, an important aspect of repeated bleeding as in hemophilia. After a single bleeding, these synovial effects are considered transient,7 whereas effects on cartilage are devastating. A single bleeding is able to induce chondrocyte apoptosis8 and results in long-lasting impaired matrix turnover, which eventually results in progressive joint damage becoming clinically evident over years.
The pathogenesis of blood-induced cartilage damage is still not fully understood.7 Cytokines such as interleukin (IL) 1β and tumor necrosis factor (TNF) α are produced by blood-derived mononuclear cells,9,10 the subsequently activated synoviocytes,11,12 and stimulated chondrocytes,13,14 and they induce a variety of biological activities. Importantly, IL-1 and TNFα are considered upstream in the cascade of cytokines and induce the production of several other proinflammatory cytokines such as IL-8 and IL-6, as well as the synthesis of proteases including matrix metalloproteinases.15 The production of those cytokines provides a transient imbalance in joint homeostasis but is unable to induce irreversible damage.16,17 However, the presence of erythrocyte-derived iron in addition to the proinflammatory cytokines provides a mechanism for more permanent damage. The catalytic heme-derived iron is involved in the Fenton reaction, resulting in the formation of reactive oxygen species in the vicinity of chondrocytes activated by proinflammatory cytokines. These oxygen radicals induce apoptosis of the chondrocytes, the only cell type of cartilage and responsible for maintenance of the extracellular matrix.8 Considering the relatively low number of chondrocytes within an abundant extracellular matrix18 and the extremely low replenishment of these chondrocytes in adult human articular cartilage,19 the induced apoptosis is rather devastating. It leads to a permanent disturbance in cartilage matrix turnover, causing loss of proteoglycans essential for resilience of cartilage tissue. Diminished mechanical properties of the cartilage make it more vulnerable to subsequent mechanical damage initiating a vicious circle ultimately leading to progressive joint destruction.
Despite increasing insight into the mechanisms involved in the devastating effects of blood on joint tissues, targeted therapies are lacking. The aim of the current study is to dissect the role of IL-1β and TNFα in the pathophysiology of blood-induced cartilage damage. Understanding the contribution of individual cytokines to the development of blood-induced arthropathy is important to provide leads for targeted therapy.
Materials and methods
Cartilage culture technique
Healthy human articular cartilage tissue was obtained within 24 hours postmortem of donors without a known history of joint disorders (n = 31; age 60.6 ± 16.6 years; 17 males and 14 females). Procedures were according to the medical ethical regulations of the University Medical Center Utrecht. Full-thickness cartilage was cut aseptically from the humeral head excluding the underlying bone and kept in phosphate-buffered saline (PBS; pH 7.4). Within 1 hour after dissection, slices were cut into full-thickness square pieces and weighed aseptically (range 5-15 mg; accuracy ±0.1 mg). Each explant was cultured individually in a 96-well round-bottomed microtiter plate (at 5% CO2 in air, 37°C, 95% humidity) in 200 μL culture medium per well. Culture medium consisted of Dulbecco’s modified Eagle medium (Invitrogen), supplemented with glutamine (2 mM), penicillin (100 IU/mL), streptomycin (100 μL/mL; all Paisley, UK), ascorbic acid (85 μM; Sigma), and 10% heat-inactivated pooled human male AB serum (Invitrogen).
For each experiment, fresh blood was drawn from healthy human donors (n = 19; age 28.3 ± 7.1 years; 9 males and 10 females) into Vacutainer tubes (Becton Dickinson). Explants were exposed to 50% volume to volume ratio (v/v) whole blood for 4 days, which is supposed to be the natural evacuation time of blood from the joint cavity.20 After blood exposure, cartilage explants were washed twice for 45 minutes under culture conditions to remove all additives and cultured for an additional 12 days without additives (recovery period, to differentiate transient from permanent effects). Medium was refreshed every 4 days.
Experimental design
In the first set of experiments, the effect of IL-1β blockade on blood-induced cartilage damage was investigated by addition of an IL-1β monoclonal antibody (IL-1βmAb; R&D Systems, Minneapolis, MN) or IL-1 receptor antagonist (IL-1RA; anakinra; Swedish Orphan International Ltd.) in a broad concentration range during blood exposure. In the second set of experiments, 100 ng/mL IL-1βmAb or 1000 ng/mL IL-1RA was added directly after addition of blood, or 2, 4, 8, 24, or 48 hours later (therapeutic approach). In those 2 experiments, long-term effects (after 12 days of recovery) on cartilage matrix turnover were assessed. Thirdly, to determine the direct effects of IL-1βmAb on cartilage in the absence of blood, explants were cultured for 4 days in the presence of 10 ng/mL IL-1βmAb. Both short-term (immediate after the 4-day culture) and long-term effects (after 12 days of recovery) on cartilage matrix turnover were determined.
To investigate the effect of blocking TNFα on blood-induced cartilage damage, 10 μg/mL TNFα monoclonal antibody (TNFαmAb; adalimumab, AbbVie; at this concentration completely inhibiting TNFα production by lipopolysaccharide [LPS]-stimulated monocytes21 ) was added to blood-cartilage cocultures for 4 days, and proteoglycan turnover was determined after 12 days of recovery. As no effect was demonstrated, the efficacy of the TNFαmAb was investigated by culturing cartilage explants for 4 days in the presence of recombinant TNFα (R&D Systems) with/without 10 μg/mL TNFαmAb. As TNFα alone exhibits only reversible effects on proteoglycan turnover,17 proteoglycan synthesis rate was determined after 4 days of culture.
