Mice lacking thrombopoietin (TPO) or its receptor c-Mpl are severely thrombocytopenic, consistent with a dominant physiological role for this cytokine in megakaryocytopoiesis. However, these mice remain healthy and show no signs of spontaneous hemorrhage, implying that TPO-independent mechanisms for platelet production exist and are sufficient for hemostasis. To investigate the roles of cytokines that act through the gp130 signaling chain in the residual platelet production of mpl-/- mice, mpl-/-IL-6-/-, mpl-/-LIF-/-, andmpl-/-IL-11R-/-double-mutant mice were generated. In each of these compound mutants, the number of circulating platelets was no lower than that observed in mice lacking only the c-mpl gene. Moreover, the deficits in the numbers of megakaryocytes and megakaryocyte progenitor cells in the bone marrow and spleen were no further exacerbated inmpl-/-IL-6-/-,mpl-/-LIF-/-, ormpl-/-IL-11R-/-double-mutant mice compared with those in Mpl-deficient animals. In single IL-6-/-, LIF-/-, andIL-11R-/- mutant mice, platelet production was normal. These data establish that, as single regulators, IL-6, IL-11, and LIF have no essential role in normal steady-state megakaryocytopoiesis, and are not required for the residual megakaryocyte and platelet production seen in thec-mpl-/- mouse.
Megakaryocytopoiesis is the tightly regulated process by which multipotential hemopoietic cells commit to the production of megakaryocytes, which undergo an ordered process of maturation, ultimately resulting in the release of platelets into the circulation. Thrombopoietin (TPO), acting through its receptor c-Mpl, is the major physiological regulator of megakaryocyte and platelet production. TPO has actions both in vitro and in vivo on megakaryocyte proliferation and maturation. In vitro TPO stimulates the production of megakaryocytes from human or murine progenitors in liquid or semisolid media.1-4 It also acts to induce megakaryocyte maturation. Large megakaryocytes of high DNA ploidy can be generated from mouse bone marrow or human CD34+ cells cultured in the presence of TPO,1,2,4-6 and these cells are able to develop proplatelets and shed platelets into the growth medium.7,8Administration of TPO to laboratory animals or humans results in a striking increase in platelets, megakaryocytes, and their progenitors.4,9-12 The indispensable in vivo functions of TPO have been elaborated in mice genetically manipulated to lack this cytokine or its receptor. TPO-/- ormpl-/- mice are severely thrombocytopenic and deficient in megakaryocytes and their progenitors.13-15These data indicate that the primary role of TPO is to regulate megakaryocyte numbers through the control of progenitor cell production and maturation.
Although TPO is the primary regulator of megakaryocyte and platelet production, mice lacking this cytokine or its receptor retain the capacity to produce sufficient platelets to prevent spontaneous hemorrhage.13-15 This implies that alternative regulators of megakaryocyte and platelet production exist in the absence of TPO signaling. A number of other cytokines have been shown to influence megakaryocytopoiesis. IL-3 is a potent in vitro stimulus of megakaryocyte colony formation and can also modestly elevate megakaryocyte and platelet numbers in vivo.16 However, we and others have shown that megakaryocytopoiesis is normal in IL-3-deficient mice,17,18 and that IL-3 does not contribute to the residual thrombopoiesis in animals lacking c-Mpl.17,19 Granulocyte-macrophage colony-stimulating factor (GM-CSF) is also capable of stimulating megakaryocyte colony formation in vitro and increased numbers of megakaryocytes in vivo.20 Stem cell factor (SCF) has a similar activity, although both these molecules are considerably less potent than IL-3.21
IL-6, IL-11, and LIF are members of a group of cytokines, the receptors for which are composed of ligand specific alpha chains and a common receptor subunit, the 130 kd glycoprotein (gp130).22 Acting alone, these regulators have little or no megakaryocyte colony stimulating activity, but can amplify the actions of IL-3 in megakaryocyte colony assays.23 Within this lineage, they appear to act most potently as stimuli of maturation. In vitro, IL-6, IL-11, and LIF each induce megakaryocytes to enlarge, become increasingly polyploid and to begin the cytoplasmic reorganization that typically preceeds platelet release.24,25 In vivo, these actions have the effect of elevating megakaryocyte and platelet numbers, usually to levels approaching twice those in untreated mice.26-28 Despite these actions, mice lacking IL-6, LIF, or the specific IL-11 α-chain are not thrombocytopenic.29-31
Although these cytokines are dispensable for the generation of normal platelet numbers in the presence of TPO signaling, their contribution to this lineage may become evident in the absence of the dominant regulator. To better explore the in vivo roles for IL-6, LIF, and IL-11 in megakaryocytopoiesis, and to determine whether these cytokines contribute to residual platelet production inmpl-/- mice, we have generated a series of compound mutant mice that are deficient in c-Mpl in addition to IL-6, LIF, or the IL-11Rα chain. Despite the loss of the actions of these cytokines, no exacerbation of the Mpl-deficient thrombocytopenia was observed in the compound mutants, nor were there further reductions in the number of megakaryocytes or their progenitors. Thus, IL-6, IL-11, and LIF appear to have no essential role in normal steady-state megakaryocytopoiesis, and even in the absence of TPO signaling are not required for the residual megakaryocyte and platelet production seen in the c-mpl-/- mouse.
Materials and methods
Mice
The c-Mpl–deficient mice15 and mice lacking functional LIF,30 IL-11Rα,31 or IL-632 genes have all been described previously. Compound heterozygote progeny (eg,mpl+/-LIF+/-) were produced by mating homozygous mutant parents. These were subsequently interbred to produce offspring, 1 in 16 of which was expected to be homozygous mutant at both loci. As all mice were of mixed C57Bl/6 and 129/Sv genetic background, wild-type controls included a combination of data collected concurrently from mice of each of these parental strains. All mice were analyzed at between 2 and 4 months of age.
