Key Points
Metformin use was associated with significantly fewer SCD-related health care utilization encounters and clinical events.
Our findings provide the first evidence to suggest potential clinical benefits associated with metformin use in patients with SCD.
Abstract
Metformin was recently found to increase fetal hemoglobin, which is protective in sickle cell disease (SCD). We tested the hypothesis that, among adults with SCD and diabetes mellitus (DM), metformin use is associated with fewer adverse SCD clinical outcomes and lower health care utilization. This is a retrospective cohort study using the MarketScan Medicaid claims database for 2006 to 2016, comparing metformin users and nonusers. Patients on hydroxyurea, insulin, or iron chelation were excluded. Main outcomes included annual rates of all-cause inpatient encounters, all-cause emergency department (ED) encounters, inpatient and ED encounters with SCD codes, vaso-occlusive episodes (VOEs), strokes, acute chest syndrome (ACS), avascular necrosis (AVN), and gallstones. Of 457 adults (median age [interquartile range], 43 years [33-52 years]; 72% female), 142 (31%) were treated with metformin. Adjusted for age, sex, and Charlson Comorbidity Index, metformin users had significantly lower rate ratios of all-cause inpatient encounters (0.68; 95% confidence interval [CI], 0.52-0.88; P < .01), inpatient encounters with SCD codes (0.45; 95% CI, 0.30-0.66; P < .01), ED encounters with SCD codes (0.34; 95% CI, 0.21-0.54; P < .01), VOE (0.22; 95% CI, 0.12-0.41; P < .01), ACS (0.17; 95% CI, 0.05-0.60; P = .01), and AVN (0.30; 95% CI, 0.11-0.87; P = .03). A subgroup analysis of 54 enrollees preinitiation and postinitiation of metformin did not indicate significant changes in rates of clinical events. Metformin was associated with significantly fewer inpatient and ED SCD encounters in adults with SCD and DM; however, confounding of underlying SCD severity cannot be excluded.
Introduction
Sickle cell disease (SCD) is an inherited hemoglobin disorder affecting ∼100 000 individuals in the United States, primarily those of African American descent.1 SCD was estimated to affect 312 000 neonates with homozygous sickle hemoglobin globally in 2010 alone, suggesting an important public health problem worldwide.2 SCD is a chronic debilitating health condition that extends from childhood to adulthood and has been associated with substantial morbidity and mortality.3-5 Patients with SCD suffer from intermittent sickling with vaso-occlusion, which leads to a number of complications, including acute and chronic pain, lung and heart disease, cerebrovascular disease, kidney injury, and avascular necrosis (AVN) of joints.3,4 Patients with SCD have increased health care utilization, with frequent hospitalizations and emergency department (ED) visits.6-9 Hydroxyurea is efficacious in preventing these complications and decreasing health care utilization in this population by increasing fetal hemoglobin (HbF) levels.10,11 However, hydroxyurea has a number of side effects, and its uptake and adherence is suboptimal.12-15
Although the prevalence of diabetes mellitus (DM) in the United States is relatively high, affecting nearly 30.3 million individuals,16 earlier studies suggested that DM is relatively rare in patients with SCD,17-23 which was not supported in a recent study by Zhou et al that reported a prevalence of 16.5% of DM in SCD patients.24 Therefore, the prevalence of DM in patients with SCD remains unclear. Metformin is a commonly used drug for the initial treatment of type 2 DM25,26 as well as for the management of overweight and obese nondiabetic patients.27,28 Metformin was recently suggested to have potential benefits for SCD through HbF induction with upregulation of FOXO3 and γ globin expression, anti-inflammatory properties, and increased nitric oxide production.29,30 However, these findings are based on laboratory experiments with no clinical data. Two recent retrospective studies reported in vivo evidence of an increase in HbF level in response to metformin use in patients with SCD (N = 18)31 and those without SCD (N = 7),32 although neither reported statistically significant findings. Both studies were limited to a small number of patients, and neither examined the relationship between metformin use and SCD-related outcomes or health care utilization.31,32 Therefore, the relation of metformin utilization to clinical outcomes and health care utilization in adults with SCD and DM, to date, has not been studied.