Determination of proteoglycan turnover
Each experiment was performed with cartilage from a single donor. To correct for possible biological variations between samples, the mean value of 8 to 10 cartilage explants (depending on the amount of cartilage tissue available) per parameter per donor, obtained randomly and handled individually, was taken as a representative value. Proteoglycan turnover was evaluated at the end of each experiment. Proteoglycans, consisting of negatively charged glycosaminoglycans (GAGs) attached to a core protein, attract water into the cartilage, therewith exerting a swelling pressure to withstand loading.22 So a decreased proteoglycan synthesis, release of GAGs, and an overall loss of GAG content result in a diminished resilience of cartilage and consequently in tissue degeneration. Assessment of quantitative biochemical proteoglycan turnover is more sensitive than histologic Alcian Blue staining.23 For proteoglycan synthesis rate, sulfate incorporation was determined by addition of Na235SO4 (NEX-041-H carrier free; DuPont; 74 kBq per well) for 4 hours to pulse label the sulfated GAGs. Subsequently, the cartilage explants were washed twice in cold PBS, digested for 2 hours at 65°C with 2% papain (Sigma), and stored at −20°C. Proteoglycan synthesis rate was determined by precipitation of GAGs with 0.3 M hexadecylpyridinium chloride monohydrate (Sigma) in 0.2 M NaCl. The precipitate was dissolved in 3 M NaCl, and the amount of radioactivity measured by liquid scintillation counting. Radioactivity was normalized to the specific activity of the medium, labeling time, and wet weight of the explant. Results were expressed as nanomoles of sulfate incorporated per gram wet weight of cartilage tissue (nmol/g per hour). The proteoglycan content of each cartilage explant and release of proteoglycans into culture medium were established by staining and precipitation of GAGs with Alcian Blue (Sigma) in the papain digest of cartilage samples and in culture medium, respectively. Staining was quantified by spectrophotometry at 620 nm using chondroitin sulfate (Sigma) as a reference. Results were normalized to the wet weight of cartilage tissue.
Immunohistochemistry
For immunohistochemistry, human cartilage explants were cultured for 4 days with/without 50% v/v whole blood, and 100 ng/mL IL-1βmAb or 10 μg/mL TNFαmAb was added. Explants were harvested, snap frozen in liquid nitrogen, and stored at −80°C. Frozen sections were sliced and fixed with 4% paraformaldehyde and 80% methanol.
Apoptosis of chondrocytes was detected using an anti–single-strand DNA (ssDNA) monoclonal antibody (clone F7–26/apostain; Alexis Corporation, The Netherlands). Tissue was permeabilized with 0.2 mg/mL saponin (Sigma) and heated to 56°C in formamid to denature unstable DNA and subsequently transferred to ice-cold PBS. The sections were incubated with anti-ssDNA antibody, and the antibody complex was visualized using 3,3′-diaminobenzidine (Vector). The tissue was counterstained with hematoxylin. Representative images of a limited number of experiments are provided.
Blood cultures
To study the effect of IL-1βmAb, IL-1RA, and TNFαmAb on the production of proinflammatory cytokines, separate whole blood (50% v/v) cultures without cartilage were performed. It was previously demonstrated that the production of proinflammatory cytokines by healthy cartilage itself is negligible,24 and that the production of these cytokines is not different between 50% blood cultures with and without cartilage.25 Blood of 6 healthy donors (age 30.7 ± 9.1 years; 4 males and 2 females) was cultured for 4 days. IL-1βmAb, IL-1RA, or TNFαmAb was added in a concentration of 100 ng/mL, 1000 ng/mL, or 10 μg/mL respectively (ie, the highest concentrations tested in blood-cartilage cocultures). After 4 days, samples were collected and centrifuged at 1500g for 10 minutes, and supernatants were stored at −80°C. The concentrations of IL-1β, IL-6, and TNFα were determined with commercially available enzyme-linked immunosorbent assay (for IL-1β and IL-6, Invitrogen; for TNFα, Sanquin), and analyses were performed according to the manufacturer’s instructions.
Statistical analyses
Statistical analysis was performed using SPSS statistics package for Windows (Version 21.0; IBM Inc., Chicago, IL). As data were nonnormally distributed, the Wilcoxon-signed rank test for related samples was performed to determine statistical significance between treatments and controls of cartilage or blood from the same donor sample. All statistical analyses were performed using absolute data. Values are expressed as percentage of control culture conditions and depicted as median values ± interquartile range, unless stated otherwise. P values <.05 were considered statistically significant.
