Abstract
BCR-ABL1 compound mutations can confer high-level resistance to imatinib and other ABL1 tyrosine kinase inhibitors (TKIs). The third-generation ABL1 TKI ponatinib is effective against BCR-ABL1 point mutants individually, but remains vulnerable to certain BCR-ABL1 compound mutants. To determine the frequency of compound mutations among chronic myeloid leukemia patients on ABL1 TKI therapy, in the present study, we examined a collection of patient samples (N = 47) with clear evidence of 2 BCR-ABL1 kinase domain mutations by direct sequencing. Using a cloning and sequencing method, we found that 70% (33/47) of double mutations detected by direct sequencing were compound mutations. Sequential, branching, and parallel routes to compound mutations were common. In addition, our approach revealed individual and compound mutations not detectable by direct sequencing. The frequency of clones harboring compound mutations with more than 2 missense mutations was low (10%), whereas the likelihood of silent mutations increased disproportionately with the total number of mutations per clone, suggesting a limited tolerance for BCR-ABL1 kinase domain missense mutations. We conclude that compound mutations are common in patients with sequencing evidence for 2 BCR-ABL1 mutations and frequently reflect a highly complex clonal network, the evolution of which may be limited by the negative impact of missense mutations on kinase function.
Key Points
For CML patients on TKI therapy, 70% of double mutations in the BCR-ABL1 kinase domain detected by direct sequencing are compound mutations.
Sequential, branching, and parallel routes to compound mutations were observed, suggesting complex patterns of emergence.
Introduction
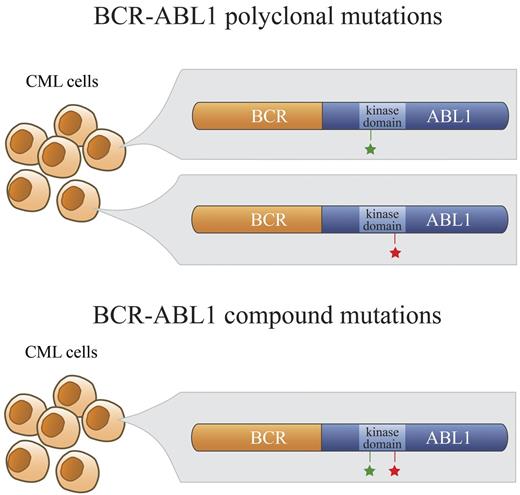
Tyrosine kinase inhibitors (TKIs) targeting the BCR-ABL1 oncoprotein are the standard therapy for patients with chronic myeloid leukemia (CML). Imatinib, nilotinib, and dasatinib are approved for the treatment of newly diagnosed CML patients.1-3 However, an estimated 20%-40% of patients receiving first-line imatinib therapy will eventually require an alternative treatment because of intolerance or resistance to TKIs.3-6 Recent studies in newly diagnosed chronic-phase patients have reported lower failure rates with dasatinib and nilotinib,1,2 but some patients will still require salvage treatment. The best-characterized mechanism of resistance is point mutations within the BCR-ABL1 kinase domain that impair or prevent TKI binding.7-9 Nilotinib and dasatinib were developed to overcome imatinib resistance and, with the exception of the multiresistant T315I mutant, these TKIs exhibit activity against many BCR-ABL1 kinase domain mutations.10,11 Sanger sequencing, the method most widely used for mutation detection, reveals only 1 mutation in the majority of cases of BCR-ABL1 kinase domain mutant-mediated resistance. However, in a subset of patients, ≥ 2 mutations were detected by conventional sequencing, reflecting either multiple BCR-ABL1 mutant clones (polyclonal mutations) or ≥ 2 mutations in the same BCR-ABL1 molecule (compound mutations; Figure 1). It has been suggested that sequential therapy with different ABL1 TKIs may inadvertently foster the development or selection of BCR-ABL1 compound mutations.12 Although each of multiple mutant clones is expected to retain its individual sensitivity to a given TKI, compound mutations can dramatically affect TKI sensitivity and catalytic fitness of the tyrosine kinase.12-14 Therefore, the distinction between compound versus polyclonal mutations is clinically important because it may influence the selection of the most suitable TKI to overcome resistance.14 Several compound mutations have been shown to confer resistance to ponatinib, and this is likely to apply to other third-line TKIs as well.13 Because the methods currently used for BCR-ABL1 kinase domain mutation screening cannot definitively distinguish compound from polyclonal mutations, there is little information available regarding their respective frequencies and clonal relationships.15 Therefore, in the present study, we used a cloning and sequencing approach to establish the frequency and clonal relationships of compound mutations in a cohort of CML patients defined by clear evidence of more than 1 BCR-ABL1 kinase domain mutation in their conventional Sanger sequencing trace.