Cytokines
Recombinant murine SCF was produced in Pichia pastoris and was purified before use. Recombinant human erythropoietin (EPO) was a kind gift from Amgen (Thousand Oaks, CA), recombinant murine GM-CSF was purchased from Schering (Kenilworth, NJ) and recombinant murine IL-3 was purchased from PeproTech (Rocky Hill, NJ).
Hematologic and progenitor cell analysis
Peripheral blood was collected from the retro-orbital sinus and diluted into 2 mL of 3% acetic acid, containing methylene blue (white cells) or 1% ammonium oxalate (platelets) for manual cell counts using hemocytometer chambers and standard microscopy. Megakaryocytes were enumerated by microscopic examination of hematoxylin and eosin–stained histologic sections of sternal bone marrow and spleen. A minimum of 30 microscopic fields was scored. Manual differential cell counts were performed using May-Grunwald Giemsa–stained thin blood smears and cytocentrifuge preparations of bone marrow and spleen.
The clonal culture of hemopoietic progenitor cells was performed in 1 mL cultures of 2.5 × 104 (bone marrow) or 105 (spleen) cells formpl-/-IL-6-/-andmpl-/-LIF-/-mice and 5 × 104mpl-/-IL-11Rα-/- bone marrow cells. All cells were cultured in 0.3% agar in Dulbecco's modified Eagles medium (DMEM) containing 20% foetal calf serum (FCS). Cytokines were used at the final concentrations of: 10 ng/mL murine IL-3, 100 ng/mL murine SCF, 2 U/mL human EPO, and 10 ng/mL murine GM-CSF. The cultures were incubated for 7 days at 37°C in fully a humidified atmosphere of 5% CO2 in air. Agar cultures were then fixed with 2.5% glutaraldehyde and sequentially stained with acetylcholinesterase, Luxol Fast Blue and hematoxylin, and the composition of each colony was determined at 100- to 400-fold magnifications.
Results
Production of mutant mice
To explore the roles of IL-6, IL-11, and LIF in megakaryocytopoiesis in the absence of TPO signaling, mice deficient in IL-6, LIF, or the IL-11R α chain in addition to c-Mpl were generated. Progeny of the 9 genotypes possible were obtained in numbers predicted by a normal Mendelian pattern of allele segregation from matings between mpl+/-IL-6+/- parental mice as well as from intercrosses of mpl+/-IL-11Rα+/- mice. Double mutantmpl-/-IL-6-/- andmpl+/-IL-11Rα-/-mice were indistinguishable from their littermates at birth and developed normally. Moreover, no lethality or illness was observed in adult mice, suggesting that neither IL-6 nor IL-11 was critical for the health or survival of mpl-/-mice. In our colony, in intercrosses ofLIF+/- mice, more than 80% of mice homozygous for the mutant LIF allele died in utero. The reason for this lethality is unclear, although analysis suggests that death occurs around the time of birth (L. Robb, unpublished data). Accordingly, in offspring of mpl+/- LIF+/-parents, the number ofmpl-/-LIF-/- mice was fewer than anticipated. However, a deficit in production ofmpl+/+LIF-/- mice occurred to a similar degree, suggesting that prenatal lethality was not exacerbated by the combined lack of LIF and c-Mpl. Thempl-/-LIF-/- mice that were born appeared normal and developed to adulthood in a manner indistinguishable from their normal littermates.
Peripheral blood
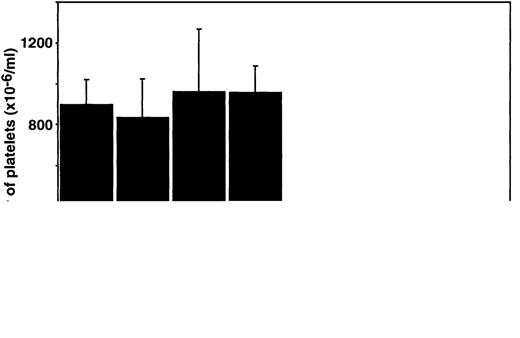
Analysis of platelet numbers in all mice lacking c-Mpl showed the expected thrombocytopenia.15 However,mpl-/- mice also lacking LIF, IL-6, or IL-11Rα had no fewer platelets than animals lacking only c-Mpl (Figure 1). As has been shown previously,29-31 the numbers of platelets in mice lacking LIF, IL-6, or the IL-11Rα chain alone were not significantly different to wild-type mice (Figure 1). The hematocrit and number of white blood cells were normal in mice of all genotypes. The relative numbers of circulating lymphocytes, granulocytes, eosinophils, and monocytes were also all within the normal ranges (Table1).
Platelet numbers in double mutant mice.
Platelet counts in wild-type (wt) mice and mice lacking c-mpl, LIF, IL-6, IL-11 receptor alpha chain (IL-11Rα), or combinations of these regulators are shown. The means ± SD were determined from measurements from 3 to 9 mice per genotype. No statistically significant differences were observed (P > .05, Studentt test) for comparison of data from LIF-/-, IL-6-/-, or IL-11Rα-/- mice with wild-type controls or of LIF-/-mpl-/-, IL-6-/-mpl-/-, or IL-11Rα-/-mpl-/-mice withmpl-/- data.
Platelet numbers in double mutant mice.