Results
Dose-dependent protection of blood-induced cartilage damage by IL-1βmAb and IL-1RA
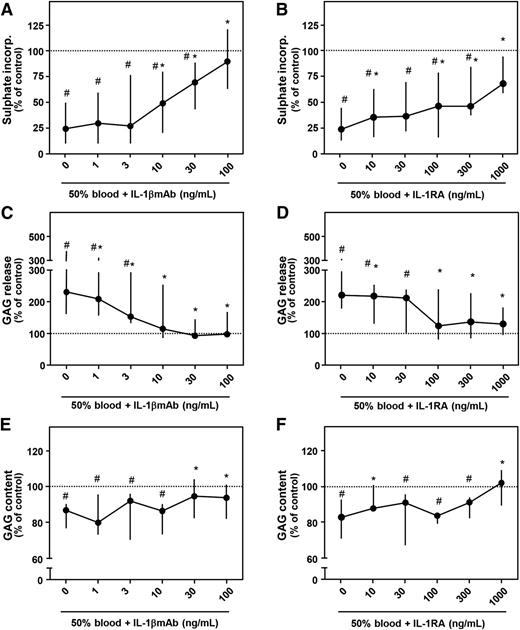
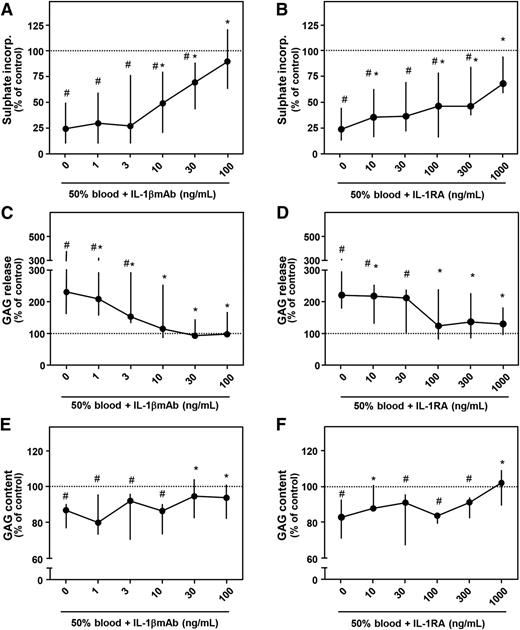
Culturing cartilage explants in the presence of 50% v/v whole blood for 4 days strongly impacted proteoglycan turnover determined 12 days later (Figure 1). Proteoglycan synthesis rate was inhibited 75% (P = .012; Figure 1A) and 76% (P = .018; Figure 1B), GAG release was increased 129% (P = .017; Figure 1C) and 125% (P = .018; Figure 1D), and GAG content decreased 14% (P = .017; Figure 1E) and 18% (P = .028; Figure 1F), all compared with control condition (100%; dotted line). Addition of IL-1βmAb during the 4-day blood exposure resulted in a dose-dependent recovery of proteoglycan synthesis rate, with a statistically significant increase from 10 ng/mL compared with blood exposure without additives (this and all higher concentrations P = .012, asterisks). At a concentration of 100 ng/mL, proteoglycan synthesis rate was not different anymore compared with control (91%; P = .401). GAG release decreased with addition of 1 ng/mL IL-1βmAb (all concentrations P < .03 vs blood alone, asterisks). From a concentration of 10 ng/mL on, GAG release was not different anymore compared with control (P > .441). Proteoglycan content improved significantly in the presence of 30 ng/mL IL-1βmAb or higher (both P = .025 compared with blood alone, asterisks).
Concentration-dependent effect of antagonizing IL-1 activity. Healthy human cartilage was cultured for 4 days in the absence (control; dotted line) or presence of 50% blood. During blood exposure, an IL-1βmAb (A, C, E; n = 8) or an IL-1RA (B, D, F; n = 7) was added. Proteoglycan synthesis rate (A, B), release (C, D), and content (E, F) are depicted. #P < .05 compared with control values; *P < .05 compared with blood-exposed cartilage without IL-1βmAb or IL-1RA addition.
Concentration-dependent effect of antagonizing IL-1 activity. Healthy human cartilage was cultured for 4 days in the absence (control; dotted line) or presence of 50% blood. During blood exposure, an IL-1βmAb (A, C, E; n = 8) or an IL-1RA (B, D, F; n = 7) was added. Proteoglycan synthesis rate (A, B), release (C, D), and content (E, F) are depicted. #P < .05 compared with control values; *P < .05 compared with blood-exposed cartilage without IL-1βmAb or IL-1RA addition.
To confirm that the protective effects of IL-1βmAb were IL-1 specific, we repeated the experiments with addition of IL-1RA, a natural inhibitor of both IL-1β and IL-1α. Similar effects with the antagonist as with the neutralizing antibody were found. Addition of IL-1RA prevented blood-induced cartilage damage in a concentration-dependent way. In nearly all concentrations tested, proteoglycan synthesis rate improved (Figure 1B, asterisks). GAG release improved at the lowest concentration of IL-1RA added and was not significantly different from control condition at a concentration of 100 ng/mL and higher (Figure 1D). Addition of 1000 ng/mL significantly improved GAG content compared with blood exposure only (+2% compared with control; P = .018; Figure 1F, asterisks). Preincubation of cartilage explants with IL-1RA before blood exposure did not further improve the antagonizing efficacy (data not shown).