Our objectives were to identify prevalent cases of DM in a cohort of publicly insured adult patients with SCD and to assess the association of metformin with clinical outcomes and health care utilization in this population. We hypothesized that adults with SCD and DM who take metformin have less frequent SCD-related complications and lower utilization of hospital care for those complications than similar adults with SCD who do not take metformin.
Methods
Data source
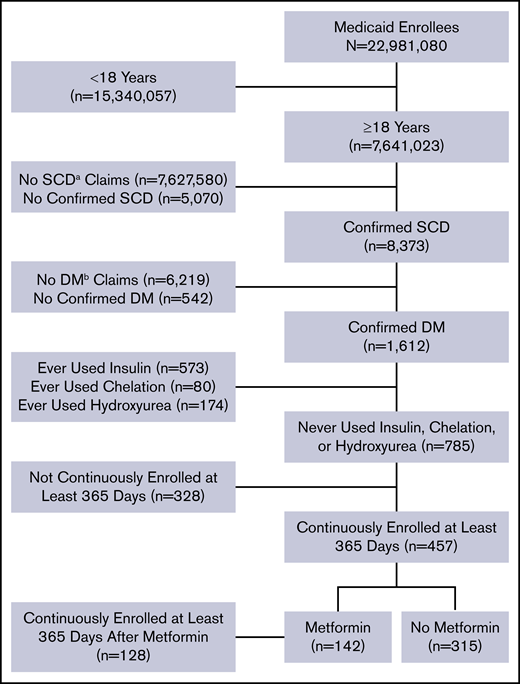
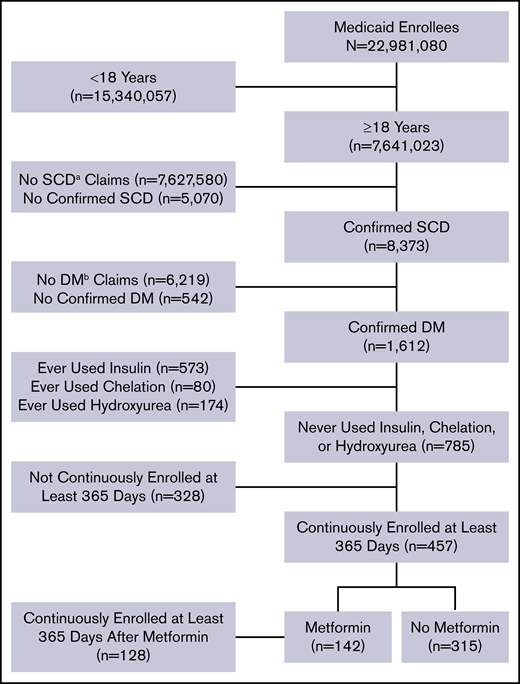
The data were extracted from the Truven Health Analytics MarketScan Medicaid Multi-State Database for the period from 1 January 2006 to 30 June 2016. The database pools claims from ∼23 million Medicaid enrollees from 8 to 12 states, varying by year. The database was accessed on 2 October 2017 via the Treatment Pathways 4.0 tool online analytic interface, and data were extracted to construct a cohort (Figure 1).
Flow diagram of study participants and their eligibility for inclusion in the analysis.aSCD, sickle cell disease; bDM, diabetes mellitus.
Flow diagram of study participants and their eligibility for inclusion in the analysis.aSCD, sickle cell disease; bDM, diabetes mellitus.
Inclusion and exclusion criteria
Medicaid enrollees who were at least 18 years of age as of 1 January 2006 and were enrolled at any point in the study period were identified with SCD and DM based on medical claims coded using International Classification of Disease (ICD), ninth or tenth revision codes. Enrollees who met all of the following criteria were included in the analysis: (1) at least 2 outpatient SCD claims separated by at least 7 days during a 2-year period from the first encounter or at least 1 inpatient SCD claim; (2) at least 2 outpatient DM claims separated by at least 30 days during a 2-year period starting from the first encounter, 1 outpatient DM claim and 1 DM drug claim, or at least 1 inpatient diabetes claim; and (3) continuous enrollment at least 365 days after the first diabetes claim. These criteria of requiring 2 outpatient visits or 1 inpatient visit have been used to identify SCD and DM patients in other studies utilizing administrative data.33-36 A list of ICD-9 and ICD-10 codes used to identify SCD and diabetes claims are listed in supplemental Table 1.