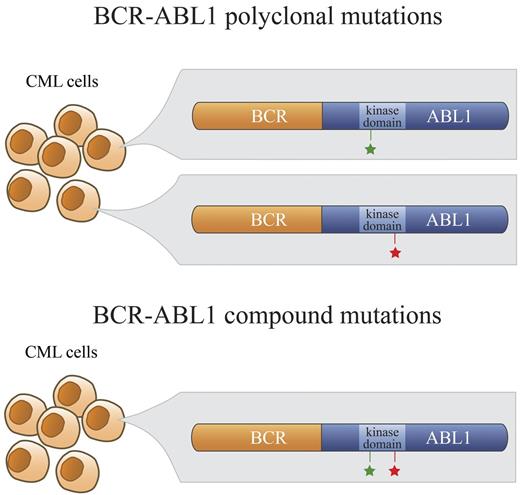
Polyclonal versus compound mutations. In a subset of patients who develop clinical resistance to ABL1 TKIs, more than 1 point mutation in the kinase domain of BCR-ABL1 is detectable by direct sequencing. In the case of polyclonal mutations, these BCR-ABL1 mutations (green and red stars; top panel) exist separately in different clones. In contrast, BCR-ABL1 compound mutants exhibit 2 mutations within the same BCR-ABL1 molecule (green and red stars; bottom panel).
Polyclonal versus compound mutations. In a subset of patients who develop clinical resistance to ABL1 TKIs, more than 1 point mutation in the kinase domain of BCR-ABL1 is detectable by direct sequencing. In the case of polyclonal mutations, these BCR-ABL1 mutations (green and red stars; top panel) exist separately in different clones. In contrast, BCR-ABL1 compound mutants exhibit 2 mutations within the same BCR-ABL1 molecule (green and red stars; bottom panel).
Methods
Patients
We analyzed samples from 47 CML patients treated with various ABL1 TKIs. The unifying selection criterion was the presence of more than 1 BCR-ABL1 kinase domain mutation detected by Sanger sequencing. Archived RNA or cDNA from the University of Utah (18 patients), Oregon Health & Science University (7 patients), University of Leipzig (5 patients), Hammersmith Hospital, Imperial College London (9 patients), and the University of Bologna (8 patients) was used for analysis. The institutional review boards of the participating centers approved this study and informed consent was obtained according to the Declaration of Helsinki where applicable. Serial samples were available for 5 patients, facilitating investigation of the kinetics and evolution of BCR-ABL1 mutations.
BCR-ABL1 kinase domain amplification, cloning, and sequencing
For the RNA samples, cDNA was synthesized as described previously.16 The BCR-ABL1 kinase domain was amplified in a 2-step nested PCR reaction that does not amplify nontranslocated ABL1.16 The amplified fragments were cloned using the TOPO TA cloning system (Invitrogen) and introduced into E coli.17 In the standard procedure, 10 bacterial colonies corresponding to BCR-ABL1 kinase domain amplicons from each patient were selected at random and grown in Luria-Bertani medium, and plasmids were extracted using the QIAGEN Plasmid Miniprep kit. The BCR-ABL1 kinase domain was sequenced in both directions using BigDye terminator chemistry on an ABI3730 instrument (Applied Biosystems)17 and compared against the ABL1 sequence (ENST00000318560) from Ensembl Genome Browser using the BLAST alignment tool (National Center for Biotechnology Information). If more than 1 mutation was detected at similar levels among the initially surveyed 10 colonies, a greater number of bacterial colonies were examined to gain a higher resolution for representation of the clonal relationship. Specifically, an expanded number of clones were sequenced in 2 cases: (1) sample CML#14 (55 in total) for which the V299L/M351T compound mutation was detected in similar frequency to the predominant compound mutation T315I/F359V of the original 10 clones sequenced but was not detected by direct sequencing, and (2) CML#46 (15 in total) for which 4 different mutations were detected by direct sequencing.
Definition of mutations
Mutations identified by direct sequencing.
Direct sequencing of PCR products is the clinical standard and detects mutant alleles if they represent at least 20%-30% of the amplicon pool. We refer to all mutations detected with this method as predominant mutations. One mutation detected by direct sequencing is referred to as a single mutation, whereas 2 mutations detected by direct sequencing are referred to as a double mutation (Table 1).
Mutations identified by sequencing of individual cloned PCR products.
Each bacterial clone carries an mRNA transcript from 1 individual BCR-ABL1 molecule. Mutations detected by this method but not by direct sequencing are referred to as low-level mutations. Two or more mutations present in the same molecule are referred to as a compound mutation, in contrast to polyclonal mutations, which refer to those present in different molecules. An individual mutation that is part of a compound mutation is designated as a component. In cases in which a low-level mutation (detected only by sequencing of individual PCR products) is detected in addition to a predominant mutation (detected by direct sequencing), it is referred to as an add-on compound mutation.
Statistical analysis
Frequency differences across multiple groups were assessed with the Fisher exact test; differences within single groups were compared with the exact binomial test. For testing the association of various factors with the number of mutant colonies, it is important to account for the fact that the probability of observation of a given factor (eg, the type of mutation) will increase with the number of mutations being observed. This expected probability is equivalent to 1 − (1 − x)n, where n is the number of mutations being assessed and x is the rate of occurrence of the factor when only a single mutation is being observed. A χ2 test was used to assess the deviation of observations from the expected value based on this calculation. A 2-sided P value of .05 was used to determine statistical significance unless otherwise noted. Statistical computations were performed using SAS Version 9.2 software.