Platelet counts in wild-type (wt) mice and mice lacking c-mpl, LIF, IL-6, IL-11 receptor alpha chain (IL-11Rα), or combinations of these regulators are shown. The means ± SD were determined from measurements from 3 to 9 mice per genotype. No statistically significant differences were observed (P > .05, Studentt test) for comparison of data from LIF-/-, IL-6-/-, or IL-11Rα-/- mice with wild-type controls or of LIF-/-mpl-/-, IL-6-/-mpl-/-, or IL-11Rα-/-mpl-/-mice withmpl-/- data.
Hematocrit and white blood cell counts in double mutant mice
| . | Genotype . | |||||||
|---|---|---|---|---|---|---|---|---|
| Wild Type . | LIF−/− . | IL-6−/− . | IL-11Ra−/− . | mpl−/− . | mpl−/− LIF−/− . | mpl−/− IL-6−/− . | mpl−/− IL-11Ra−/− . | |
| Hematocrit (%) | 53 ± 2 | 49 ± 2 | 51 ± 3 | 48 ± 1* | 50 ± 2 | 49 ± 4 | 51 ± 1 | 48 ± 2 |
| White cell count (×10−6/mL) | 4.7 ± 1.5 | 6.6 ± 1.9 | 3.5 ± 1.2 | 8.2 ± 2.7* | 3.9 ± 1.2 | 6.5 ± 2.4 | 4.3 ± 1.2 | 6.7 ± 2.0 |
| Neutrophils (%) | 7 ± 2 | 15 ± 2 | 11 ± 4 | 15 ± 8 | 9 ± 9 | 16 ± 10 | 17 ± 16 | 14 ± 13 |
| Lymphocytes (%) | 84 ± 4 | 80 ± 1 | 84 ± 4 | 76 ± 9 | 86 ± 10 | 78 ± 9 | 75 ± 20 | 82 ± 14 |
| Monocytes (%) | 7 ± 4 | 4 ± 1 | 3 ± 1 | 8 ± 4 | 4 ± 3 | 5 ± 3 | 7 ± 5 | 3 ± 2 |
| Eosinophils (%) | 2 ± 2 | 1 ± 1 | 2 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 |
| . | Genotype . | |||||||
|---|---|---|---|---|---|---|---|---|
| Wild Type . | LIF−/− . | IL-6−/− . | IL-11Ra−/− . | mpl−/− . | mpl−/− LIF−/− . | mpl−/− IL-6−/− . | mpl−/− IL-11Ra−/− . | |
| Hematocrit (%) | 53 ± 2 | 49 ± 2 | 51 ± 3 | 48 ± 1* | 50 ± 2 | 49 ± 4 | 51 ± 1 | 48 ± 2 |
| White cell count (×10−6/mL) | 4.7 ± 1.5 | 6.6 ± 1.9 | 3.5 ± 1.2 | 8.2 ± 2.7* | 3.9 ± 1.2 | 6.5 ± 2.4 | 4.3 ± 1.2 | 6.7 ± 2.0 |
| Neutrophils (%) | 7 ± 2 | 15 ± 2 | 11 ± 4 | 15 ± 8 | 9 ± 9 | 16 ± 10 | 17 ± 16 | 14 ± 13 |
| Lymphocytes (%) | 84 ± 4 | 80 ± 1 | 84 ± 4 | 76 ± 9 | 86 ± 10 | 78 ± 9 | 75 ± 20 | 82 ± 14 |
| Monocytes (%) | 7 ± 4 | 4 ± 1 | 3 ± 1 | 8 ± 4 | 4 ± 3 | 5 ± 3 | 7 ± 5 | 3 ± 2 |
| Eosinophils (%) | 2 ± 2 | 1 ± 1 | 2 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 |
Mean ± SD, n = 2 to 9 mice of each genotype.
P < .05 for comparison of data from LIF−/−, IL-6−/−, or IL-11Rα−/− mice with wild-type controls or of LIF−/− mpl−/−, IL-6−/− mpl−/−, or IL-11Rα−/− mpl−/− mice with mpl−/− data. No other statistically significant differences were observed.
Megakaryocytopoiesis
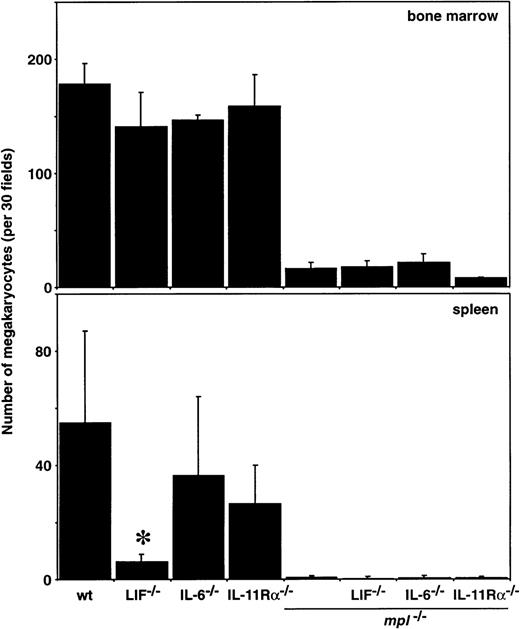
To determine the effects of multiple cytokine deficiencies on the production of megakaryocytes, the numbers of these cells in the sternal marrow and spleen were enumerated from histologic sections. Consistent with previous results,15 megakaryocyte numbers were reduced up to 10-fold in the bone marrow, and essentially absent in the spleens, of all mice lacking c-Mpl (Figure2). The loss of IL-6 or the IL-11Rα chain alone had little effect on the number of megakaryocytes in either of these tissues. Mice deficient solely for LIF exhibited normal bone marrow megakaryocyte numbers but a reduced number of these cells in the spleen (Figure 2). Hemopoietic deficiencies confined to the spleen have previously been observed in LIF-/- mice and may relate to impaired stem cell function in these mice.30
Megakaryocytes in the bone marrow and spleen of double mutant mice.