We used additional exclusion criteria to eliminate potential sources of confounding. In particular, we excluded enrollees with drug claims for iron chelation drugs, insulin, or hydroxyurea. SCD patients receiving chronic blood transfusions to reduce the occurrence of SCD-related complications are at risk for iron overload–related endocrinopathies, including DM. We also excluded patients using insulin to avoid any potential interaction with metformin that could affect our interpretation of the study findings. Finally, hydroxyurea has known protective effects in patients with SCD, and we wanted to avoid any interaction or confounding effects related to its use along with metformin. Furthermore, a preliminary analysis indicated that inclusion of enrollees with drug claims for iron chelation drugs, insulin, or hydroxyurea altered the summary effect estimate by >10%, providing evidence of possible confounding.37
Metformin use
Among the enrollees identified with DM, metformin use was identified using drug claims data, and metformin users were contrasted with metformin nonusers. In addition, a secondary analysis was done of the association of metformin adherence with outcomes among metformin users. Adherence was assessed for those subjects enrolled at least 365 days after their first metformin claim based on the medication possession ratio (MPR).38 The MPR was calculated as the sum of days supplied with metformin, divided by the number of days of follow-up, less the number of days hospitalized.38-41 It was assumed that enrollees received a full supply of metformin and had 100% adherence during hospitalization. Adherence was categorized as high (MPR ≥ 80%), moderate (MPR < 80% to ≥ 40%), and/or low (MPR < 40%).
Outcome events
Outcome events following the first diabetes claim in the study period were identified using a method similar to Candrilli et al.42 ICD-10 codes were mapped to ICD-9 codes using the ICD-9 to ICD-10 Crosswalk Tool (http://www.icd10codesearch.com/). We identified inpatient encounters by searching for any claims in the inpatient setting. Inpatient encounters with SCD codes were identified by searching for any claims listing an SCD code (supplemental Table 1) in the inpatient setting. Similarly, ED encounters not resulting in an inpatient admission were identified by searching for any claims in the outpatient ED setting, and ED encounters with SCD codes were identified by searching for any claims listing an SCD code in the outpatient ED setting. We identified vaso-occlusive episodes (VOEs) by searching for the VOE-related claims (supplemental Table 2). Similarly, acute chest syndrome (ACS), AVN, and gallstone events were identified by searching for claims listing corresponding ICD-9 or ICD-10 codes for those outcomes (supplemental Table 2). We identified stroke events by searching for stroke-related claims within the inpatient setting (supplemental Table 2).
Statistical analysis
For the comparison of metformin users and nonusers, the numbers of events occurring after the first diabetes claim were calculated for each eligible enrollee. Rates of events were calculated as the number of events divided by the total number of enrollment days. The number of enrollees with at least 1 event was compared between groups using the χ2 test. The median rate of events was compared between groups using the nonparametric Mann-Whitney U test and adherence levels were compared using the Kruskal-Wallis test. Rate ratios (RRs) were calculated using negative binomial regression, with the natural logarithm of enrollment time as the offset.
We also conducted a subgroup analysis to examine the effect of adherence among metformin users. Data were limited to the 365 days after the first metformin claim in this subgroup analysis. The numbers of events occurring after the first metformin claim were calculated for each eligible enrollee. Rates of events were calculated as the number of events divided by 365, minus the number of inpatient days.