Results
The majority of double mutations are compound mutations
The frequency of occurrence of ≥ 2 mutations among a total of 1700 samples harboring BCR-ABL1 kinase domain mutations detectable by direct sequencing at the 5 participating centers was 11.4% (194/1700). The samples with ≥ 2 mutations pertained to a total of 47 patients, for which the most recent clinical sample was investigated for this study. We sequenced a total of 583 colonies (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and identified 218 different mutations, 167 (77%) of which were missense mutations encompassing 157 residues in the kinase domain (supplemental Table 1). The remaining 51 nucleotide changes (23%) were silent mutations. The residues most frequently involved in missense mutations were F317 (5.4%), T315 (4.9%), G250 (3.5%), M351 (3.2%), F359 (2.7%), and V299 (2.4%; supplemental Table 1). The predominant mutations detected by direct sequencing have all previously been associated with resistance to second-generation ABL1 TKIs, except M351T, which has been reported in imatinib failure but has not been reported to confer significant resistance to either nilotinib or dasatinib.10,11,18 Ninety percent of the mutations were transitions, with the most common nucleotide changes being A→G and T→C (supplemental Table 2). We found that the majority of double mutations detected by direct sequencing (70.2%; 33/47) were in fact compound mutations as established by sequencing of individually cloned PCR products (Table 2). Thirty different compound mutations were detected among the 33 patients shown to harbor compound mutations (Table 2); the T315I/F359V, Y253H/F317L, and G250E/V299L mutations were each detected in more than 1 patient. The most common components of the compound mutations were (by frequency): T315I (27%; 8/30), V299L (20%; 6/30), F359V (17%; 5/30), and M351T (17%; 5/30). In total, these 4 substitutions accounted for 40% (24/60) of the components in these 30 compound mutations. Only 13 of 47 patients contained low-level independent mutations that were detected by cloning and sequencing but not by direct sequencing. Although 1 in 10 clones with a novel mutation could result from PCR artifact or other technical issues, it is noteworthy that 1 such sample (CML#24) contained 2 of 10 clones with the same mutation (P310S) that was not detected by direct sequencing. In addition, all mutations detected by direct sequencing were detected by the cloning and sequencing method, indicating the high fidelity of this method.
Compound mutations and exposure to multiple TKIs
Detailed clinical annotation was available for 28 patients: 20 with compound mutations and 8 with polyclonal mutations. Among these, 15 of 28 had received at least 2 sequential ABL1 TKI therapies (Table 3): 3 from the polyclonal mutation group and 12 from the compound mutation group. There was no pronounced overrepresentation of BCR-ABL1 compound mutations among advanced- or blastic-phase patients (69%; 9/13) compared with chronic-phase patients (11/15; 73%). However, the sample number available for this study does not permit a detailed analysis of the correlation between the phase of disease and the presence of multiple mutations.
In patients treated sequentially with dasatinib, nilotinib, or both TKIs after imatinib failure who had developed resistance to second-line treatment, analysis of the individual components of the compound mutations revealed that the identities of component mutations reflected the type of prior drug exposure. Therefore, in all patients treated with dasatinib, at least 1 component of the compound mutations was V299L, F317L, or E255K, all of which have been reported in clinical or in vitro resistance to dasatinib.10,11,19 Among these, the role of the E255K mutation in conferring clinical resistance to dasatinib is the least certain.20,21 In patients treated with nilotinib, at least 1 component was associated with clinical or in vitro resistance (E255K, Y253H, or F359V; Table 3).10,11 Analogous observations were made in the patients with polyclonal mutations (supplemental Table 3).
Low-level mutations
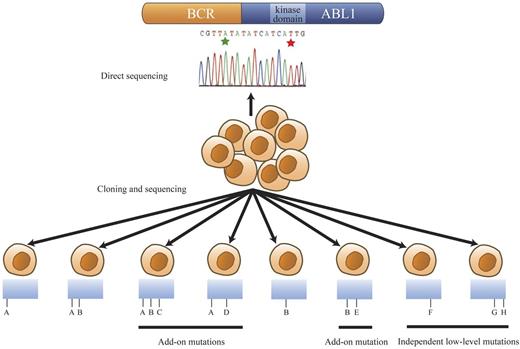
Cloning and sequencing of individual clones revealed many additional mutations that were not detectable by direct sequencing. The categories of mutation patterns that we observed among our samples are shown as a model in Figure 2. The majority of additional mutations are components of compound mutations and presumably originate from a BCR-ABL1 clone that carried a predominant mutation. We refer to this group of mutations as add-on compound mutations (Figure 2 mutations C-E) because they are acquired in addition to a preexisting predominant mutation. A second group of mutations present in clones separate from the predominant mutations are referred to as independent low-level mutations (Figure 2 mutations F-H). Based on the substantial underlying mutational complexity observed in our study, we reasoned that patients with multiple mutations may have a heightened risk of acquiring further mutations. Therefore, for comparison, we applied the cloning and sequencing approach to 12 additional patients with single mutations as detected by direct sequencing to screen for the presence of low-level mutations. However, we found no statistically significant difference in the prevalence of low-level single or compound mutations in patients with a single predominant mutation (25%; 3/12) compared with the main study group of patients with 2 or more predominant mutations detectable by direct sequencing (32%; 15/47; P = .74). The clinical significance of these low-level mutations is unknown. A recent study focusing on a selected set of 31 common BCR-ABL1 kinase domain mutations demonstrated a clear association between the presence of multiple low-level mutations and poor response to subsequent ABL1 TKI therapy.22-24 Parker et al specifically looked for the presence of known TKI-resistant mutants.22,24 The present study did not examine the impact of low-level mutants on TKI sensitivity.
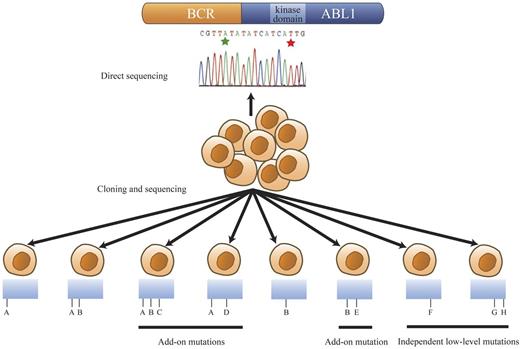
Mutational patterns revealed by cloning and sequencing of the BCR-ABL1 kinase domain region. Hypothetical mutation identities are designated A through H for discussion/explanation purposes. The range of mutation patterns revealed by cloning and sequencing when 2 BCR-ABL1 kinase domain mutations, A and B (green and red stars in the trace), are detected by direct sequencing are represented. This model encompasses all observed mutational patterns among the patients with 2 mutations evident by direct sequencing in this study. Detection of mutations A and B by direct sequencing can reflect their presence in the same BCR-ABL1 molecule (detected in 1 clone) or in different BCR-ABL1 molecules (detected in separate clones). The presence of both A and B in the same or different clones is signified as A/B or as A and B, respectively. In some compound mutant patients, clone A and clone B were observed separately in addition to clone A/B. Mutation A or B was sometimes coexistent with add-on mutations, for example, A/D and B/E. Predominant compound mutants could also acquire further add-on mutations as exemplified by A/B/C. Some mutations, such as F (single independent low-level mutants) or G/H (compound independent low-level mutants), were observed in clones that did not carry either predominant mutation, A or B.
Mutational patterns revealed by cloning and sequencing of the BCR-ABL1 kinase domain region. Hypothetical mutation identities are designated A through H for discussion/explanation purposes. The range of mutation patterns revealed by cloning and sequencing when 2 BCR-ABL1 kinase domain mutations, A and B (green and red stars in the trace), are detected by direct sequencing are represented. This model encompasses all observed mutational patterns among the patients with 2 mutations evident by direct sequencing in this study. Detection of mutations A and B by direct sequencing can reflect their presence in the same BCR-ABL1 molecule (detected in 1 clone) or in different BCR-ABL1 molecules (detected in separate clones). The presence of both A and B in the same or different clones is signified as A/B or as A and B, respectively. In some compound mutant patients, clone A and clone B were observed separately in addition to clone A/B. Mutation A or B was sometimes coexistent with add-on mutations, for example, A/D and B/E. Predominant compound mutants could also acquire further add-on mutations as exemplified by A/B/C. Some mutations, such as F (single independent low-level mutants) or G/H (compound independent low-level mutants), were observed in clones that did not carry either predominant mutation, A or B.
Clonal relationships
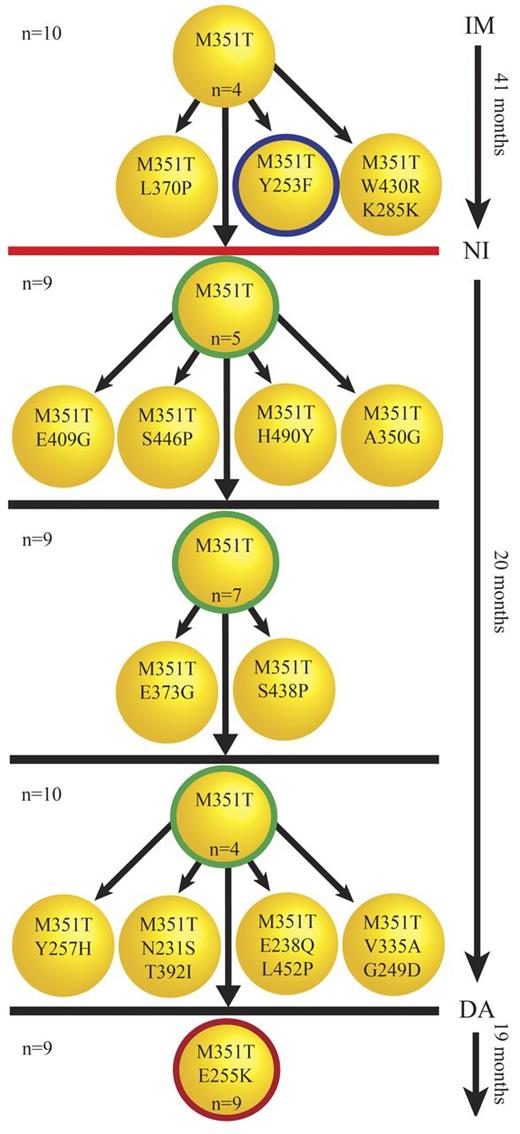
It is commonly thought that BCR-ABL1 kinase domain mutant clones evolve in a linear fashion through successive acquisition of different point mutations that incrementally increase their fitness to withstand selective pressure, for example, by conferring resistance to TKI therapy. Figure 3 gives an example of this linear evolution (shown for patient CML#19), and the clonal relationships for 61% (20/33) of compound mutant samples were compatible with this model. However, cloning and sequencing of individual PCR amplicons revealed the presence of each individual component as a single mutation in some patients. For example L387M, T315I, and the compound mutation L387M/T315I were all detected in separate clones originating from sample CML#44, suggesting that either L387M or T315I must have been acquired via 2 independent events. This unexpected pattern was observed in 39% (13/33) of compound mutation samples (Table 4) and may indicate that certain patients are predisposed toward a highly restricted range of mutations.
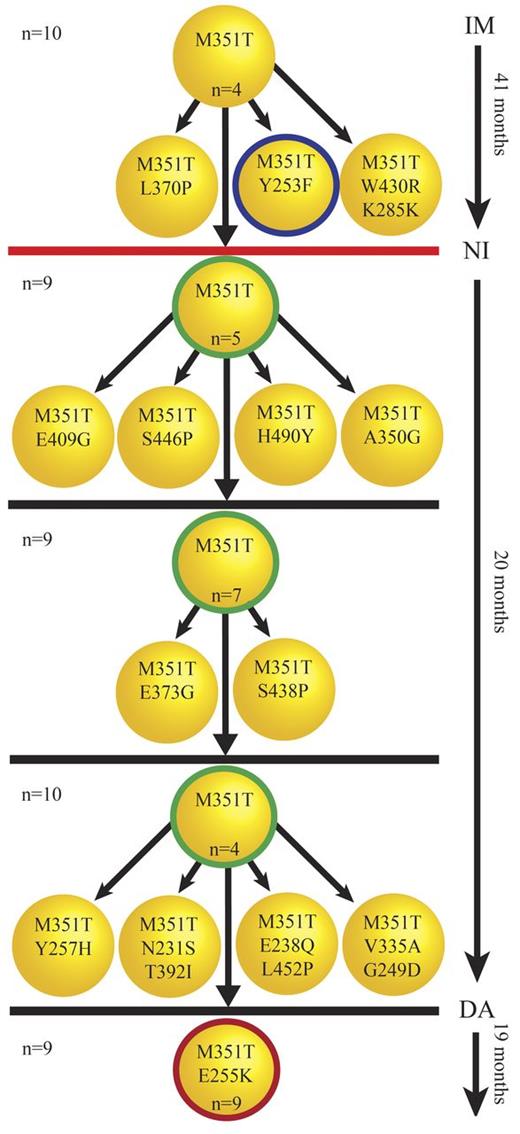
Successive acquisition of mutations. Five serial samples were available for patient CML#19. The clones with thick circles represent the mutations detected by direct sequencing. The number of sequenced clones for each sample is shown on the left. The number of each mutated clone is shown inside each clone unless it was detected only once. The unmutated or independent low-level mutant clones are not shown here. M351T and Y253F were detected by direct sequencing in the first sample under imatinib (IM) therapy (41 months). Cloning and sequencing confirmed M351T/Y253F as a compound mutation but also revealed other low-level clones presumably derived from M351T that were undetectable by direct sequencing. The red line indicates a 30-month gap between stoppage of imatinib therapy and start of nilotinib (NI) therapy, during which time the patient was treated with homoharringtonine. Direct sequencing in the next 3 samples taken after the start of nilotinib therapy detected only M351T. Similar to the first sample, cloning and sequencing revealed add-on compound mutants derived from M351T not detected by direct sequencing. An E255K/M351T compound mutation was first observed under dasatinib (DA) therapy. E255K/M351T was the only mutation detected in the last sample, and was evident by direct sequencing and the more sensitive cloning and sequencing method. M351T/E255K developed along with resistance to dasatinib in this patient. The patient's disease progressed to blast crisis a few months after detection of M351T/E255K and loss of response to dasatinib.
Successive acquisition of mutations. Five serial samples were available for patient CML#19. The clones with thick circles represent the mutations detected by direct sequencing. The number of sequenced clones for each sample is shown on the left. The number of each mutated clone is shown inside each clone unless it was detected only once. The unmutated or independent low-level mutant clones are not shown here. M351T and Y253F were detected by direct sequencing in the first sample under imatinib (IM) therapy (41 months). Cloning and sequencing confirmed M351T/Y253F as a compound mutation but also revealed other low-level clones presumably derived from M351T that were undetectable by direct sequencing. The red line indicates a 30-month gap between stoppage of imatinib therapy and start of nilotinib (NI) therapy, during which time the patient was treated with homoharringtonine. Direct sequencing in the next 3 samples taken after the start of nilotinib therapy detected only M351T. Similar to the first sample, cloning and sequencing revealed add-on compound mutants derived from M351T not detected by direct sequencing. An E255K/M351T compound mutation was first observed under dasatinib (DA) therapy. E255K/M351T was the only mutation detected in the last sample, and was evident by direct sequencing and the more sensitive cloning and sequencing method. M351T/E255K developed along with resistance to dasatinib in this patient. The patient's disease progressed to blast crisis a few months after detection of M351T/E255K and loss of response to dasatinib.
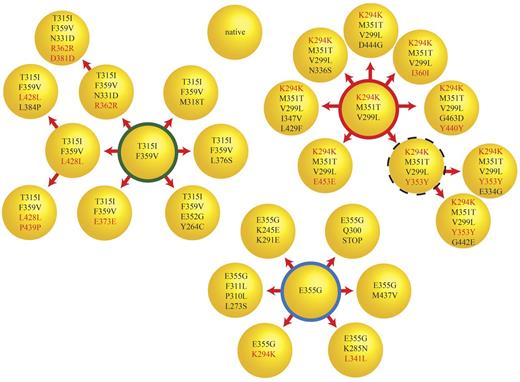
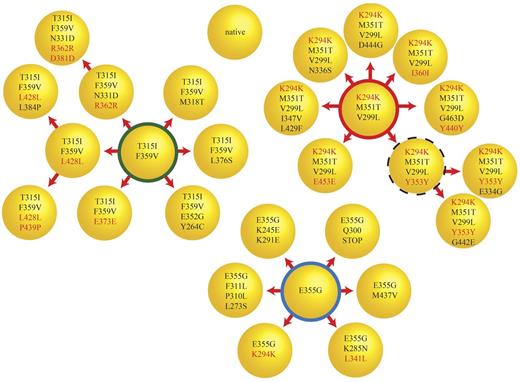
In 1 sample (CML#14), cloning and sequencing of 10 colonies revealed a compound mutation with a frequency that was similar to that of the predominant mutation but was not detected by direct sequencing. To better understand the complexity of clonal relationships in this sample, we sequenced the BCR-ABL1 kinase domain in an additional 45 bacterial colonies. We identified 3 independent major clones, each of which could be implicated in further mutational changes (Figure 4). In total, we found 26 different mutant clones, which is consistent with a high level of mutational complexity. None of the clones harbored more than 4 missense mutations and the number of silent mutations increased disproportionately with the total number of mutations in each clone. Based on this observation, we investigated whether there may be a limit to the number of missense mutations tolerated by BCR-ABL1.
Simultaneous evolution of compound mutant clones at comparable levels. Cloning and sequencing of 55 colonies for CML#14 revealed a complex pattern of compound mutations. There were at least 3 major classes of clones (F359V/T315I, K294K/M351T/V299L, and E355G) and their derivatives, which formed the majority of the BCR-ABL1 clones in this sample. The thick red, green, and blue circles represent 11, 6, and 14 clones, respectively. The dotted circle represents a clone that was not detected but was expected to be the parent of the 2 observed clones. The remaining circles represent clones detected a single time. These single clone results should be interpreted with caution because PCR error can never be rigorously excluded. Silent mutations are labeled in red. There is a marked increase in the proportion of silent mutations as clones acquire additional mutations. One clone presumably destined for immediate elimination has a stop mutation (E355G/Q300Stop) expected to abolish kinase activity in the middle of the kinase domain.
Simultaneous evolution of compound mutant clones at comparable levels. Cloning and sequencing of 55 colonies for CML#14 revealed a complex pattern of compound mutations. There were at least 3 major classes of clones (F359V/T315I, K294K/M351T/V299L, and E355G) and their derivatives, which formed the majority of the BCR-ABL1 clones in this sample. The thick red, green, and blue circles represent 11, 6, and 14 clones, respectively. The dotted circle represents a clone that was not detected but was expected to be the parent of the 2 observed clones. The remaining circles represent clones detected a single time. These single clone results should be interpreted with caution because PCR error can never be rigorously excluded. Silent mutations are labeled in red. There is a marked increase in the proportion of silent mutations as clones acquire additional mutations. One clone presumably destined for immediate elimination has a stop mutation (E355G/Q300Stop) expected to abolish kinase activity in the middle of the kinase domain.
Limited mutation tolerance of the BCR-ABL1 kinase domain
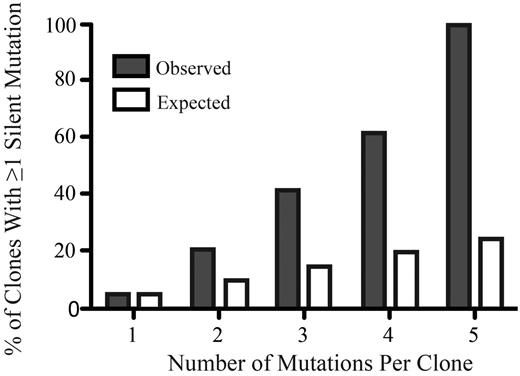
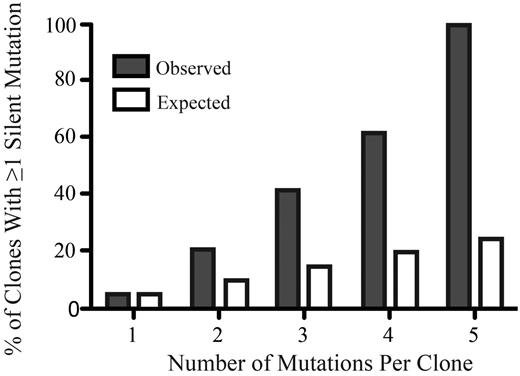
Add-on compound mutations were found in polyclonal and compound mutant samples, with some samples harboring up to 6 different components within the context of a compound mutation. The majority of missense add-on mutations (supplemental Table 4) were at residues that have not been associated previously with resistance, and most of these mutations were nonrecurrent, suggesting that they were passenger mutations that did not confer a competitive advantage or increased resistance to TKIs.9,18,25-29 We hypothesized that successively acquired missense mutations will eventually impair or abrogate kinase function. Given that such reduced-fitness clones will be eliminated in vivo, analyzing the frequency of silent mutations that are neutral with respect to kinase function can test this hypothesis only indirectly. If additional missense mutations are detrimental, then the likelihood of an additional mutation being a silent mutation should increase disproportionately relative to the total number of mutations in a given clone. We found that the proportion of silent mutations increased with the total number of mutations significantly beyond the calculated expectation, suggesting that additional missense mutations are poorly tolerated by the kinase (Figure 5 and supplemental Methods).