Megakaryocyte numbers in histologic sections of sternal marrow and spleen from wild-type (wt) mice and mice lacking c-mpl, LIF, IL-6, IL-11 receptor alpha-chain (IL-11Rα) or combinations of these genes are shown. The means ± SD of megakaryocyte numbers per 30 high power fields (bone marrow, × 400; spleen, × 200) were calculated from samples taken from 2 to 9 mice per genotype. *P < .05 for comparison of LIF-/-, IL-6-/-, or IL-11Rα-/-with wt or of LIF-/-mpl-/-, IL-6-/-mpl-/-, or IL-11Rα-/-mpl-/- withmpl-/-. No other statistically significant differences were observed.
Megakaryocytes in the bone marrow and spleen of double mutant mice.
Megakaryocyte numbers in histologic sections of sternal marrow and spleen from wild-type (wt) mice and mice lacking c-mpl, LIF, IL-6, IL-11 receptor alpha-chain (IL-11Rα) or combinations of these genes are shown. The means ± SD of megakaryocyte numbers per 30 high power fields (bone marrow, × 400; spleen, × 200) were calculated from samples taken from 2 to 9 mice per genotype. *P < .05 for comparison of LIF-/-, IL-6-/-, or IL-11Rα-/-with wt or of LIF-/-mpl-/-, IL-6-/-mpl-/-, or IL-11Rα-/-mpl-/- withmpl-/-. No other statistically significant differences were observed.
Morphologically recognizable cells of other hemopoietic lineages in the bone marrow and spleen were examined in cytocentrifuge preparations. The numbers of blast cells, neutrophils, monocytes, lymphocytes, eosinophils, and erythroid precursors were normal in mice of all genotypes (Table 2).
Hematologic analysis of double mutant mice
| . | Genotype . | |||||||
|---|---|---|---|---|---|---|---|---|
| Wild Type . | LIF−/− . | IL-6−/− . | IL-11Rα−/− . | mpl−/− . | mpl−/− LIF−/− . | mpl−/− IL-6−/− . | mpl−/− IL-11Rα−/− . | |
| Bone marrow | ||||||||
| Blasts (%) | 4 ± 1 | 3 ± 0 | 4 ± 1 | 4 ± 1 | 3 ± 1 | 3 ± 1 | 3 ± 2 | 1 ± 1 |
| Promyelocytes/myelocytes (%) | 6 ± 2 | 7 ± 4 | 6 ± 3 | 7 ± 2 | 2 ± 2 | 7 ± 4 | 4 ± | 6 ± 1 |
| Metamyelocytes/neutrophils (%) | 23 ± 2 | 21 ± 7 | 25 ± 3 | 24 ± 5 | 26 ± 4 | 22 ± 9 | 28 ± 6 | 50 ± 18* |
| Lymphocytes (%) | 26 ± 3 | 24 ± 5 | 28 ± 4 | 26 ± 7 | 37 ± 5 | 28 ± 3* | 31 ± 6 | 21 ± 12* |
| Monocytes (%) | 10 ± 5 | 7 ± 1 | 7 ± 2 | 8 ± 2 | 5 ± 3 | 10 ± 4 | 8 ± 4 | 4 ± 1 |
| Eosinophils (%) | 4 ± 3 | 5 ± 4 | 4 ± 1 | 4 ± 3 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 |
| Nucleated erythroid cells (%) | 29 ± 6 | 33 ± 10 | 26 ± 3 | 27 ± 6 | 26 ± 4 | 29 ± 8 | 25 ± 1 | 17 ± 7* |
| Spleen | ||||||||
| Weight (mg) | 77 ± 14 | 83 ± 18 | 71 ± 22 | 70 ± 16 | 82 ± 20 | 94 ± 8 | 80 ± 12 | 75 ± 11 |
| Blasts (%) | 4 ± 2 | 2 ± 2 | 3 ± 2 | 3 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 2 | 0 |
| Promyelocytes/myelocytes (%) | 0 | 0 | 0 | 0 | 0 | 1 ± 1 | 0 | 1 ± 1 |
| Metamyelocytes/neutrophils (%) | 6 ± 3 | 3 ± 2 | 4 ± 3 | 1 ± 1 | 2 ± 1 | 2 ± 2 | 7 ± 4 | 9 ± 9 |
| Lymphocytes (%) | 82 ± 7 | 89 ± 2 | 81 ± 5 | 89 ± 6 | 83 ± 14 | 86 ± 6 | 83 ± 4 | 77 ± 10 |
| Monocytes (%) | 3 ± 3 | 3 ± 1 | 3 ± 1 | 2 ± 2 | 1 ± 1 | 4 ± 3 | 3 ± 2 | 3 ± 2 |
| Eosinophils (%) | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 0 | 0 | 1 ± 1 | 0 |
| Nucleated erythroid cells (%) | 4 ± 3 | 2 ± 2 | 8 ± 5 | 4 ± 6 | 12 ± 14 | 5 ± 5 | 4 ± 3 | 10 ± 4 |
| . | Genotype . | |||||||
|---|---|---|---|---|---|---|---|---|
| Wild Type . | LIF−/− . | IL-6−/− . | IL-11Rα−/− . | mpl−/− . | mpl−/− LIF−/− . | mpl−/− IL-6−/− . | mpl−/− IL-11Rα−/− . | |
| Bone marrow | ||||||||
| Blasts (%) | 4 ± 1 | 3 ± 0 | 4 ± 1 | 4 ± 1 | 3 ± 1 | 3 ± 1 | 3 ± 2 | 1 ± 1 |
| Promyelocytes/myelocytes (%) | 6 ± 2 | 7 ± 4 | 6 ± 3 | 7 ± 2 | 2 ± 2 | 7 ± 4 | 4 ± | 6 ± 1 |
| Metamyelocytes/neutrophils (%) | 23 ± 2 | 21 ± 7 | 25 ± 3 | 24 ± 5 | 26 ± 4 | 22 ± 9 | 28 ± 6 | 50 ± 18* |