Adjusted RRs were produced using negative binomial regression, adjusting for age group (young adults [18 years ≤ age < 40 years] vs adults [age ≥ 40 years]),43,44 sex, and Charlson Comorbidity Index,45 which have been used in several prior SCD studies.42,46-49 The Charlson Comorbidity Index is a weighted index to predict risk of death within 1 year of hospitalization for patients with 19 specific comorbid conditions.45 The Charlson Comorbidity Index was calculated using claims data (see supplemental Table 3 for list of claims corresponding to each index item).45 Given the small sample size, we did not disaggregate results by sex. For the comparison of metformin users to nonusers, nonusers were considered the referent group. For the subgroup analysis of the associations of metformin adherence with events, nonadherent metformin users were considered the referent group. An additional subgroup analysis examined within-subject changes in rates of events before and after metformin initiation. Enrollees with 365 days of continuous enrollment before and after their first metformin claim were included. For each outcome, rates of events were calculated as the number of events in a year. Analyses were done using SAS 9.4 and GraphPad Prism 6. P < .05 was considered statistically significant.
Results
Study population
We identified 8373 patients with SCD and 1612 of them (19%) also had a diagnosis of DM. Our final cohort totaled 457 patients who met all of our predefined criteria (Figure 1). Of them, 142 were metformin users (31%) and 315 were nonusers (69%). Thirty-eight metformin users (27%) and 10 nonusers (3%) had claims for other oral glucose-lowering medications. The majority of patients were female (330; 72%). The median age [interquartile range] was 43 years [33-52 years] and the median Charlson Comorbidity Index score [interquartile range] was 1 [0-4] (Table 1). There were no statistically significant differences in patient characteristics according to metformin status or adherence level using the MPR percentage (Table 1). All clinical events in our cohort are summarized in Table 2, including all-cause inpatient encounters, inpatient encounters with SCD codes, all-cause ED encounters, ED encounters with SCD codes, VOE events, ACS episodes, and stroke, AVN, and gallstone events.
Clinical outcomes by metformin use
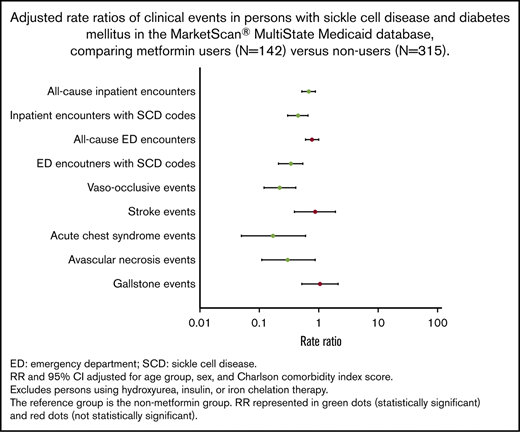
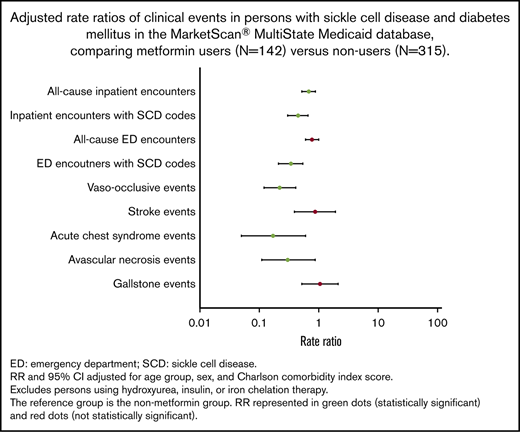
Overall, patients in the metformin group had clinically meaningful and statistically significantly less frequent clinical events compared with those in the nonmetformin group (Table 2). Patients in the metformin group had a significantly lower rate per year of all-cause inpatient encounters (P < .01), inpatient encounters with SCD codes (P < .01), ED encounters with SCD codes (P = .01), VOE events (P < .01), and ACS episodes (P = .01) (Table 2; supplemental Figure 1). Moreover, patients in the metformin group had significantly lower frequencies of 1 or more VOE (P < .01) and ACS episodes (P < .01) (Table 2).