Time-dependent protection of blood-induced cartilage damage by IL-1βmAb and IL-1RA
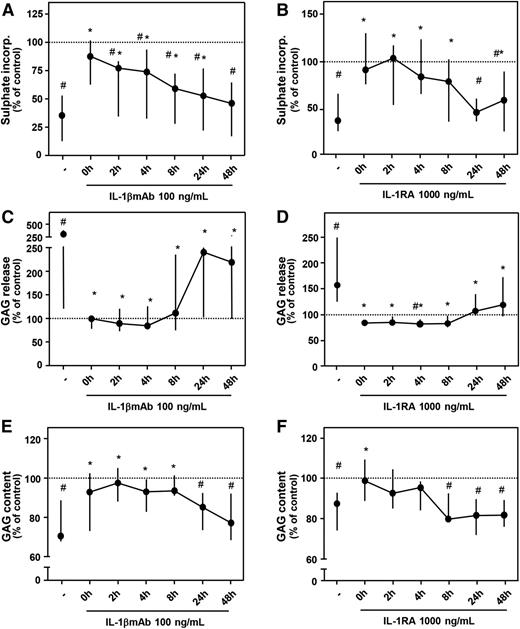
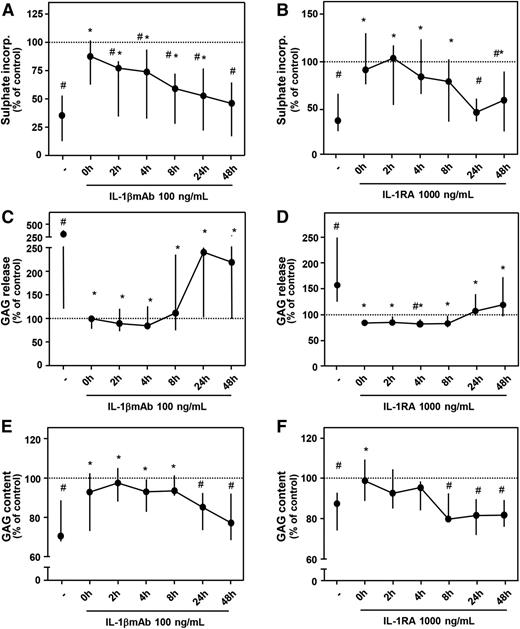
In a separate set of experiments, it was shown again that addition of a high concentration of IL-1βmAb (100 ng/mL) or IL-1RA (1000 ng/mL) immediately after blood exposure resulted in a complete restoration of proteoglycan turnover, matching control values again (Figure 2; all parameters P > .05 compared with control). Blocking IL-1 within 8 hours after blood exposure was most effective; thereafter, the effect diminished. Proteoglycan synthesis improved in a time-dependent manner (Figure 2A-B). A significant decrease in GAG release by addition of IL-1βmAb or IL-1RA was seen at all time points (Figure 2C-D), but when the antibody was added after 24 hours, the effect was clearly less pronounced (Figure 2C). GAG content significantly improved when IL-1βmAb was added within 8 hours after blood exposure (Figure 2E; P ≤ .043, asterisks) but for IL-1RA only when added shortly after blood exposure (Figure 2F; P = .018). Compared with control cartilage, GAG content was significantly lower (namely, additives were not effective anymore) when IL-1RA was added after 8 hours or later (all P = .018, pound signs) and for IL-1βmAb when added after 24 hours or later (P ≤ .028).
Time-dependent effect of antagonizing IL-1 activity. Cartilage was cultured as described for Figure 1. IL-1βmAb (A, C, E; n = 7) or IL-1RA (B, D, F; n = 7) was added at the moment of blood exposure or 2 to 48 hours later. Proteoglycan synthesis rate (A, B), release (C, D), and content (E, F) are depicted. Dotted line represents control conditions (cartilage cultures without additives). #P < .05 compared with control values; *P < .05 compared with blood-exposed cartilage without IL-1βmAb or IL-1RA addition.
Time-dependent effect of antagonizing IL-1 activity. Cartilage was cultured as described for Figure 1. IL-1βmAb (A, C, E; n = 7) or IL-1RA (B, D, F; n = 7) was added at the moment of blood exposure or 2 to 48 hours later. Proteoglycan synthesis rate (A, B), release (C, D), and content (E, F) are depicted. Dotted line represents control conditions (cartilage cultures without additives). #P < .05 compared with control values; *P < .05 compared with blood-exposed cartilage without IL-1βmAb or IL-1RA addition.
No direct effect of IL-1βmAb on healthy cartilage
IL-1 not only induces cartilage degradation, but also is important for maintaining cartilage homeostasis.26 Therefore, it was investigated whether addition of IL-1βmAb had a direct effect on healthy cartilage. Proteoglycan turnover was assessed 12 days later for long-term effects, showing no direct effects of 10 ng/mL IL-1βmAb in the absence of blood (P > .50 for all 3 parameters; Table 1). Because it is known that the impact on proteoglycan synthesis rate of IL-1β in the absence of an iron source is reversible,7 the short-term effect of addition of IL-1βmAb was also tested. No change in proteoglycan synthesis rate was demonstrated (n = 3; 4.39 [2.33-8.50] vs 4.33 [2.49-9.04] nmol/g per hour). For IL-1RA, it has been demonstrated previously that it has no direct effects on proteoglycan synthesis rate.27,28
Antagonizing TNFα does not reverse blood-induced cartilage damage
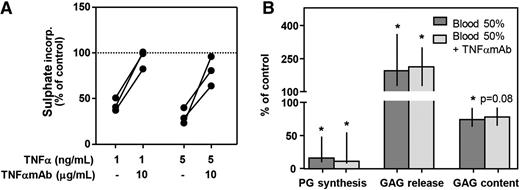
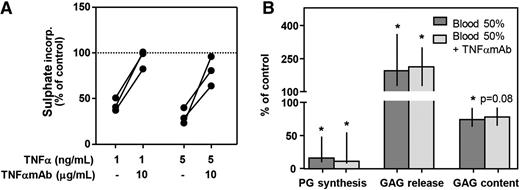
To investigate the role of TNFα, we tested the influence of antagonizing TNFα with a monoclonal antibody (TNFαmAb; adalimumab) on proteoglycan turnover. TNFα in the absence of blood clearly inhibited proteoglycan synthesis rate assessed at day 4 (1 ng/mL, −57%; 5 ng/mL, −69%; Figure 3A). Concomitant addition of TNFαmAb (10 μg/mL) reversed this effect almost completely (Figure 3A; 1 ng/mL TNFα, 99% of control; 5 ng/mL TNFα, 81% of control), demonstrating its functionality. However, in blood-cartilage cocultures, addition of TNFαmAb (10 μg/mL) did not affect any of the proteoglycan turnover parameters assessed after 12 days of recovery (Figure 3B). Proteoglycan synthesis rate decreased to 16% of control values, and addition of TNFαmAb did not change this (11% of control; P = .500). GAG release increased upon blood exposure to 194% of control and to 213% in the presence of TNFαmAb (P = .500). Also GAG content after blood exposure was not affected by addition of TNFαmAb (74% vs 78% of control; P = .225).