| Lymphocytes (%) | 26 ± 3 | 24 ± 5 | 28 ± 4 | 26 ± 7 | 37 ± 5 | 28 ± 3* | 31 ± 6 | 21 ± 12* |
| Monocytes (%) | 10 ± 5 | 7 ± 1 | 7 ± 2 | 8 ± 2 | 5 ± 3 | 10 ± 4 | 8 ± 4 | 4 ± 1 |
| Eosinophils (%) | 4 ± 3 | 5 ± 4 | 4 ± 1 | 4 ± 3 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 |
| Nucleated erythroid cells (%) | 29 ± 6 | 33 ± 10 | 26 ± 3 | 27 ± 6 | 26 ± 4 | 29 ± 8 | 25 ± 1 | 17 ± 7* |
| Spleen | ||||||||
| Weight (mg) | 77 ± 14 | 83 ± 18 | 71 ± 22 | 70 ± 16 | 82 ± 20 | 94 ± 8 | 80 ± 12 | 75 ± 11 |
| Blasts (%) | 4 ± 2 | 2 ± 2 | 3 ± 2 | 3 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 2 | 0 |
| Promyelocytes/myelocytes (%) | 0 | 0 | 0 | 0 | 0 | 1 ± 1 | 0 | 1 ± 1 |
| Metamyelocytes/neutrophils (%) | 6 ± 3 | 3 ± 2 | 4 ± 3 | 1 ± 1 | 2 ± 1 | 2 ± 2 | 7 ± 4 | 9 ± 9 |
| Lymphocytes (%) | 82 ± 7 | 89 ± 2 | 81 ± 5 | 89 ± 6 | 83 ± 14 | 86 ± 6 | 83 ± 4 | 77 ± 10 |
| Monocytes (%) | 3 ± 3 | 3 ± 1 | 3 ± 1 | 2 ± 2 | 1 ± 1 | 4 ± 3 | 3 ± 2 | 3 ± 2 |
| Eosinophils (%) | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 0 | 0 | 1 ± 1 | 0 |
| Nucleated erythroid cells (%) | 4 ± 3 | 2 ± 2 | 8 ± 5 | 4 ± 6 | 12 ± 14 | 5 ± 5 | 4 ± 3 | 10 ± 4 |
Mean ± SD, n = 2 to 6 mice of each genotype.
P < .05 for comparison of data from LIF−/−, IL-6−/−, or IL-11Rα−/− mice with wild-type controls or of LIF−/− mpl−/−, IL-6−/− mpl−/−, or IL-11Rα−/− mpl−/− mice with mpl−/− data. No other statistically significant differences were observed.
Hemopoietic progenitor cells
To assess the effects of the loss of LIF, IL-6, and the IL-11Rα chain on more primitive cell populations, the numbers and lineage commitment of progenitor cells from the bone marrow of mutant mice were analyzed in semisolid agar cultures stimulated with IL-3 and SCF, a combination of growth factors known to stimulate colony formation by a wide range of committed progenitors.15
Although previous studies suggested that IL-6-/-mice have a mild megakaryocyte progenitor cell defect,29 in our analysis the number of these committed cells was normal, as were megakaryocyte progenitor cell numbers in LIF-/-marrow (Figure 3). Previous studies have demonstrated that in mice lacking IL-11Rα, megakaryocyte progenitor numbers were normal.31 As expected, the deficit in megakaryocyte progenitor cells characteristic ofmpl-/- mice15 was evident inmpl-/-IL-6-/-,mpl-/-LIF-/-, andmpl-/-IL-11Rα-/- double mutants, but in none of these mice was the deficiency exacerbated (Figures 3 and 5). A similar picture emerged from analysis of spleen cells: The reduced number of megakaryocyte colonies typical ofmpl-/- cultures was not altered in cultures ofmpl-/-IL-6-/- ormpl-/-LIF-/- splenocytes (Figure 3).
Megakaryocyte progenitor cells in double mutant mice.
The means ± SD of acetylcholinesterase-positive colonies in semisolid agar cultures of 2.5 × 104 femoral bone marrow cells or 105 spleen cells are shown. Cells were stimulated with the combination of IL-3, SCF, and EPO. No statistically significant differences were observed (P > .05, Students' t test) for comparison of data from LIF-/-, IL-6-/-, or IL-11Rα-/-mice with wild-type controls or of LIF-/-mpl-/-, IL-6-/-mpl-/-, or IL-11Rα-/-mpl-/- mice withmpl-/ -data.
Megakaryocyte progenitor cells in double mutant mice.
The means ± SD of acetylcholinesterase-positive colonies in semisolid agar cultures of 2.5 × 104 femoral bone marrow cells or 105 spleen cells are shown. Cells were stimulated with the combination of IL-3, SCF, and EPO. No statistically significant differences were observed (P > .05, Students' t test) for comparison of data from LIF-/-, IL-6-/-, or IL-11Rα-/-mice with wild-type controls or of LIF-/-mpl-/-, IL-6-/-mpl-/-, or IL-11Rα-/-mpl-/- mice withmpl-/ -data.