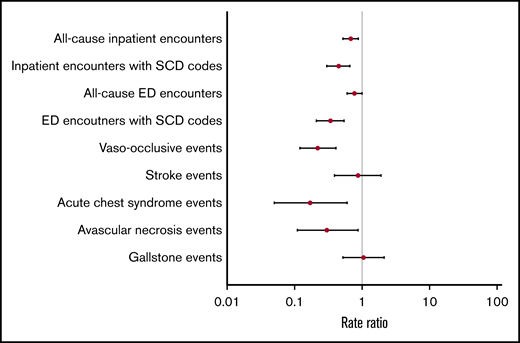
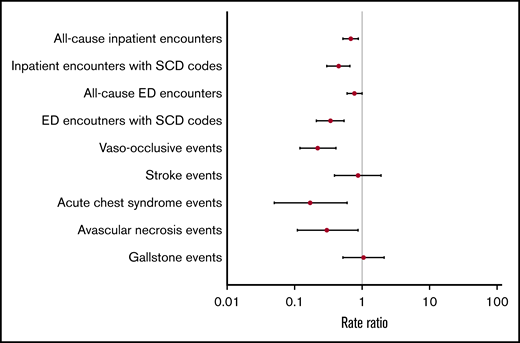
Overall, adjusted RRs of clinical events were lower in the metformin group compared with the nonmetformin group (Figure 2). Patients in the metformin group had significantly lower adjusted RRs of all-cause inpatient encounters (P < .01), inpatient encounters with SCD codes (P < .01), ED encounters with SCD codes (P < .01), VOE events (P < .01), ACS episodes (P = .01), and AVN events (P=.03) (Table 3). Similar trends of clinical events were also observed when we stratified patients by age groups as young adults (<40 years) vs adults (≥40 years) (Table 3).
Evaluating consistency of clinical outcomes with metformin use
To further evaluate the consistency of the association of metformin use with clinical outcomes and health care utilization, in a first subgroup analysis we assessed adherence levels in a subset of metformin users with at least 12 months of follow-up after their first metformin claim. We calculated adherence rates for most metformin users using the MPR (128 of 142; 90%). Adherence to metformin varied across different subgroups as follows (n, %): high (37, 29%), moderate (49, 38%), and low (42, 33%) adherence (Table 1). Overall, patients with higher adherence to metformin had less frequent clinical events, compared with those with lower adherence levels. In particular, patients with high and moderate adherence to metformin had significantly fewer inpatient encounters with SCD codes compared with those with low adherence (P < .01) (Table 4). Using negative binomial regression, adjusted for age, sex, and Charlson Comorbidity Index score, patients with high or moderate metformin adherence had less frequent all-cause inpatient encounters and inpatient encounters with SCD codes as well as all-cause ED encounters and ED encounters with SCD codes, compared with those with low adherence levels, although these differences were not statistically significant (Table 5; supplemental Figure 2).
A second subgroup analysis excluded 48 patients who took other oral glucose-lowering drugs; results were essentially unchanged (not reported). Another subgroup analysis conducted among 54 enrollees with 365 days of continuous enrollment before and after initiation of metformin did not indicate significant changes in rates of clinical events (Table 6; supplemental Figure 3).
Discussion
To our knowledge, this study is the first to examine clinical associations of metformin use with SCD-related complications in adults with SCD and DM covered by Medicaid. We found that metformin might have protective effects with less frequent hospital-treated clinical events. Among adults with SCD and DM, metformin users had significantly fewer all-cause inpatient encounters as well as inpatient and ED encounters with SCD codes. In particular, metformin users had significantly fewer VOEs, ACS episodes, and AVN events. A supplemental analysis among 90% of our original sample of metformin users suggested that patients with higher adherence to metformin had fewer clinical events than patients with low adherence.