Antagonizing TNFα with a monoclonal antibody (TNFαmAb) cannot protect cartilage from blood-induced damage. (A) Healthy human cartilage (n = 3) was cultured for 4 days in the absence (control; dotted line) or presence of TNFα and TNFαmAb. Proteoglycan synthesis rate was determined to check the activity of the antibody. (B) Cartilage was cultured in the absence (control; 100% level) or presence of whole blood (50% v/v) with/without TNFαmAb (10 μg/mL) (n = 5). Proteoglycan turnover was determined after 12 days of recovery. *P < .05 compared with control values. No statistically significant effects between cultures with and without TNFαmAb were seen.
Antagonizing TNFα with a monoclonal antibody (TNFαmAb) cannot protect cartilage from blood-induced damage. (A) Healthy human cartilage (n = 3) was cultured for 4 days in the absence (control; dotted line) or presence of TNFα and TNFαmAb. Proteoglycan synthesis rate was determined to check the activity of the antibody. (B) Cartilage was cultured in the absence (control; 100% level) or presence of whole blood (50% v/v) with/without TNFαmAb (10 μg/mL) (n = 5). Proteoglycan turnover was determined after 12 days of recovery. *P < .05 compared with control values. No statistically significant effects between cultures with and without TNFαmAb were seen.
Blood-induced chondrocyte apoptosis is limited by blocking IL-1β
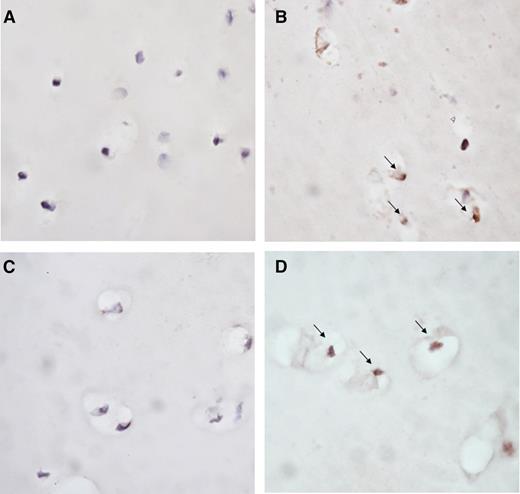
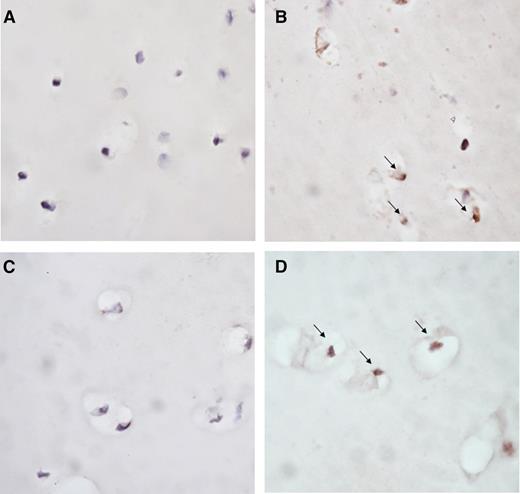
Exposure of cartilage to blood for 4 days led to apoptosis of chondrocytes as compared with cartilage cultured in medium without additives (representative images in Figure 4A-B). Addition of IL-1βmAb prevented this blood-induced apoptosis (representative image in Figure 4C), whereas TNFαmAb did not affect it (representative image in Figure 4D).
Blocking IL-1 limits blood-induced chondrocyte apoptosis. Representative photomicrographs of chondrocyte apoptosis in human articular cartilage explants cultured in medium (A) or exposed to 50% v/v whole blood (B-D) for 4 days. IL-1βmAb (C) or TNFαmAb (D) was added during blood exposure. Apoptosis of chondrocytes was determined by immunohistochemical staining with ssDNA (brown staining, indicated by arrow). Original magnification ×40.
Blocking IL-1 limits blood-induced chondrocyte apoptosis. Representative photomicrographs of chondrocyte apoptosis in human articular cartilage explants cultured in medium (A) or exposed to 50% v/v whole blood (B-D) for 4 days. IL-1βmAb (C) or TNFαmAb (D) was added during blood exposure. Apoptosis of chondrocytes was determined by immunohistochemical staining with ssDNA (brown staining, indicated by arrow). Original magnification ×40.