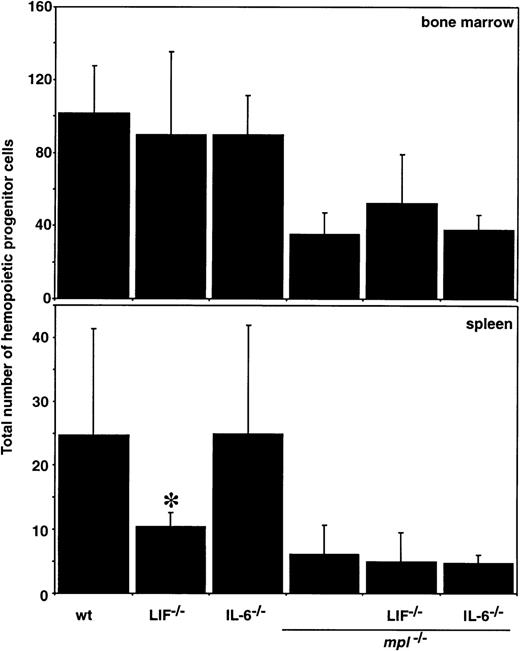
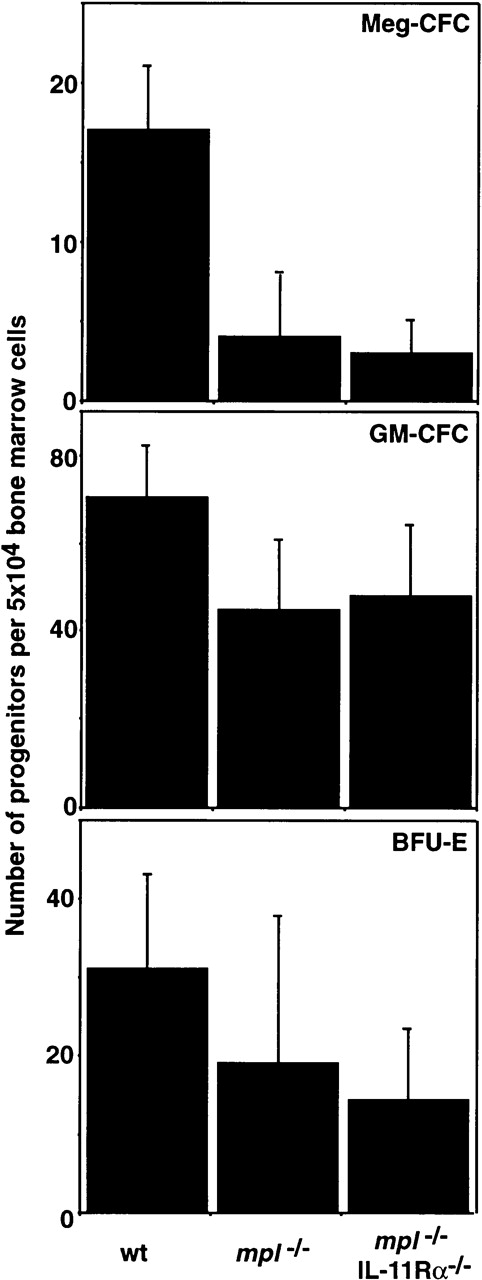
The effects of ablating multiple cytokine signaling pathways on the number and lineage commitment of other hemopoietic progenitor cells was also assessed in semisolid agar cultures. The total number of hemopoietic progenitor cells as well as the relative frequency of preprogenitors and cells committed to the granulocyte, macrophage, and eosinophil lineages was normal in mice lacking IL-6 or LIF. As expected, mpl-/- marrow lacked normal numbers of progenitor cells of all lineages, but this was not exacerbated inmpl-/-LIF-/- ormpl-/-IL-6-/- mice (Figure4 and Table 3). GM-CFC and BFU-E were assessed in IL-11Rα-/- andmpl-/-IL-11Rα-/-mice and no significant differences in the number of these progenitor cells were observed relative to wild-type ormpl-/- controls, respectively (Figure5).
Hemopoietic progenitor cells in double mutant mice.
The means ± SD of the total number of colonies that developed in semisolid agar cultures of 2.5 × 104 femoral bone marrow cells or 105 spleen cells is shown. Cells were stimulated with the combination of IL-3, SCF, and EPO. *P < .05 for comparison of LIF-/-, IL-6-/-, or IL-11Rα-/- with wild type (wt) or of LIF-/-mpl-/-, IL-6-/-mpl-/-, or IL-11Rα-/-mpl-/-withmpl-/-. No other statistically significant differences were observed.
Hemopoietic progenitor cells in double mutant mice.
The means ± SD of the total number of colonies that developed in semisolid agar cultures of 2.5 × 104 femoral bone marrow cells or 105 spleen cells is shown. Cells were stimulated with the combination of IL-3, SCF, and EPO. *P < .05 for comparison of LIF-/-, IL-6-/-, or IL-11Rα-/- with wild type (wt) or of LIF-/-mpl-/-, IL-6-/-mpl-/-, or IL-11Rα-/-mpl-/-withmpl-/-. No other statistically significant differences were observed.