The percentage of silent mutations increases disproportionately with the total number of mutations per clone. The x-axis represents the number of mutations per clone and the y-axis represents the percentage of clones with at least 1 silent mutation (gray bars) compared with the expected percentage (white bars).
The percentage of silent mutations increases disproportionately with the total number of mutations per clone. The x-axis represents the number of mutations per clone and the y-axis represents the percentage of clones with at least 1 silent mutation (gray bars) compared with the expected percentage (white bars).
Discussion
BCR-ABL1 kinase domain mutations are the most common and best-characterized mechanism of TKI resistance in BCR-ABL1+ leukemia. Depending on the clinical setting (primary or secondary resistance and chronic or advanced phase), kinase domain mutations have been detected in 30%-90% of patients with imatinib resistance and in 20%-80% of patients who failed nilotinib or dasatinib.12,30-34 Although the latter 2 drugs overcome the majority of mutants associated with imatinib resistance, they are not universally active and exhibit some vulnerabilities. It has been suggested that sequential therapy with different ABL1 TKIs may predispose patients to the acquisition or selection of clones harboring multiple mutations, including mutations in the same BCR-ABL1 molecule (compound mutations).12 Certain compound mutations may pose a significant clinical problem because of their ability to confer cross-resistance to multiple TKIs, in contrast to mutations in different clones that are individually susceptible to 1 or more TKIs. If sequential TKI therapy (imatinib followed by dasatinib and/or nilotinib) predisposed a patient to multiresistant compound mutations, this would be a strong argument for the use of dasatinib or nilotinib as first-line therapy. Although double or multiple BCR-ABL1 mutations have been reported in many studies, it is unknown which proportion of these represent compound mutations.
In the present study, we investigated the frequency of compound mutations among 47 CML patients harboring 2 or more BCR-ABL1 kinase domain mutations as detected by direct Sanger sequencing. We confirmed through cloning and sequencing that 70% of the double mutations were in fact compound mutations. However, for polyclonal mutations, both mutations should be present at higher than 20% to be detectable by direct sequencing. When a stark unequal representation occurs (eg, 15% and 85%), then 1 of the mutations in a polyclonal mutation will become undetectable by direct sequencing. From a clinical point of view, direct sequencing may be biased toward the detection of compound mutations compared with polyclonal mutations.
We found that the composition of the compound mutations clearly reflected the TKI exposure history, with at least 1 component of each compound mutant in patients exposed to more than 1 TKI being associated with clinical or in vitro resistance to nilotinib or dasatinib. Examining the complexity of the clonality of BCR-ABL1 compound mutations according to disease phase is an interesting next step, but requires profiling within a larger focused cohort of samples not available from the current study.
We observed different patterns of mutations, which are shown in Figure 2. One concern regarding low-level mutations is the genuineness of these mutations. Because they are detected at a very low level, one must consider that they could be artifacts associated with cDNA synthesis or PCR. However, we have evidence that supports the genuineness of these mutations. First, we did not see any low-level mutations in the sequenced colonies for 3 samples. Specifically, these 3 samples contained exactly and only the mutations detected by direct sequencing, arguing against frequent introduction of PCR artifact with our methods. Second, these low-level mutations are not random: 39 low-level silent or missense mutations were detected in more than 1 patient (supplemental Table 5). Finally, the rate of silent mutations was lower than expected (supplemental Methods). However, technical artifact can never be ruled out with PCR-based analysis because a replication error early in the reaction will be perpetuated as the population of product is expanded.
We observed compound mutations with up to 6 components in individual clones. However, if more than 2 missense mutations were detected in a single clone, this particular combination was always limited to 1 of the 10 sequenced clones from each patient, suggesting that the add-on mutations failed to promote the clone's expansion. Moreover, the prevalence of silent mutations increased disproportionately with the total number of accumulated mutations. Because silent mutations are not subject to selection pressure, they are a convenient measure of mutation rate35 ; their disproportionately increased frequency in clones with multiple mutations suggests that clones that acquire additional missense mutations are not competitive, possibly because of impaired kinase function. These data suggest that the BCR-ABL1 kinase domain may have a limited tolerance for missense mutations. The evolution of mutant clones will be influenced both by the selection pressure imposed by TKIs and by the need to maintain kinase activity. We and others have previously reported that kinase domain mutations can alter kinase activity and downstream signaling.36,37 Although it is conceivable that, in some cases, fortuitous combinations of mutations may not attenuate kinase function,12 our data suggest that a lack of deleterious effects on kinase activity as the number of mutations increases must be the exception. Conversely, a surprisingly high proportion of double mutations represent compound mutations. Given that certain compound mutations such as G250E/T315I and E255V/T315I confer high-level resistance to ponatinib or DCC-2036 in vitro, our data suggest that these mutations may emerge as a significant clinical challenge in patients on third-line TKIs.13,14 Comprehensive in vitro TKI resistance profiles of BCR-ABL1 compound mutants have not been established. Among the 30 different BCR-ABL1 compound mutations detected in the present study (Table 2), 8 unique BCR-ABL1T315I–inclusive compound mutations were identified among 9 patients. There were 2 occurrences of BCR-ABL1T315I/F359V (CML#7 and CML#14). We recently identified and reported BCR-ABL1T315I–inclusive compound mutants recovered in cell-based screens for resistance to ponatinib13 or DCC-2036.38 Consultation of this dataset revealed that 75% (6/8) and 38% (3/8) of the BCR-ABL1T315I–inclusive mutants from the present study were recovered in the ponatinib or DCC-2036 resistance screen, respectively, and 38% (3/8) were recovered in both resistance screens (supplemental Table 6). The standard mutation detection technologies in current use cannot distinguish between polyclonal and compound mutations, and this limitation hampers clinicians' ability to choose between TKI options.
In the clonal dominance model, selective pressure exerted by TKIs drives the linear evolution of clones that acquire ever greater fitness to withstand TKIs and expand at the expense of less competitive clones. However, our data reveal a high level of clonal diversity. For example, in sample CML#46 from the polyclonal mutation group, we detected 3 clones, each with a different mutation at the same residue (ie, F317C, F317L, and F317V), indicating independent acquisition of mutations at the same residue by at least 3 different clones. Unexpectedly, this does not seem to be a rare event. In 39% of the patients with compound mutations, transcripts with each of the individual component mutations coexisted, indicating at least 2 independent events. This is similar to the pattern observed for some patients with myeloproliferative disorders in which JAK2 and TET2 mutations were observed in the same clone and also in different clones.39 One practical implication of this is that one cannot assume identical clones when a patient relapses with the same mutation after a transient response to TKI therapy.
It has been suggested that cancer development depends on the continuous acquisition of genetic variation by random mutations, with environmental selection acting on the resultant phenotype diversity.40 The evolution of BCR-ABL1 kinase domain mutant clones in the TKI environment is an example of the complex interplay between random mutation and selection in CML. Fortunately, there appear to be strong constraints on the evolution of the causal oncoprotein BCR-ABL1, which may contribute to the remarkable success of TKI therapy in CML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Suzanne Wickens for clerical assistance.
This work was supported by the National Institutes of Health (grants HL082978-01 and CA04963920A2 to M.W.D.), the Leukemia & Lymphoma Society (grant 7036-01 to M.W.D.), and the Howard Hughes Medical Institute. A.M.E. is a Fellow and M.W.D. is a Scholar in Clinical Research of the Leukemia & Lymphoma Society.
National Institutes of Health
Authorship
Contribution: J.S.K., T.O., and M.W.D. wrote the manuscript; T.W.K., S.S., C.A.E., T.L., D.M., and B.J.D. provided the clinical information for the samples; J.S.K., P.S., S.S., L.T.A., C.A.E., M.S.Z., J.C.E., and M.A. performed the experiments; T.W.K., B.J.D., S.S., A.G.R., and L.F. provided materials; J.S.K. and C.C.M. analyzed the data; J.S.K., A.D.P., and I.L.K. produced the figures; C.A.E., A.D.P., A.M.E., D.J.A., Z.G., A.G.R., and D.M. commented on the manuscript; and T.O. and M.W.D. designed the experiments.
Conflict-of-interest disclosure: S.S. is a consultant for Novartis, BMS, and ARIAD. T.L. is a consultant for Novartis, BMS, and Pfizer and receives research funding from Novartis. B.J.D. is a principal investigator or coinvestigator on Novartis, Bristol-Myers Squibb, and ARIAD clinical trials and his institution has contracts with these companies to pay for patient costs, nurse and data manager salaries, and institutional overhead; he does not derive salary, nor does his laboratory receive funds from these contracts. M.W.D. is a consultant for BMS, Novartis, ARIAD, and Incyte and his laboratory receives research funding from BMS and Novartis. The Oregon Health & Science University (OHSU) and B.J.D. have a financial interest in MolecularMD and OHSU has licensed technology used in some of these clinical trials to MolecularMD. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. The remaining authors declare no competing financial interests.
Correspondence: Michael W. Deininger, MD, PhD, Huntsman Cancer Institute, 2000 Circle of Hope, Salt Lake City, UT 84112-5550; e-mail: michael.deininger@hci.utah.edu.
References
Author notes
T.O. and M.W.D. contributed equally to this work.