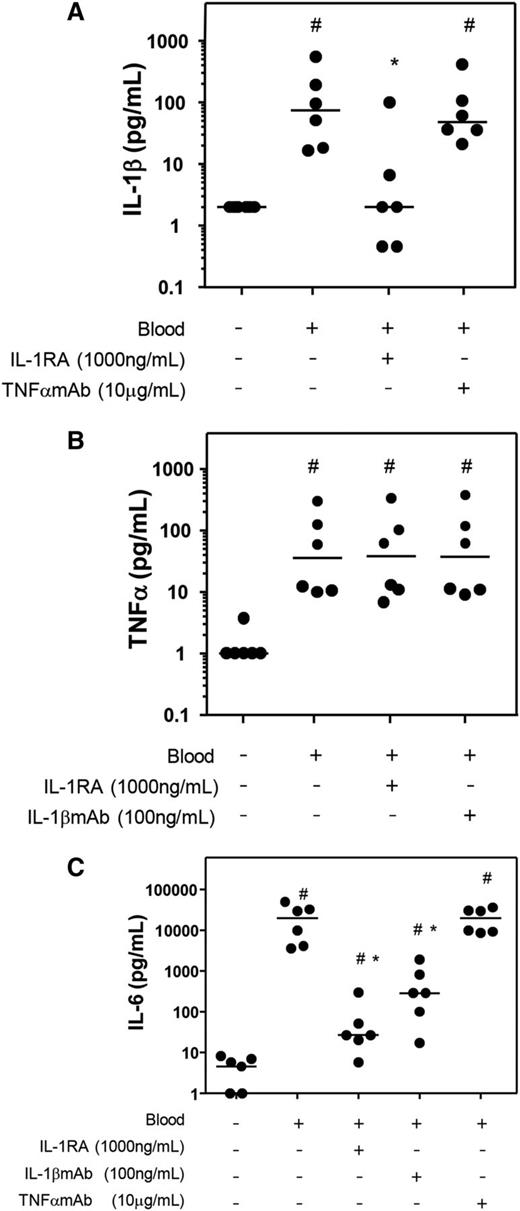
Blocking the activity of IL-1β limits the production of IL-6 but not TNFα
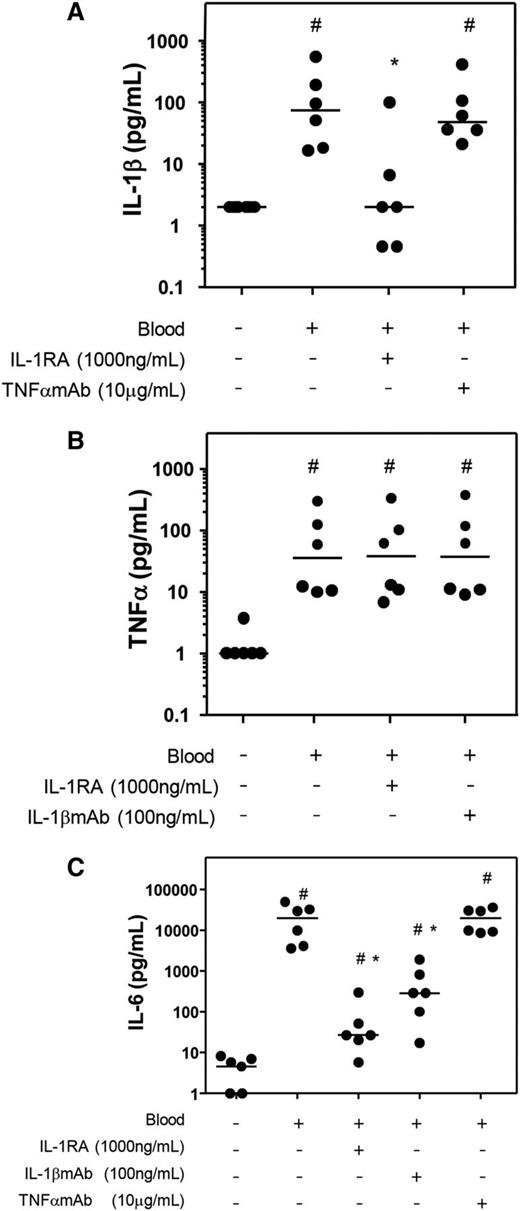
To further elucidate the role of the individual cytokines, in 4-day whole-blood cultures the effect of blocking either IL-1β or TNFα on levels of other proinflammatory cytokines was investigated. The production of IL-1β was limited by the addition of IL-1RA but not by TNFαmAb (Figure 5A; P = .028 and P = .345 compared with blood only). TNFα levels were not influenced in the presence of IL-1 blocking agents (Figure 5B; both P = .753 compared with blood only). Addition of IL-1RA or IL-1βmAb, but not TNFαmAb, limited the production of IL-6 compared with the blood culture without additives (Figure 5C; P = .028 for both IL-1RA and IL-1βmAb; P = .753 for TNFαmAb).
Effect of antagonizing IL-1 or TNFα on proinflammatory cytokine production in whole blood cultures. Fifty percent v/v whole blood of 6 donors was cultured for 4 days. IL-1RA, IL-1βmAb, or TNFαmAb was added, and the production of the proinflammatory cytokines IL-1β (A), TNFα (B), and IL-6 (C) was measured. Data are shown as individual (dots) and median (dash) values. Control culture conditions without blood and additives is shown as well. #P < .05 compared with control; *P < .05 compared with blood without additions.
Effect of antagonizing IL-1 or TNFα on proinflammatory cytokine production in whole blood cultures. Fifty percent v/v whole blood of 6 donors was cultured for 4 days. IL-1RA, IL-1βmAb, or TNFαmAb was added, and the production of the proinflammatory cytokines IL-1β (A), TNFα (B), and IL-6 (C) was measured. Data are shown as individual (dots) and median (dash) values. Control culture conditions without blood and additives is shown as well. #P < .05 compared with control; *P < .05 compared with blood without additions.
Discussion
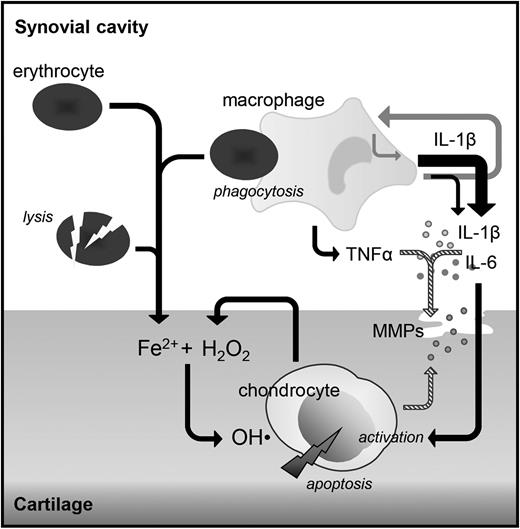
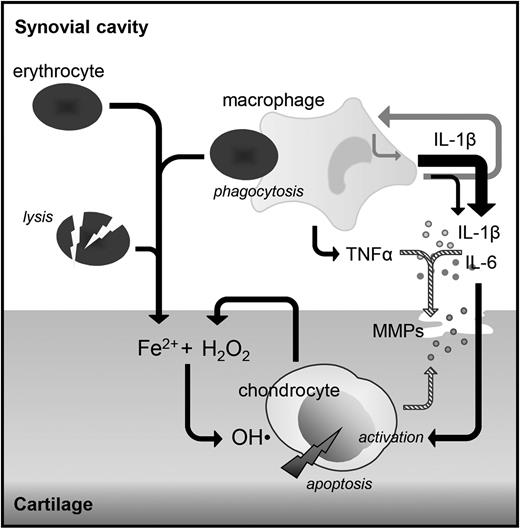
This human in vitro study demonstrates that IL-1β, and not TNFα, is a key mediating cytokine in the harmful direct effects of blood on cartilage. Blocking IL-1β with either an IL-1βmAb or IL-1RA competing with both IL-1α and IL-1β for receptor binding reversed blood-induced cartilage damage in a dose- and time-dependent manner up to complete normalization. In contrast, blocking TNFα with a monoclonal antibody did not demonstrate any efficacy. In addition, we showed that blocking IL-1β resulted in a reduction of IL-6, whereas TNFα levels were unaffected. Blocking TNFα did not change levels of IL-1β or IL-6, advocating for 2 separate pathways (Figure 6).
Suggested role of cytokines in the pathogenesis of synovial independent blood-induced cartilage damage. Monocyte/macrophages activated by the phagocytosis of erythrocytes produce IL-1β and TNFα, which both induce the production of proteases causing reversible cartilage damage (striped arrows). IL-1β also induces the production of IL-6 and IL-1β itself (gray arrows). These cytokines are able to activate the chondrocyte to produce hydrogen peroxide (H2O2), which in the presence of iron leads to the production of hydroxyl radicals (OH·) causing chondrocyte apoptosis and as such irreversible cartilage damage, whereas TNFα does not. Local inhibition of IL-1β (directly in case of major surgery) or within the same day (in case of trauma or hemophilia-induced joint bleeds) may provide a mechanism to prevent cartilage damage and with that joint destruction later on.
Suggested role of cytokines in the pathogenesis of synovial independent blood-induced cartilage damage. Monocyte/macrophages activated by the phagocytosis of erythrocytes produce IL-1β and TNFα, which both induce the production of proteases causing reversible cartilage damage (striped arrows). IL-1β also induces the production of IL-6 and IL-1β itself (gray arrows). These cytokines are able to activate the chondrocyte to produce hydrogen peroxide (H2O2), which in the presence of iron leads to the production of hydroxyl radicals (OH·) causing chondrocyte apoptosis and as such irreversible cartilage damage, whereas TNFα does not. Local inhibition of IL-1β (directly in case of major surgery) or within the same day (in case of trauma or hemophilia-induced joint bleeds) may provide a mechanism to prevent cartilage damage and with that joint destruction later on.
IL-1βmAb in the absence of blood did not have any effect on cartilage proteoglycan turnover, which is an important observation as IL-1β not only can induce cartilage matrix breakdown27,29 but also has a role in maintaining cartilage homeostasis.26 The lack of influence on healthy cartilage is in line with previous studies showing that chondrocytes from healthy cartilage produce only low levels of proinflammatory cytokines,24,25 and IL-1RA does not exert any effect on healthy cartilage in the absence of IL-1.27,28
With a molecular weight of ∼17 kDa for IL-1RA compared with 160 kDa for the IL-1βmAb, ∼100-fold higher molar concentration of IL-1RA was necessary to elicit a similar response on blood-induced cartilage damage. This excess of IL-1RA compared with the amounts of IL-1 produced is needed to block all IL-1 receptors on chondrocytes and on blood cells (especially monocytes/macrophages). Its activity relies mainly on antagonizing the activity of IL-1β, not IL-1α, as selective blocking of IL-1β with a monoclonal antibody also fully prevented cartilage destruction. Heme is a potent inducer of IL-1β secretion by macrophages,30,31 and IL-1β production by synoviocytes is demonstrated in case of repeated joint bleeds as in hemophilic arthropathy.32 Moreover, a hemarthrosis induced in hemophilic mice results in elevated levels of IL-1β.33,34 An upregulation of the nuclear factor–κB pathway, which is induced by IL-1β, has been emphasized in hemophilic mice upon joint bleeding34 and in synovial tissue of patients with hemophilic arthropathy.35 In experimental arthritis, blocking IL-1 prevents cartilage damage upon inflammation36,37 as is also observed in a posttraumatic osteoarthritis model.38 Therapeutic efficacy of IL-1RA is demonstrated in hand osteoarthritis39 and in 2 cases suffering from another form of iron-induced arthropathy in case of hemochromatosis.40
Opposing IL-1 was most effective when performed within 8 hours after blood exposure, but benefit was still achieved when performed until 24 hours after exposure to blood. This is in line with the observation that IL-1 production in blood-cartilage coculture is detected within 24 hours and prevented by addition of IL-4 and IL-10 within 4 to 8 hours.25 Addition of those anti-inflammatory cytokines after 8 hours does not improve cartilage turnover anymore, whereas blocking IL-1 still shows benefit several hours later. IL-1β is capable of inducing its own production as well as the production of other proinflammatory cytokines.9,15 So inhibiting the initial release of IL-1 by monocytes/macrophages is essential to abrogate the positive feedback loop (Figure 6).
Our group previously demonstrated that long-lasting inhibition of proteoglycan synthesis induced by lysed erythrocytes together with IL-1β is less pronounced than the effect of mononuclear cells together with erythrocytes, or whole blood.8 TNFα was suggested as a possible factor explaining these differences, its production being demonstrated in blood-cartilage cocultures24 and shown to increase hydroxyl radical formation by chondrocytes in the presence of iron.41,42 In the present study, however, blocking TNFα, even though completely preventing TNFα induced short-term inhibition of proteoglycan synthesis in the absence of blood (reversible changes because of upregulation of cartilage degrading enzymes17 ), did not affect long-term blood-induced cartilage damage (irreversible changes because of chondrocyte apoptosis43 ). The concentrations of TNFα produced in the blood-cartilage coculture system (maximum 350 pg/mL in a previous study24 and the current study) are relatively low, probably too low to stimulate hydroxyl radical formation, which is a prerequisite for inducing irreversible cartilage changes (in the study demonstrating this hydroxyl inducing capacity, 100 ng/mL TNFα was used to stimulate chondrocytes41 ).
TNFα is produced as a membrane-bound precursor that is released through proteolytic cleavage by the TNF-converting enzyme (TACE). Adalimumab, the TNFαmAb used in this study, binds and inactivates both the membrane-bound and soluble forms.44 TNFα production by synovial tissue will also contribute to end-stage hemophilic arthropathy.32 However, the increase in TNFα upon a joint bleeding (in a murine hemophilia model) is either absent33 or very minimal,34 questioning its role in the early stages of blood-induced joint damage. Probably, TNFα is mainly a mediator in synovial inflammation instead of direct cartilage destruction. Also in murine models of inflammatory arthritis and posttraumatic osteoarthritis, no effect on cartilage destruction upon TNFα blockade has been found, corroborating this hypothesis.36-38 Nevertheless, this study does not exclude a role for TNFα in inducing the reversible (short-term) changes to cartilage.
The present study advocates for IL-6 as a contributing factor to the effects of IL-1β and iron on cartilage. We show that its production is suppressed in the presence of an IL-1–blocking agent, whereas TNFα levels are unaffected. Also in osteoarthritic synovium45 and monocyte cultures,9 addition of IL-1RA showed a dose-dependent reduction in IL-6 production. A protective effect of blocking IL-6 on cartilage damage and synovitis upon joint bleeding in a hemophilia mouse model has been shown when administered in combination with clotting factor replacement.46
The main limitations of this study are related to the use of an in vitro model. Besides the lack of involvement of synovial tissue as mentioned previously, also the replenishment of blood cells in an ongoing bleed is not included in this system. This is particularly important for IL-1RA treatment as cells with unblocked receptors will enter the joint and as such higher doses of IL-1RA will be required. Moreover, it needs to be investigated whether a single injection is sufficient to prevent cartilage damage, and as clinical observations demonstrate differences in vulnerability to the effects of intra-articular bleeding,47 it remains to be investigated which patients will benefit from this treatment.
Taken together, these data suggest IL-1β to play a critical role in blood-induced cartilage damage, whereas this could not be demonstrated for TNFα. Opposing the activity of IL-1β within the same day or next morning after blood exposure prevents the initiation of a self-amplifying loop resulting in progressive cartilage damage. As therapeutic agents opposing the activity of IL-1β are available and safe for application,48-50 local treatment with such drugs upon a joint bleed might be relevant to prevent progressive joint damage over time. However, more (in vivo) evidence is needed that interfering with these proinflammatory cytokines released and induced upon a joint bleed halts the degenerative process.
Presented in abstract form at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, December 6, 2014.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Pathology Department of the University Medical Center Utrecht for assistance in obtaining cartilage tissue, G. H. Wassink for his contribution to the cytokine assessments, and M. J. G. Wenting-van Wijk for her contribution to the immunohistochemistry.
This work was supported by an unrestricted grant from CSL Behring. Parts of the research described are awarded the Access to Insight Harold R. Roberts Award 2014 by NovoNordisk.
CSL Behring, the funding source, was not involved in any part of the study, the writing of the manuscript, or the decision to submit the manuscript for publication.
Authorship
Contribution: L.F.D.v.V., R.E.G.S., S.C.M., and F.P.J.G.L. designed the research; L.F.D.v.V., K.C., and E.C.A. performed the experiments; L.F.D.v.V., K.C., E.C.A., S.C.M., and F.P.J.G.L. analyzed the data; L.F.D.v.V., G.R., R.E.G.S., S.C.M., and F.P.J.G.L. interpreted the data; L.F.D.v.V. drafted the manuscript; G.R., R.E.G.S., S.C.M., and F.P.J.G.L. participated in manuscript preparation; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lize F. D. van Vulpen, University Medical Center Utrecht, Room F.02.127, PO Box 85500, 3508 GA Utrecht, The Netherlands; e-mail: l.f.d.vanvulpen-2@umcutrecht.nl.