Hemopoietic progenitor cells in double mutant mice
| Colony Number . | Genotype . | |||||
|---|---|---|---|---|---|---|
| Wild Type . | LIF−/− . | IL-6−/− . | mpl−/− . | mpl−/−LIF−/− . | mpl−/−IL-6−/− . | |
| Bone marrow (per 25 000 cells) | ||||||
| Blast | 15 ± 3 | 14 ± 1 | 9 ± 0 | 2 ± 1 | 2 ± 2 | 2 ± 2 |
| Granulocyte | 36 ± 7 | 20 ± 43-150 | 31 ± 17 | 13 ± 8 | 20 ± 13 | 20 ± 1 |
| Mixed granulocyte/macrophage | 41 ± 15 | 22 ± 10 | 21 ± 5 | 8 ± 5 | 18 ± 6 | 13 ± 7 |
| Macrophage | 27 ± 6 | 20 ± 10 | 32 ± 22 | 13 ± 8 | 24 ± 12 | 28 ± 53-150 |
| Eosinophil | 5 ± 1 | 2 ± 13-150 | 3 ± 1 | 0.5 ± 0.6 | 1 ± 2 | 2 ± 2 |
| Spleen (per 100 000) cells | ||||||
| Blast | 3 ± 3 | 1 ± 1 | 3 ± 4 | 0.3 ± 0.5 | 1 ± 2 | 0 |
| Granulocyte | 3 ± 2 | 0.5 ± 0.7 | 2 ± 0 | 2 ± 1 | 0.3 ± 0.6 | 1 ± 1 |
| Mixed granulocyte/macrophage | 1 ± 1 | 0 | 0.5 ± 0.7 | 0 | 0.3 ± 0.6 | 0.3 ± 0.6 |
| Macrophage | 2 ± 2 | 0 | 2 ± 0 | 0.3 ± 0.5 | 0.3 ± 0.6 | 0.3 ± 0.6 |
| Eosinophil | 0.3 ± 0.5 | 0 | 0 | 0 | 0 | 0 |
| Colony Number . | Genotype . | |||||
|---|---|---|---|---|---|---|
| Wild Type . | LIF−/− . | IL-6−/− . | mpl−/− . | mpl−/−LIF−/− . | mpl−/−IL-6−/− . | |
| Bone marrow (per 25 000 cells) | ||||||
| Blast | 15 ± 3 | 14 ± 1 | 9 ± 0 | 2 ± 1 | 2 ± 2 | 2 ± 2 |
| Granulocyte | 36 ± 7 | 20 ± 43-150 | 31 ± 17 | 13 ± 8 | 20 ± 13 | 20 ± 1 |
| Mixed granulocyte/macrophage | 41 ± 15 | 22 ± 10 | 21 ± 5 | 8 ± 5 | 18 ± 6 | 13 ± 7 |
| Macrophage | 27 ± 6 | 20 ± 10 | 32 ± 22 | 13 ± 8 | 24 ± 12 | 28 ± 53-150 |
| Eosinophil | 5 ± 1 | 2 ± 13-150 | 3 ± 1 | 0.5 ± 0.6 | 1 ± 2 | 2 ± 2 |
| Spleen (per 100 000) cells | ||||||
| Blast | 3 ± 3 | 1 ± 1 | 3 ± 4 | 0.3 ± 0.5 | 1 ± 2 | 0 |
| Granulocyte | 3 ± 2 | 0.5 ± 0.7 | 2 ± 0 | 2 ± 1 | 0.3 ± 0.6 | 1 ± 1 |
| Mixed granulocyte/macrophage | 1 ± 1 | 0 | 0.5 ± 0.7 | 0 | 0.3 ± 0.6 | 0.3 ± 0.6 |
| Macrophage | 2 ± 2 | 0 | 2 ± 0 | 0.3 ± 0.5 | 0.3 ± 0.6 | 0.3 ± 0.6 |
| Eosinophil | 0.3 ± 0.5 | 0 | 0 | 0 | 0 | 0 |
Mean ± SD, n = 2 to 4 mice of each genotype.
P < .05 for comparison of data from LIF−/−, IL-6−/−, or IL-11R−/− mice with wild-type controls or of LIF−/− mpl−/−, IL-6−/− mpl−/−, or IL-11Rα−/− mpl−/− mice with mpl−/− data. No other statistically significant differences were observed.
Hemopoietic progenitor cells inmpl−/−IL-11R−/−double mutant mice.
The mean ± SD of the total number of colonies that developed in semisolid agar cultures of 2.5 × 104 femoral bone marrow cells or 105 spleen cells is shown. Cells were stimulated with IL-3 and SCF (Meg-CFC), GM-CSF, IL-3, and SCF (GM-CFC) or SCF and EPO (BFU-E). No statistically significant differences were observed (P > .05, Students' t test) for comparison of IL-11Rα-/- with wild type (wt) or of IL-11Rα-/-mpl-/- withmpl-/-.
Hemopoietic progenitor cells inmpl−/−IL-11R−/−double mutant mice.
The mean ± SD of the total number of colonies that developed in semisolid agar cultures of 2.5 × 104 femoral bone marrow cells or 105 spleen cells is shown. Cells were stimulated with IL-3 and SCF (Meg-CFC), GM-CSF, IL-3, and SCF (GM-CFC) or SCF and EPO (BFU-E). No statistically significant differences were observed (P > .05, Students' t test) for comparison of IL-11Rα-/- with wild type (wt) or of IL-11Rα-/-mpl-/- withmpl-/-.
The total number of hemopoietic progenitor cells in the spleens of LIF-/- mice was slightly reduced, consistent with previous observations.30 Spleens fromIL-6-/- mice contained normal numbers of progenitor cells of all hemopoietic lineages (Figure 4). Despite some variability in the differential counts, compounded by the low progenitor cell levels, there were no major differences in splenic blast-CFC, nor progenitors of granulocytes, macrophages, or eosinophils inmpl-/-IL-6-/- ormpl-/-LIF-/- mice in comparison with mpl-/- controls (Table 3).