DM has not been commonly studied in patients with SCD, with published data mainly from case reports or case series17-23 and single-center studies.50-54 In our study, one-fifth of our cohort with a diagnosis of SCD also had a probable diagnosis of DM (19%). Our findings were comparable to the reported prevalence of DM in a recent study by Zhou et al (16.5%)24 and among African Americans, using data from the National Health and Nutrition Examination Survey (NHANES) (18.2%).55 In contrast, earlier studies suggested a much lower prevalence of DM among adults with different SCD genotypes, ranging from 3.5% to 7%.50-52,56 The relatively higher prevalence of DM in our cohort as well as others24,55 could be multifactorial. First, as SCD patients have an increasing life expectancy over the past 3 decades as a result of advances in therapy (ie, hydroxyurea and stem cell transplant),57,58 they will be at higher risk of developing chronic medical conditions, especially metabolic ones, such as DM, dyslipidemia, and hypertension.24,55 Second, a number of common risk factors have been associated with these metabolic conditions, including poor diet, sedentary lifestyle, and central obesity, all of which have been a growing problem in our American society.16,55,59-61 Third, the higher prevalence of DM in our study could be related to being an exclusively Medicaid population. Finally, confirming the diagnosis of DM in patients with SCD can be challenging. Because of the shortened lifespan of red blood cells in patients with SCD, glycosylated hemoglobin (HbA1c), a commonly used laboratory marker for uncontrolled hyperglycemia and DM, might be at falsely low levels.62 In support, other reports suggested that HbA1c is an unreliable screening test for hyperglycemia and DM in SCD patients as well as those with sickle cell trait.63,64 These findings suggest that previous studies may have underestimated the burden of these metabolic conditions, particularly DM, in SCD patients.
Hydroxyurea, approved by the US Food and Drug Administration, is a medication for SCD that has been associated with improvements in clinical outcomes.9,65-69 Previous studies have shown that patients with SCD who were on hydroxyurea had significantly lower rates of hospitalizations by 27% to 58%, ED visits by 43%, and VOEs by 36% to 44%.66,67,70 In our study, we found that metformin use was likewise associated with significantly lower rates of SCD-related clinical events compared with no metformin use. Metformin use was associated with significantly lower median rates of inpatient encounters with SCD codes by 55%, ED encounters with SCD codes by 66%, VOEs by 78%, ACS episodes by 83%, and AVN events by 70%. We also found significantly lower median rates of all-cause inpatient encounters but not ED encounters, which could be secondary to DM-related care in the ED setting (eg, diabetic foot wound or infection). In our analysis, we did not find significant differences in the Charlson Comorbidity Index scores between metformin users and nonusers, suggesting comparable health status in both groups. Furthermore, when we evaluated the effects of metformin on clinic outcomes in both groups, we adjusted for Charlson Comorbidity Index scores, which controls for non-SCD–specific comorbidity. We also found that patients with higher adherence to metformin had less frequent inpatient and ED encounters with SCD codes, albeit not statistically significant, which was consistent with a similar relationship with higher hydroxyurea adherence.42,71-73 Contraindications for metformin use include renal disease and hepatic disease. Although there were no statistically significant differences in Charlson Comorbidity Index scores between metformin users and nonusers, there were statistically significant differences in the proportion of users and nonusers with evidence of renal and/or hepatic disease in their administrative claims data. Fewer metformin users had evidence of renal and/or hepatic disease, likely reflecting the exclusion of these patients from using metformin due to contraindication. Nevertheless, a subanalysis excluding those with these contraindications produced similar results (data not shown).
Our study used a large, multistate database of public insurance claims data, which have been used in several published studies on SCD.74-78 Additionally, our study contained data across several years (2006-2016) with information from at least 300 hospitals each year that varied in size, teaching status, and geographical locations.
This study has limitations. The MarketScan data we used are composed of Medicaid data collected from contributing states. States contributing data may change year to year. For this reason, long-term follow-up of individual patients is limited. Thus, we chose to conduct a cross-sectional study to assess the association between metformin use and outcomes in persons with SCD and DM. Cross-sectional studies have limitations, including the inability to establish a temporal relationship between the exposure and the outcome. Furthermore, we lacked data to adjust for differences between metformin users and nonusers in underlying SCD severity. There is currently no validated SCD severity score that can be applied to administrative claims data; however, the Charlson Comorbidity Index has been used by other investigators to control for underlying comorbidity burden in SCD-related studies using administrative claims data.42,46-49 Therefore, although the study controlled for differences in non-SCD–specific comorbidity, we cannot exclude confounding by indication as an explanation for the findings. If patients who have a high risk of complications of SCD are less likely to be prescribed metformin, one would observe inverse associations between metformin use and the frequency of those complications even if metformin had no effect. Potential reasons for metformin prescription, in addition to DM treatment, include its antitumor, antiaging, cardiovascular-protective, and neuroprotective effects, and as a treatment of polycystic ovary syndrome.79 It is not obvious that prescription of metformin for any of these indications would be more common in patients with more severe SCD. Additionally, the MarketScan Multi-State Medicaid database is based on a convenience sample of participating states, and the enrollees in this database all have public insurance. This may limit the generalizability of our findings to other US populations, such as those who are uninsured or have private insurance, live in other states, or live outside of the United States. Furthermore, limiting inclusion to those with SCD and DM claims and to nonusers of hydroxyurea, insulin or iron chelation further limits generalizability. Another limitation is that, because claims data are collected for billing purposes, misclassification of clinical and pharmacy information can occur. The validity of claims-based algorithms for identifying individuals with probable diagnoses of SCD or DM has been shown to be acceptable, although for SCD, such data have been restricted to children.80,81 If misclassification bias is nondifferential, the associations with metformin will be biased toward the null. In addition, this database does not provide detailed information on other potentially important confounders. The identification of patients with probable SCD and DM on the basis of claims using SCD-specific and DM-specific ICD-9 codes may have excluded adults with milder SCD and DM and overrepresented those with more severe disease. On the other hand, by excluding SCD patients on hydroxyurea to avoid any interaction or confounding effects, we may have excluded SCD patients with more severe disease, who are more likely to use hydroxyurea. Lack of laboratory values for the study population, including HbF levels, is another inherent limitation in MarketScan data. Finally, our subgroup analysis finding that rates of hospital encounters with SCD codes were equivalent before and after metformin use was initiated is suggestive of the possibility of confounding by indication and contrasts with our main analysis findings. This second subgroup analysis was necessarily restricted to a select subgroup of 38% of our original metformin group who had continuous coverage 365 days before and after their initial metformin prescription. Thus, it is difficult to compare the findings with our main analysis, both because of the imprecision of the findings and the possibility that other non-SCD factors influenced health and care-seeking over time in this subgroup. Indeed, small sample size and lack of statistical power precluded us from examining the most specific SCD health events (VOEs, ACS episodes, and AVN events) in this subgroup. Furthermore, this subanalysis is itself subject to limitations and is inconsistent with another subgroup finding that higher adherence to metformin is associated with lower rates of hospital encounters with SCD codes. Thus, the findings from the current analyses do not provide sufficient information to assess to what extent confounding by indication has influenced the main study results. Nonetheless, the subgroup analyses findings highlight the need for a more rigorously designed trial in which patients with SCD are randomized to receive metformin before drawing conclusions about the causal effect of metformin use on SCD events. Furthermore, given the nature of our study, we cannot determine whether the observed benefits of metformin are a result of its effects on SCD pathophysiology, DM pathophysiology, or both.
In conclusion, the findings from this study are in agreement with the hypothesis that metformin might have protective clinical effects in adults with SCD. Moreover, the findings are in line with a previous laboratory biology study documenting positive effects of metformin on HbF induction. Nonetheless, given the inherent limitations of claims data and the conflicting results from 1 subgroup analysis, we cannot rule out the possibility of confounding by indication as an explanation for the findings presented here. Thus, these findings are best considered hypothesis-generating. Given the known safety profile of metformin and the emerging evidence of the possibility that metformin might have beneficial effects in patients with SCD, future prospective studies are warranted to evaluate its efficacy and cost-effectiveness in relation to clinical outcomes, quality of life, mortality, and health care utilization in adults with SCD.
Acknowledgments
The authors thank Scott D. Grosse, at the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, for his support and guidance during data analysis.
This work was supported by grant number K12HS023011 (S.M.B.) from the Agency for Healthcare Research and Quality.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Authorship
Contribution: S.M.B. and A.B.P. designed the research study and interpreted the data; A.B.P. analyzed the data and critically revised the paper; S.M.B. drafted the paper; and both authors approved the submitted final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sherif M. Badawy, Ann & Robert H. Lurie Children’s Hospital of Chicago, 225 E Chicago Ave, Box #30, Chicago, IL 60611; e-mail: sbadawy@luriechildrens.org.
References
Author notes
Data-sharing requests may be e-mailed to the corresponding author.
The full-text version of this article contains a data supplement.