Discussion
Although mice lacking TPO or its receptor c-Mpl are profoundly thrombocytopenic, they live a normal lifespan without overt signs of hemorrhage. This implies that important TPO-independent mechanisms can account for about 5% to 20% of normal steady-state platelet production, a level that is sufficient for hemostasis. In this study, we have explored the role of cytokines that use the gp130 signaling chain in residualmpl-/- megakaryocytopoiesis through the generation of compound mutant mice that lack IL-6, LIF, or the ligand-binding chain of the IL-11 receptor in addition to c-Mpl. The thrombocytopenia characteristic of mpl-/- mice was not exacerbated in mpl-/-IL-6-/-,mpl-/-LIF-/-, ormpl-/-IL-11Rα-/-double-mutant mice (Figure 1). The compound mutants also produced numbers of megakaryocytes and their committed progenitor cells in the bone marrow and spleen (Figures 2 and 3) that were typical of those observed in mpl-/- control mice. The data presented here also extend previous studies29-31 to show thatLIF-/-, IL-6-/-, andIL-11Rα-/- mice exhibit normal megakaryocytopoiesis. Thus, although IL-6, IL-11, and LIF are each capable of elevating platelet counts on administration into animals,26-28 as individual stimuli, they are dispensable for steady state megakaryocytopoiesis, both in normal mice as well as in mice lacking the major regulator of this hemopoietic cell lineage.
Our data cannot exclude the possibility that cytokines signaling through gp130 act in concert to promote megakaryocyte development, and that deletion of a single factor is compensated by actions of other members of the group. As the targeted deletion of gp130 in mice results in embryonic lethality,33 the physiologic role of this cytokine family as a whole on megakaryocyte and platelet production has not been investigated. Recently, however, mice engineered to conditionally lack the gp130 signaling subunit were created in which the gp130 subunit was inactivated after the postnatal period.34 These mice exhibit neurologic, cardiac, immunologic, hepatic, and pulmonary defects, consistent with the varied roles of cytokines that act through gp130. Interestingly, the number of platelets in these mice was only 70% of normal values. Megakaryocyte and progenitor cell numbers were not enumerated, so it is not clear whether this result reflects direct actions of gp130 signaling in megakaryocytopoiesis or the indirect effects of other defects in these mice. If the phenotype of the conditional gp130 knockout mice does reflect an indispensable physiological role for the gp130 family of cytokines in steady-state megakaryocytopoiesis, quantitative comparison of the platelet deficiency in these animals (30%) with that in mpl-/- mice (90%) suggests that a functional overlap exists between these 2 cytokine systems. In any event, these mice cannot provide direct proof that a gp130–dependent component of steady-state megakaryocytopoiesis is responsible for the residual platelet production in mpl-/- mice. An intercross of the gp130– and Mpl-deficient mice would be required to definitively address this issue.
Our previous studies have excluded a role for IL-3 in the residual megakaryocytopoiesis of mpl-/- mice and the current data suggest that IL-11, IL-6, and LIF are also unlikely to contribute significantly. We have now examined each of the cytokines known to have a significant impact on megakaryocyte and platelet production. This raises the possibility that previously unidentified regulator(s) exist that may have the capacity to stimulate megakaryocytopoiesis. Alternatively, residual platelet production in Mpl-deficient mice may reflect a basal level of cytokine-independent megakaryocytopoiesis. Platelet release appears to occur in the absence of cytokines and may be largely controlled by entirely cell-intrinsic processes. The action of NF-E2 appears to play a key role in platelet shedding as mice lacking this transcription factor produce megakaryocytes with normal cytokine responsiveness but which cannot release platelets.35 This raises the possibility that earlier processes of megakaryocyte production might in part be cytokine independent, possibly also driven by the actions of specific transcription factors. GATA-1, for example, is required for megakaryocyte production, with mice lacking this transcription factor capable of generating only reduced numbers of relatively immature megakaryocytes.36,37 In this regard, although mice lacking EPO, the only cytokine known to stimulate the production of maturing erythrocytes, are extremely anemic, erythrocytes are not entirely absent.38 It should be noted, however, that in vitro studies provide no evidence for cytokine-independent hemopoiesis: production of hemopoietic cells in culture is strictly dependent on the addition of extrinsic factors.
In addition to the deficiency in megakaryocyte and platelet production, mice lacking c-Mpl exhibit reduced stem cell capacity that results in the production of subnormal levels of progenitor cells of all hemopoietic lineages.39,40 Despite the fact that mice lacking IL-6 or LIF have also been reported to have compromised stem cell activity,29 30 the deficit of hemopoietic progenitor cells inmpl-/-IL-6-/-,mpl-/-LIF-/-, ormpl-/-IL-11Rα-/-double-mutant mice was no more severe than in mice lacking only c-Mpl (Figure 4 and Table 3). Although we have not measured stem cell function in mpl-/-IL-6-/-,mpl-/-LIF-/-, ormpl-/-IL-11Rα-/- mice, this observation provides an indirect suggestion that the stem cell compartment in these compound mutants is no more compromised that inmpl-/- mice. If so, the hemopoietic stem cells reliant on LIF or IL-6 appear to be included within the TPO-dependent pool. Future comparison of stem cell function in compound mutant mice using definitive in vivo assays will directly resolve this issue. Thus, in addition to defining the roles of gp130–dependent cytokines in megakaryocyte and platelet development, thempl-/-IL-6-/-,mpl-/-LIF-/-, ormpl-/-IL-11Rα-/- mice will also be valuable reagents in dissecting the cytokine control of hemopoietic stem cell function.
Acknowledgments
We thank Naomi Sprigg, Sandra Mifsud, Ladina DiRago, Janelle Mighall, Rachel Mansfield, and Bette Papaevangeliou, for their skilled technical assistance.
Supported by the National Health and Medical Research Council, Canberra, the Anti-Cancer Council of Victoria, and the National Institute of Health, Bethesda, grant nos CA22556 and HL62275, an Australian Government Cooperative Research Centres Program Grant, and the Bone Marrow Donor Institute.
Reprints:Warren S. Alexander, The Walter and Eliza Hall Institute for Medical Research, PO Royal Melbourne Hospital, Victoria 3050, Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal